Proinflammatory Polyphosphate Increases in Plasma of Obese Children with Insulin Resistance and Adults with Severe Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.1.1. Children with Normal Weight, Overweight, and Obesity
2.1.2. Patients with Severe Type 2 Diabetes and Controls
2.2. Anthropomorphic and Biochemical Parameters
2.3. Plasma and Platelet Polyphosphate Determinations
2.4. Plasma von Willebrand Factor
2.5. Statistical Analysis
3. Results
3.1. Plasma Polyphosphate in Children with Overweight/obesity
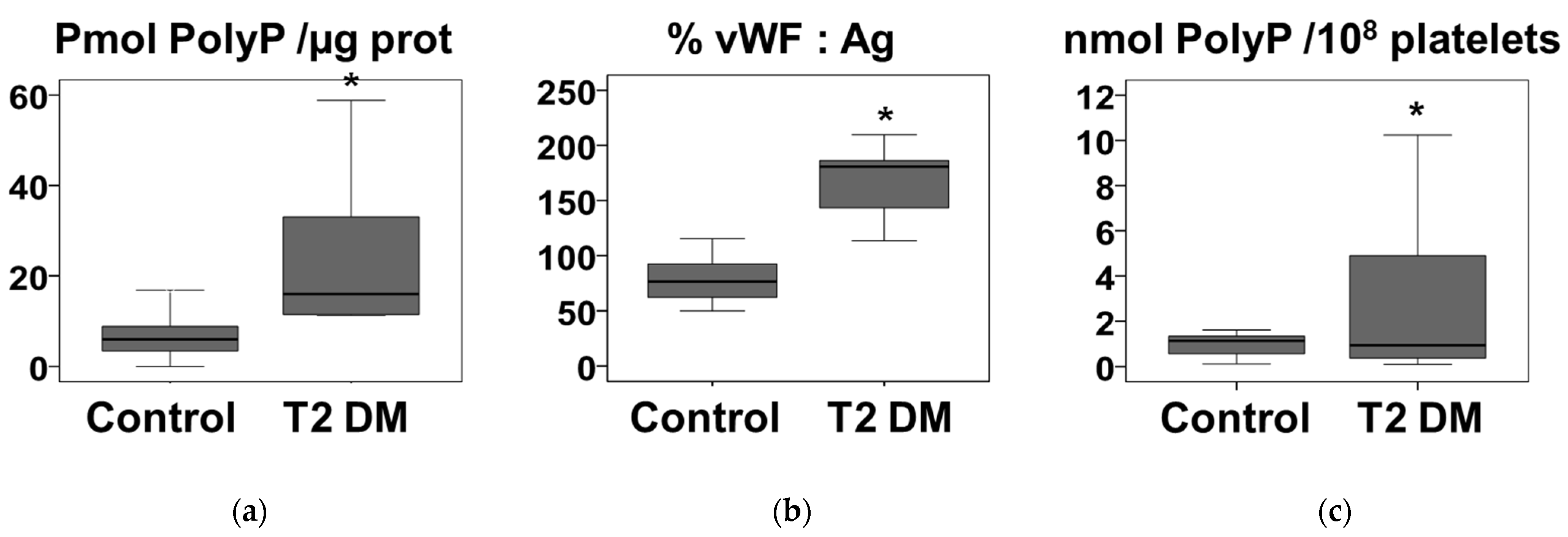
3.2. Plasma Polyphosphate in Severe Type 2 Diabetes Adult Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberto, C.A.; Swinburn, B.; Hawkes, C.; Huang, T.T.; Costa, S.A.; Ashe, M.; Zwicker, L.; Cawley, J.H.; Brownell, K.D. Patchy progress on obesity prevention: Emerging examples, entrenched barriers, and new thinking. Lancet 2015, 385, 2400–2409. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Levy-Marchal, C.; Arslanian, S.; Cutfield, W.; Sinaiko, A.; Druet, C.; Marcovecchio, M.L.; Chiarelli, F. Insulin resistance in children: Consensus, perspective, and future directions. J. Clin. Endocrinol. Metab. 2010, 95, 5189–5198. [Google Scholar] [CrossRef]
- Chiarelli, F.; Marcovecchio, M.L. Insulin resistance and obesity in childhood. Eur. J. Endocrinol. 2008, 159 (Suppl 1), S67–S74. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Insulin resistance: The link between obesity and cardiovascular disease. Med. Clin. N. Am. 2011, 95, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Cree-Green, M.; Triolo, T.M.; Nadeau, K.J. Etiology of insulin resistance in youth with type 2 diabetes. Curr. Diab. Rep. 2013, 13, 81–88. [Google Scholar] [CrossRef]
- Calle, M.C.; Fernandez, M.L. Inflammation and type 2 diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef]
- de Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef]
- Baker, C.J.; Smith, S.A.; Morrissey, J.H. Polyphosphate in thrombosis, hemostasis, and inflammation. Res. Pract. Thromb Haemost. 2019, 3, 18–25. [Google Scholar] [CrossRef]
- Rao, N.N.; Gómez-García, M.R.; Kornberg, A. Inorganic polyphosphate: Essential for growth and survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.A.; Lea, C.R.; Oldfield, E.; Docampo, R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J. Biol. Chem. 2004, 279, 44250–44257. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Mutch, N.J.; Baskar, D.; Rohloff, P.; Docampo, R.; Morrissey, J.H. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Smith, S.A.; Morrissey, J.H. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood 2011, 118, 6963–6970. [Google Scholar] [CrossRef] [PubMed]
- Wat, J.M.; Foley, J.H.; Krisinger, M.J.; Ocariza, L.M.; Lei, V.; Wasney, G.A.; Lameignere, E.; Strynadka, N.C.; Smith, S.A.; Morrissey, J.H.; et al. Polyphosphate suppresses complement via the terminal pathway. Blood 2014, 123, 768–776. [Google Scholar] [CrossRef]
- Biswas, I.; Panicker, S.R.; Cai, X.; Mehta-D’souza, P.; Rezaie, A.R. Inorganic Polyphosphate Amplifies High Mobility Group Box 1-Mediated Von Willebrand Factor Release and Platelet String Formation on Endothelial Cells. Arterioscler. Thromb Vasc. Biol. 2018, 38, 1868–1877. [Google Scholar] [CrossRef]
