The Association between Gut Microbiome and Pregnancy-Induced Hypertension: A Nested Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. DNA Extraction and Metagenomic Sequencing
2.3. Statistical Analysis
3. Results
3.1. Main Results
3.1.1. General Characteristics
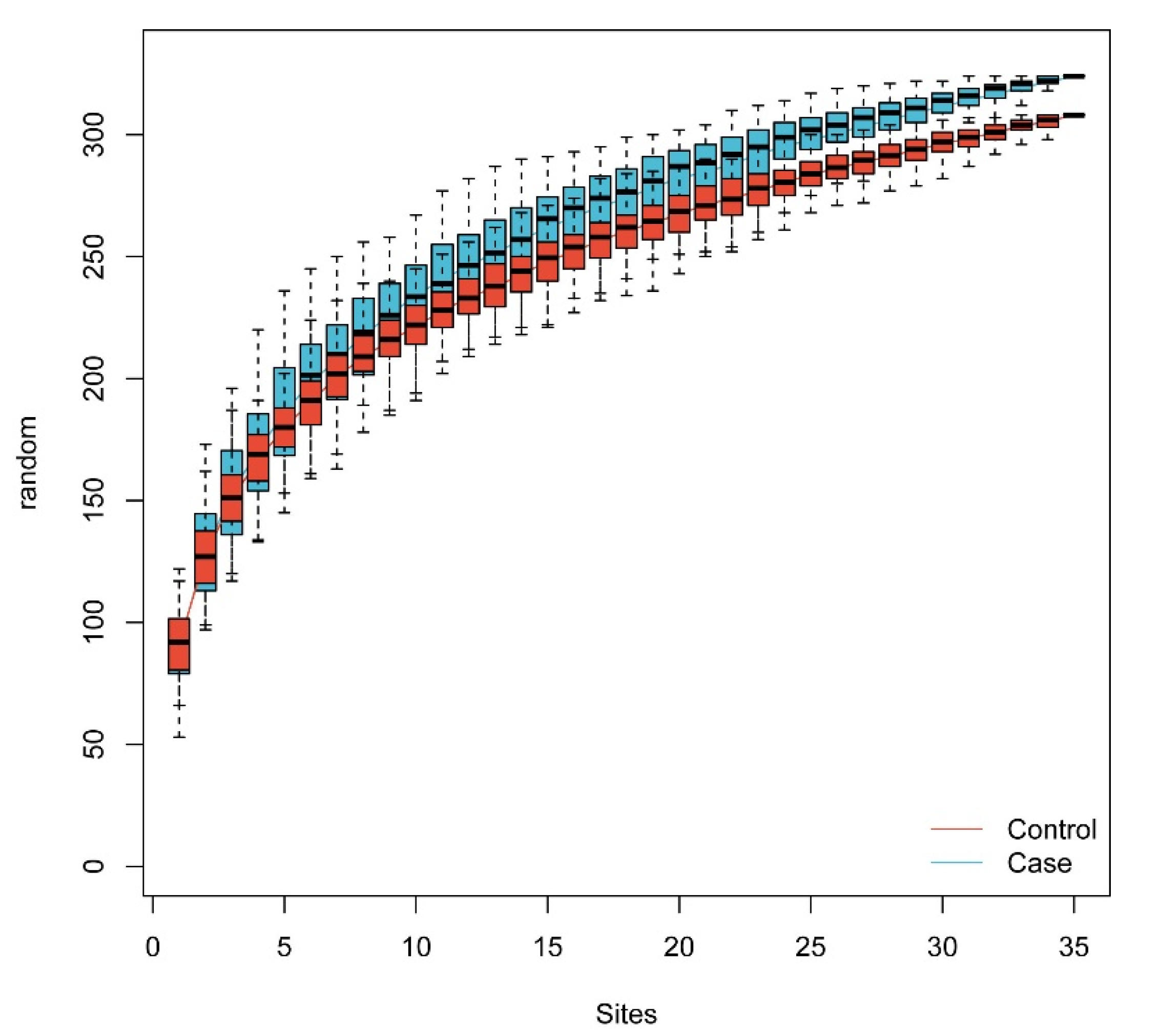
3.1.2. The Gut Microbiome Diversity Alteration
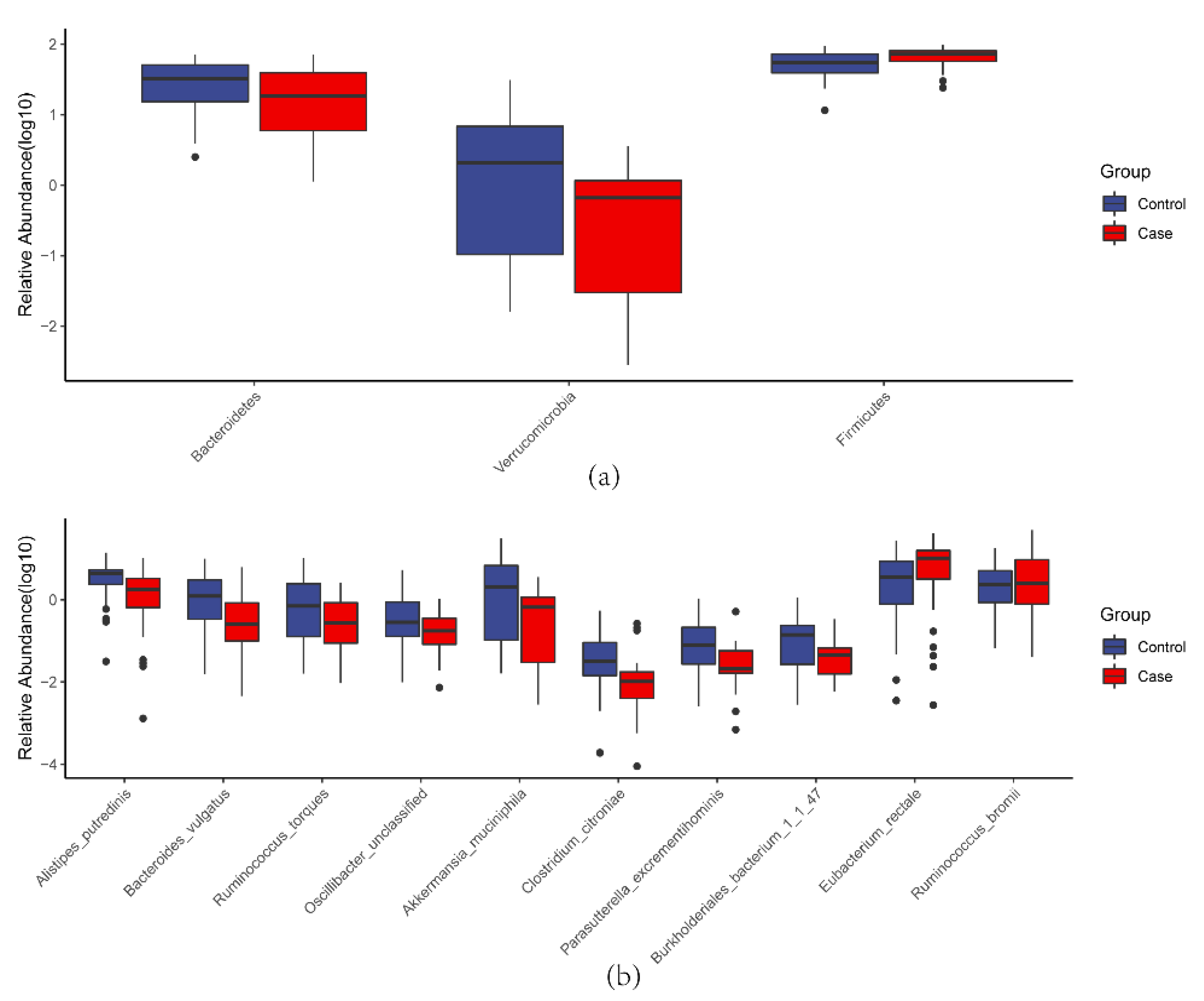
3.1.3. Microbial Taxa Alteration
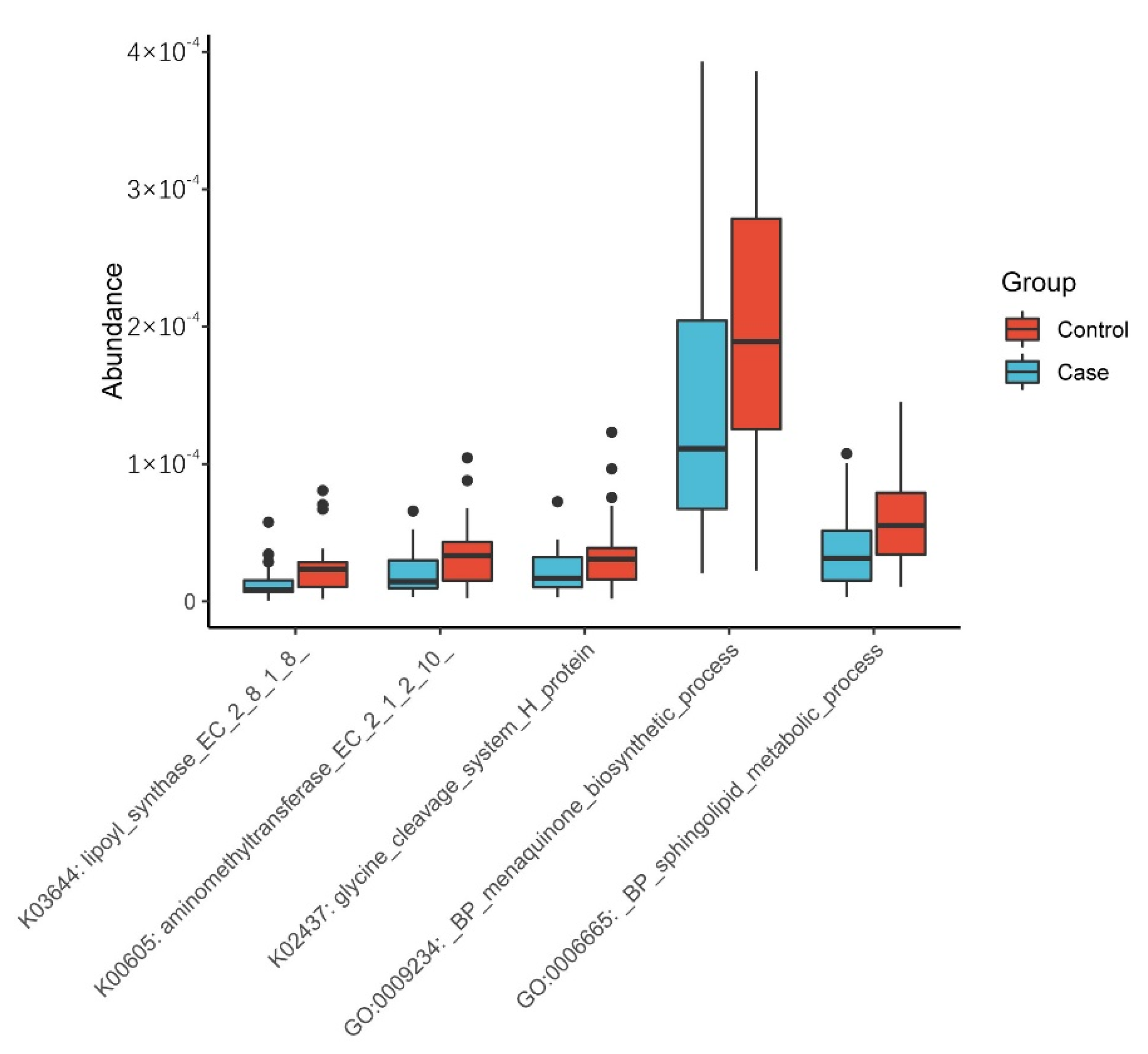
3.1.4. Functional Prediction Analysis
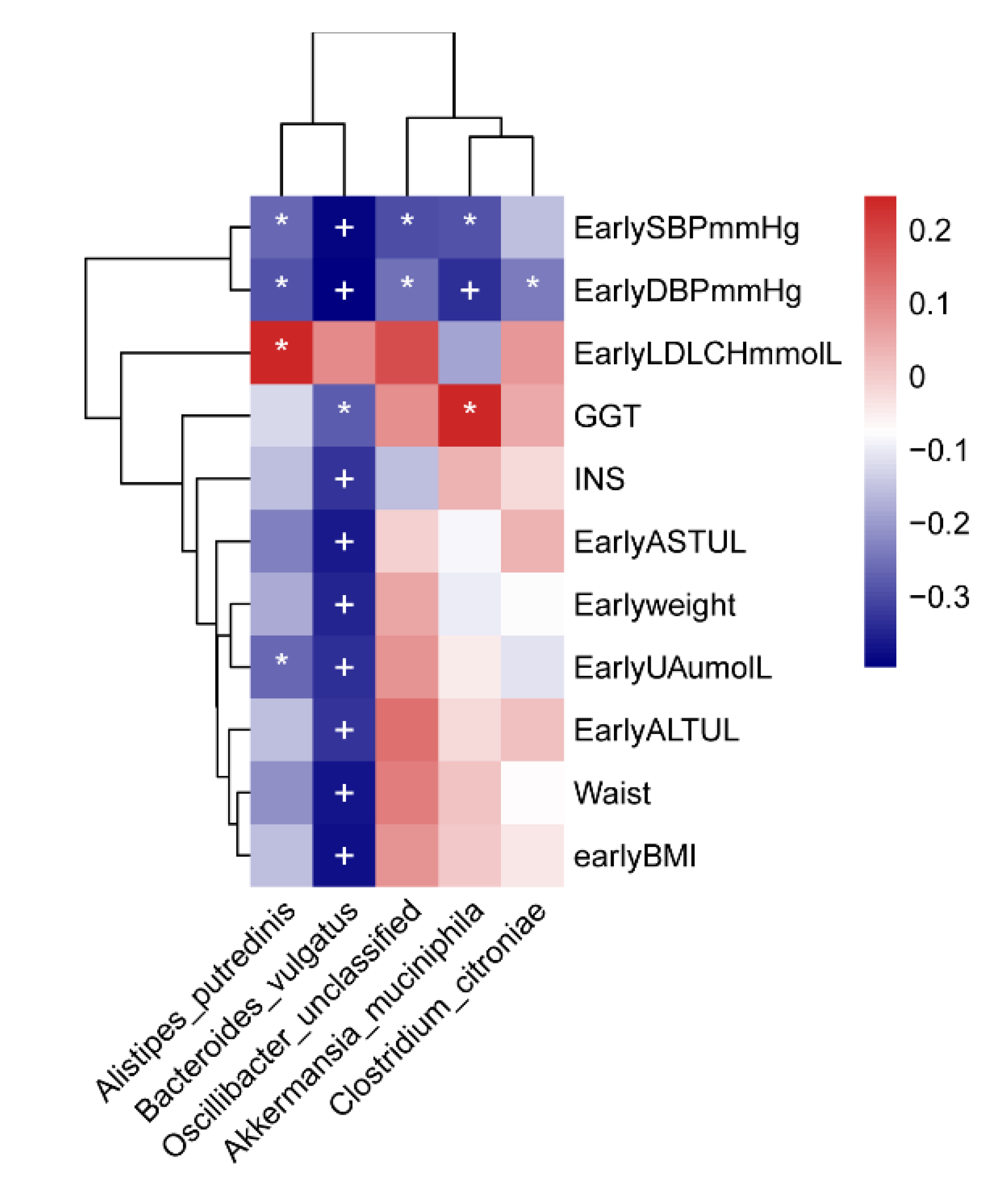
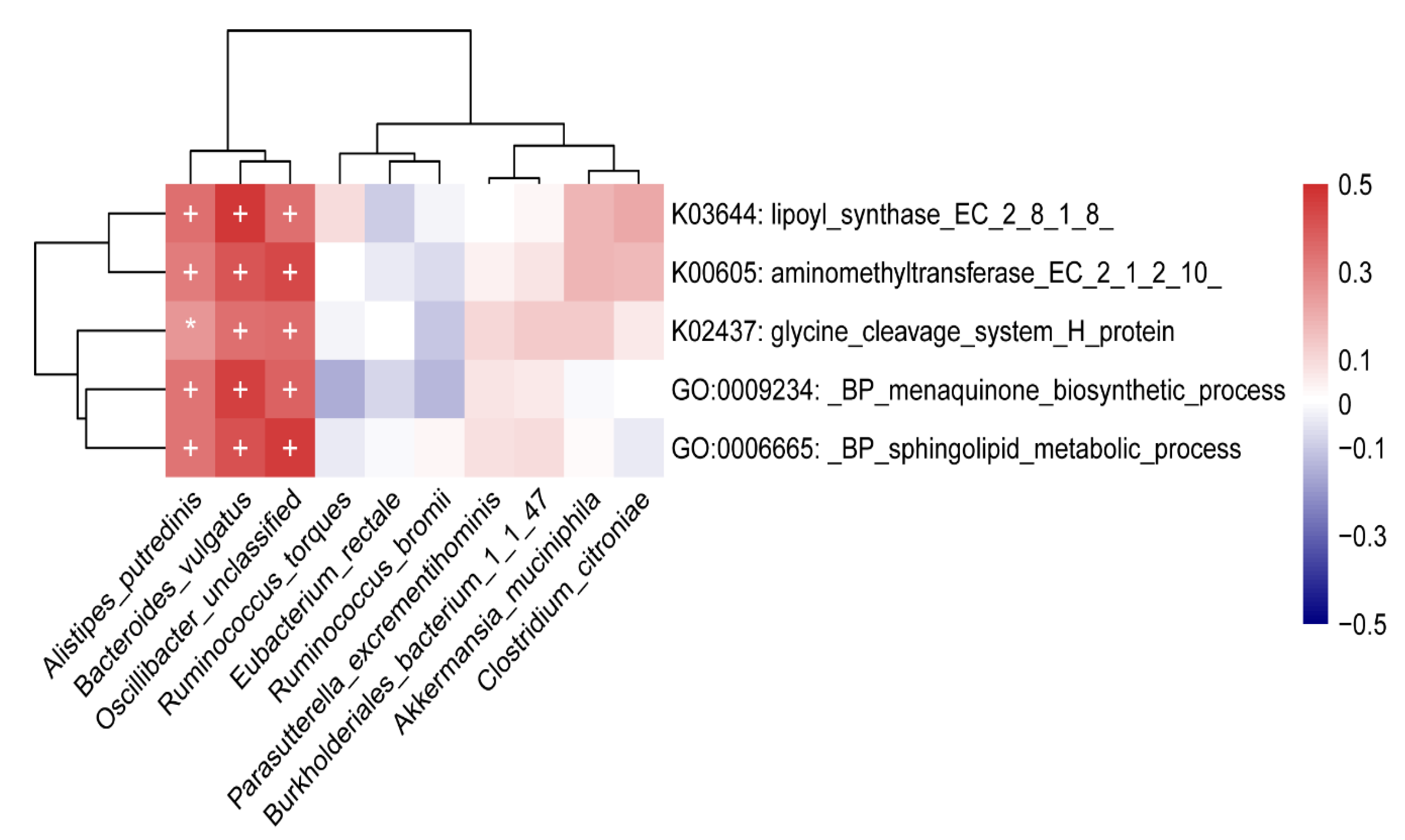
3.1.5. Correlation Analysis
Correlation between the Gut Microbiome and Clinical Factors
Correlation between Gut Microbiome and Functions
4. Discussion
4.1. Diversity Alteration
4.2. Microbial Taxa Alteration
4.3. Exploration of the Potential Mechanisms of the Gut Microbiome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kintiraki, E.; Papakatsika, S.; Kotronis, G.; Goulis, D.G.; Kotsis, V. Pregnancy-Induced hypertension. Hormones 2015, 14, 211–223. [Google Scholar] [CrossRef]
- Savitz, D.A.; Danilack, V.A.; Engel, S.M.; Elston, B.; Lipkind, H.S. Descriptive Epidemiology of Chronic Hypertension, Gestational Hypertension, and Preeclampsia in New York State, 1995–2004. Matern. Child. Health J. 2014, 18, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Umesawa, M.; Kobashi, G. Epidemiology of hypertensive disorders in pregnancy: Prevalence, risk factors, predictors and prognosis. Hypertens. Res. 2016, 40, 213–220. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Liu, Y.; An, H.; Li, Z.; Zhang, L.; Li, H.; Zhang, Y.; Ye, R.; Li, N.; Li, Z.; Zhang, L.; et al. Impact of gestational hypertension and preeclampsia on low birthweight and small-for-gestational-age infants in China: A large prospective cohort study. J. Clin. Hypertens 2021, 23, 835–842. [Google Scholar] [CrossRef]
- Abalos, E.; Cuesta, C.; Carroli, G.; Qureshi, Z.; Widmer, M.; Vogel, J.; Souza, J. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: A secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 14–24. [Google Scholar] [CrossRef]
- Khumanthem, P.D.; Chanam, M.S.; Samjetshabam, R.D. Maternal Mortality and Its Causes in a Tertiary Center. J. Obstet. Gynecol. India 2012, 62, 168–171. [Google Scholar] [CrossRef]
- You, F.; Huo, K.; Wang, R.; Xu, N.; Deng, J.; Wei, Y.; Shi, F.; Liu, H.; Cheng, G.; Zhang, Z.; et al. Maternal Mortality in Henan Province, China: Changes between 1996 and 2009. PLoS ONE 2012, 7, e47153. [Google Scholar] [CrossRef]