- Moreno-Sanchez, D.; Hernandez-Ruiz, L.; Ruiz, F.A.; Docampo, R. Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J. Biol. Chem. 2012, 287, 28435–28444. [Google Scholar] [CrossRef]
- Jimenez-Nuñez, M.D.; Moreno-Sanchez, D.; Hernandez-Ruiz, L.; Benítez-Rondán, A.; Ramos-Amaya, A.; Rodríguez-Bayona, B.; Medina, F.; Brieva, J.A.; Ruiz, F.A. Myeloma cells contain high levels of inorganic polyphosphate which is associated with nucleolar transcription. Haematologica 2012, 97, 1264–1271. [Google Scholar] [CrossRef]
- Chrysanthopoulou, A.; Kambas, K.; Stakos, D.; Mitroulis, I.; Mitsios, A.; Vidali, V.; Angelidou, I.; Bochenek, M.; Arelaki, S.; Arampatzioglou, A.; et al. Interferon lambda1/IL-29 and inorganic polyphosphate are novel regulators of neutrophil-driven thromboinflammation. J. Pathol. 2017, 243, 111–122. [Google Scholar] [CrossRef]
- Santi, M.J.; Montilla, M.; Carroza, M.A.; Ruiz, F.A. Novel assay for prothrombotic polyphosphates in plasma reveals their correlation with obesity. Thromb. Res. 2016, 144, 53–55. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Visiedo, F.; Domínguez-Riscart, J.; Durán-Ruiz, M.C.; Saez-Benito, A.; Lechuga-Sancho, A.M.; Mateos, R.M. Catalase post-translational modifications as key targets in the control of erythrocyte redox homeostasis in children with obesity and insulin resistance. Free Radic. Biol. Med. 2022, 191, 40–47. [Google Scholar] [CrossRef]
- Montilla, M.; Hernández-Ruiz, L.; García-Cozar, F.J.; Alvarez-Laderas, I.; Rodríguez-Martorell, J.; Ruiz, F.A. Polyphosphate binds to human von Willebrand factor in vivo and modulates its interaction with glycoprotein Ib. J. Thromb. Haemost. 2012, 10, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- Wurst, H.; Kornberg, A. A soluble exopolyphosphatase of Saccharomyces cerevisiae. Purification and characterization. J. Biol. Chem. 1994, 269, 10996–11001. [Google Scholar] [CrossRef]
- Xiang, Y.; Hwa, J. Regulation of VWF expression, and secretion in health and disease. Curr. Opin. Hematol. 2016, 23, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Pottinger, B.E.; Read, R.C.; Paleolog, E.M.; Higgins, P.G.; Pearson, J.D. von Willebrand factor is an acute phase reactant in man. Thromb. Res. 1989, 53, 387–394. [Google Scholar] [CrossRef]
- Meigs, J.B.; Mittleman, M.A.; Nathan, D.M.; Tofler, G.H.; Singer, D.E.; Murphy-Sheehy, P.M.; Lipinska, I.; D’Agostino, R.B.; Wilson, P.W. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: The Framingham Offspring Study. JAMA 2000, 283, 221–228. [Google Scholar] [CrossRef]
- Lim, H.S.; Lip, G.Y.; Blann, A.D. Plasma von Willebrand factor and the development of the metabolic syndrome in patients with hypertension. J. Clin. Endocrinol. Metab. 2004, 89, 5377–5381. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, G.; Yang, J.; Zheng, R.; Jiang, L.; Bao, P. The association between metabolic syndrome and vascular endothelial dysfunction in adolescents. Exp. Ther. Med. 2013, 5, 1663–1666. [Google Scholar] [CrossRef]