- Männistö, T.; Mendola, P.; Vääräsmäki, M.; Jarvelin, M.-R.; Hartikainen, A.-L.; Pouta, A.; Suvanto, E. Elevated Blood Pressure in Pregnancy and Subsequent Chronic Disease Risk. Circulation 2013, 127, 681–690. [Google Scholar] [CrossRef]
- Mitka, M. Any Hypertension during Pregnancy Raises Risk for Several Chronic Diseases. JAMA 2013, 309, 971–972. [Google Scholar] [CrossRef]
- Kajantie, E.; Eriksson, J.G.; Osmond, C.; Thornburg, K.; Barker, D.J. Pre-Eclampsia Is Associated with Increased Risk of Stroke in the Adult Offspring the Helsinki Birth Cohort Study. Stroke 2009, 40, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Miettola, S.; Hartikainen, A.-L.; Vääräsmäki, M.; Bloigu, A.; Ruokonen, A.; Järvelin, M.-R.; Pouta, A. Offspring’s blood pressure and metabolic phenotype after exposure to gestational hypertension in utero. Eur. J. Epidemiol. 2013, 28, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Kuciene, R.; Dulskiene, V. Associations of maternal gestational hypertension with high blood pressure and overweight/obesity in their adolescent offspring: A retrospective cohort study. Sci. Rep. 2022, 12, 3800. [Google Scholar] [CrossRef] [PubMed]