- Kälsch, J.; Bechmann, L.P.; Heider, D.; Best, J.; Manka, P.; Kälsch, H.; Sowa, J.P.; Moebus, S.; Slomiany, U.; Jöckel, K.H.; et al. Normal liver enzymes are correlated with severity of metabolic syndrome in a large population based cohort. Sci. Rep. 2015, 5, 13058. [Google Scholar] [CrossRef]
- Rückert, I.M.; Heier, M.; Rathmann, W.; Baumeister, S.E.; Döring, A.; Meisinger, C. Association between markers of fatty liver disease and impaired glucose regulation in men and women from the general population: The KORA-F4-study. PLoS ONE 2011, 6, e22932. [Google Scholar] [CrossRef]
- Schulze, M.B. Metabolic health in normal-weight and obese individuals. Diabetologia 2019, 62, 558–566. [Google Scholar] [CrossRef]
- Zembic, A.; Eckel, N.; Stefan, N.; Baudry, J.; Schulze, M.B. An Empirically Derived Definition of Metabolically Healthy Obesity Based on Risk of Cardiovascular and Total Mortality. JAMA Netw. Open 2021, 4, e218505. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, N.; Harper, M.T. High extracellular phosphate increases platelet polyphosphate content. Platelets 2021, 32, 992–994. [Google Scholar] [CrossRef]
- van der Vaart, A.; Yeung, S.M.H.; van Dijk, P.R.; Bakker, S.J.L.; de Borst, M.H. Phosphate and fibroblast growth factor 23 in diabetes. Clin. Sci. 2021, 135, 1669–1687. [Google Scholar] [CrossRef] [PubMed]
- van der Vaart, A.; Cai, Q.; Nolte, I.M.; van Beek, A.P.J.; Navis, G.; Bakker, S.J.L.; van Dijk, P.R.; de Borst, M.H. Plasma phosphate and all-cause mortality in individuals with and without type 2 diabetes: The Dutch population-based lifelines cohort study. Cardiovasc. Diabetol. 2022, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Shires, R.; Teitelbaum, S.L.; Bergfeld, M.A.; Fallon, M.D.; Slatopolsky, E.; Avioli, L.V. The effect of streptozotocin-induced chronic diabetes mellitus on bone and mineral homeostasis in the rat. J. Lab. Clin. Med. 1981, 97, 231–240. [Google Scholar] [PubMed]
- Imtiaz, R.; Hawken, S.; McCormick, B.B.; Leung, S.; Hiremath, S.; Zimmerman, D.L. Diabetes Mellitus and Younger Age Are Risk Factors for Hyperphosphatemia in Peritoneal Dialysis Patients. Nutrients 2017, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Mailer, R.K.W.; Hänel, L.; Allende, M.; Renné, T. Polyphosphate as a Target for Interference with Inflammation and Thrombosis. Front. Med. 2019, 6, 76. [Google Scholar] [CrossRef]
- Livermore, T.M.; Azevedo, C.; Kolozsvari, B.; Wilson, M.S.; Saiardi, A. Phosphate, inositol and polyphosphates. Biochem. Soc. Trans. 2016, 44, 253–259. [Google Scholar] [CrossRef]
- Azevedo, C.; Saiardi, A. Eukaryotic Phosphate Homeostasis: The Inositol Pyrophosphate Perspective. Trends Biochem. Sci. 2017, 42, 219–231. [Google Scholar] [CrossRef]
- Ghosh, S.; Shukla, D.; Suman, K.; Lakshmi, B.J.; Manorama, R.; Kumar, S.; Bhandari, R. Inositol hexakisphosphate kinase 1 maintains hemostasis in mice by regulating platelet polyphosphate levels. Blood 2013, 122, 1478–1486. [Google Scholar] [CrossRef]
- Jung, I.R.; Anokye-Danso, F.; Jin, S.; Ahima, R.S.; Kim, S.F. IPMK modulates hepatic glucose production and insulin signaling. J. Cell. Physiol. 2022, 237, 3421–3432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ceyhan, Y.; Kaftanovskaya, E.M.; Vasquez, J.L.; Vacher, J.; Knop, F.K.; Nathanson, L.; Agoulnik, A.I.; Ittmann, M.M.; Agoulnik, I.U. INPP4B protects from metabolic syndrome and associated disorders. Commun. Biol. 2021, 4, 416. [Google Scholar] [CrossRef] [PubMed]



| BMI < 25 (n = 5) | BMI > 25 (n = 10) | BMI > 30 (n = 10) | |
|---|---|---|---|
| Age (years) | 7.4 ± 0.2 | 9.6 ± 3.0 | 12.2 ± 1.4 a |
| Male/Female | 2/3 | 5/5 | 6/4 |
| BMI (kg/m2) | 20.7 ± 4.8 | 28.4 ± 1.6 a | 32.37 ± 3.1 a,b |
| TC (mg/dL) | 145.5 ± 34.7 | 154.5 ± 20.3 | 149.4 ± 43.6 |
| TG (mg/dL) | 78.2 ± 42.3 | 94.6 ± 40.3 | 95.0 ± 42.8 |
| LDL-C (mg/dL) | 67.3 ± 37.3 | 85.1 ± 21.6 | 83.9 ± 39.5 |
| HDL-C (mg/dL) | 64.7 ± 8.9 | 50.6 ± 11.6 | 46.5 ± 9.3 |
| Glucose (mg/dL) | 83.2 ± 11.6 | 85.9 ± 4.3 | 93.6 ± 8.0 |
| Insulin (µu/dL) | 8.0 ± 6.4 | 21.4 ± 10.2 a,c | 27.0 ± 15.9 a,c |
| HOMA-IR | 1.7 ± 1.4 | 4.4 ± 1.9 a,c | 6.2 ± 3.6 a,c |
| GOT (U/L) | 33.0 ± 8.6 | 21.7 ± 4.5 | 23.3 ± 4.4 |
| GPT (U/L) | 29.0 ± 16.0 | 20.1 ± 5.7 | 28.4 ± 9.7 |
| Univariate Analysis | Bivariate Analysis | |||
|---|---|---|---|---|
| Beta | p-Value | Beta | p-Value | |
| Age (years) | 0.318 | 0.46 | - | - |
| Male/Female | 2.481 | 0.2 | −1.72077 | 0.406 |
| BMI (kg/m2) | 0.166 | 0.47 | - | - |
| TC (mg/dL) | 0.004 | 0.904 | - | - |
| TG (mg/dL) | −0.023 | 0.43 | - | - |
| LDL-C (mg/dL) | −0.0007 | 0.981 | - | - |
| HDL-C (mg/dL) | 0.081 | 0.349 | - | - |
| Glucose (mg/dL) | 0.0181 | 0.9 | - | - |
| Insulin (µu/dL) | 0.1765 | 0.03 | 0.245938 | 0.567 |
| HOMA-IR | 0.7847 | 0.0325 | −0.60355 | 0.7629 |
| GOT (U/L) | 0.1787 | 0.315 | - | - |
| GPT (U/L) | 0.18 | 0.11 | 0.242983 | 0.0167 |
| vWF:Ag (%) | 0.0719 | 0.03 | −0.005027 | 0.8958 |
| R2 = 0.43 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montilla, M.; Liberato, A.; Ruiz-Ocaña, P.; Sáez-Benito, A.; Aguilar-Diosdado, M.; Lechuga-Sancho, A.M.; Ruiz, F.A. Proinflammatory Polyphosphate Increases in Plasma of Obese Children with Insulin Resistance and Adults with Severe Type 2 Diabetes. Nutrients 2022, 14, 4601. https://doi.org/10.3390/nu14214601
Montilla M, Liberato A, Ruiz-Ocaña P, Sáez-Benito A, Aguilar-Diosdado M, Lechuga-Sancho AM, Ruiz FA. Proinflammatory Polyphosphate Increases in Plasma of Obese Children with Insulin Resistance and Adults with Severe Type 2 Diabetes. Nutrients. 2022; 14(21):4601. https://doi.org/10.3390/nu14214601
Chicago/Turabian StyleMontilla, Marcela, Andrea Liberato, Pablo Ruiz-Ocaña, Ana Sáez-Benito, Manuel Aguilar-Diosdado, Alfonso Maria Lechuga-Sancho, and Felix A. Ruiz. 2022. "Proinflammatory Polyphosphate Increases in Plasma of Obese Children with Insulin Resistance and Adults with Severe Type 2 Diabetes" Nutrients 14, no. 21: 4601. https://doi.org/10.3390/nu14214601
APA StyleMontilla, M., Liberato, A., Ruiz-Ocaña, P., Sáez-Benito, A., Aguilar-Diosdado, M., Lechuga-Sancho, A. M., & Ruiz, F. A. (2022). Proinflammatory Polyphosphate Increases in Plasma of Obese Children with Insulin Resistance and Adults with Severe Type 2 Diabetes. Nutrients, 14(21), 4601. https://doi.org/10.3390/nu14214601