- Tuovinen, S.; Eriksson, J.G.; Kajantie, E.; Lahti, J.; Pesonen, A.K.; Heinonen, K.; Osmond, C.; Barker, D.J.; Räikkönen, K. Maternal hypertensive disorders in pregnancy and self-reported cognitive impairment of the offspring 70 years later: The Helsinki Birth Cohort Study. Am. J. Obstet. Gynecol. 2013, 208, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.; Pluymen, L.P.; Dalmeijer, G.W.; Groenhof, T.K.J.; Uiterwaal, C.S.; Smit, H.A.; Van Rossem, L. Hypertensive disorders of pregnancy and cardiometabolic outcomes in childhood: A systematic review. Eur. J. Prev. Cardiol. 2019, 26, 1718–1747. [Google Scholar] [CrossRef]
- Minter, M.R.; Zhang, C.; Leone, V.; Zhang, C.; Leone, V.; Zhang, X.; Oyler-Castrillo, P.; Zhang, X.; Musch, M.W.; Shen, X.; et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016, 6, 30018. [Google Scholar] [CrossRef]
- Hall, A.B.; Tolonen, A.C.; Xavier, R.J. Human genetic variation and the gut microbiome in disease. Nat. Rev. Genet. 2017, 18, 690–699. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Li, D.Y.; Hazen, S.L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 2019, 16, 137–154. [Google Scholar] [CrossRef]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Shi, W.; Du, N.; Xu, X.; Zhang, Y.; Ji, P.; Zhang, F.; Jia, Z.; Wang, Y.; et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 2018, 67, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Balci, S.; Tohma, Y.A.; Esin, S.; Onalan, G.; Tekindal, M.A.; Zeyneloglu, H.B. Gut dysbiosis may be associated with hyperemesis gravidarum. J. Matern. Neonatal Med. 2022, 3, 2041–2045. [Google Scholar] [CrossRef] [PubMed]
- Karbach, S.H.; Schonfelder, T.; Brandao, I.; Wilms, E.; Hörmann, N.; Jäckel, S.; Schüler, R.; Finger, S.; Knorr, M.; Lagrange, J.; et al. Gut Microbiota Promote Angiotensin II-Induced Arterial Hypertension and Vascular Dysfunction. J. Am. Heart Assoc. 2016, 5, e003698. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, H.; Gao, Y.; An, N.; Li, X.; Pan, X.; Yang, X.; Tian, L.; Sun, J.; Xiong, X.; et al. Gut microbiota-derived short-chain fatty acids and hypertension: Mechanism and treatment. Biomed. Pharmacother. 2020, 130, 110503. [Google Scholar] [CrossRef]
- Marques, F.Z.; Mackay, C.R.; Kaye, D.M. Beyond gut feelings: How the gut microbiota regulates blood pressure. Nat. Rev. Cardiol. 2018, 15, 20–32. [Google Scholar] [CrossRef]
- Tomasova, L.; Dobrowolski, L.; Jurkowska, H.; Wróbel, M.; Huc, T.; Ondrias, K.; Ostaszewski, R.; Ufnal, M. Intracolonic hydrogen sulfide lowers blood pressure in rats. Nitric Oxide 2016, 60, 50–58. [Google Scholar] [CrossRef]
- De Siena, M.; Laterza, L.; Matteo, M.V.; Mignini, I.; Schepis, T.; Rizzatti, G.; Ianiro, G.; Rinninella, E.; Cintoni, M.; Gasbarrini, A. Gut and Reproductive Tract Microbiota Adaptation during Pregnancy: New Insights for Pregnancy-Related Complications and Therapy. Microorganisms 2021, 9, 473. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. Increased Systolic and Diastolic Blood Pressure Is Associated with Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension 2016, 68, 974–981. [Google Scholar] [CrossRef]
- Chen, X.; Li, P.; Liu, M.; Zheng, H.; He, Y.; Chen, M.X.; Tang, W.; Yue, X.; Huang, Y.; Zhuang, L.; et al. Faculty Opinions recommendation of Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 2020, 69, 513–522. [Google Scholar] [CrossRef]
- Lv, L.J.; Li, S.H.; Li, S.C.; Zhong, Z.C.; Duan, H.L.; Tian, C.; Li, H.; He, W.; Chen, M.C.; He, T.W.; et al. Early-Onset Preeclampsia Is Associated with Gut Microbial Alterations in Antepartum and Postpartum Women. Front. Cell. Infect. Microbiol. 2019, 9, 224. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, C.; Li, Y.; Lam, T.-W.; Yiu, S.-M.; Kristiansen, K.; Wang, J. SOAP2: An improved ultrafast tool for short read alignment. Bioinformatics 2009, 25, 1966–1967. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.T.; Franzosa, E.A.; Tickle, T.L.; Scholz, M.; Weingart, G.; Pasolli, E.; Tett, A.; Huttenhower, C.; Segata, N. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat. Methods 2015, 12, 902–903. [Google Scholar] [CrossRef] [PubMed]
- Franzosa, E.A.; McIver, L.J.; Rahnavard, G.; Thompson, L.R.; Schirmer, M.; Weingart, G.; Lipson, K.S.; Knight, R.; Caporaso, J.G.; Segata, N.; et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat. Methods 2018, 15, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Mallick, H.; Rahnavard, A.; Mciver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar]
- Hu, J.; Zhong, X.; Yan, J.; Zhou, D.; Qin, D.; Xiao, X.; Zheng, Y.; Liu, Y. High-throughput sequencing analysis of intestinal flora changes in ESRD and CKD patients. BMC Nephrol. 2020, 21, 12. [Google Scholar] [CrossRef]
- Yan, H.-X.; An, W.-C.; Chen, F.; An, B.; Pan, Y.; Jin, J.; Xia, X.-P.; Cui, Z.-J.; Jiang, L.; Zhou, S.-J.; et al. Intestinal microbiota changes in Graves’ disease: A prospective clinical study. Biosci. Rep. 2020, 40, BSR20191242. [Google Scholar] [CrossRef]
- Wang, J.; Gu, X.; Yang, J.; Wei, Y.; Zhao, Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients With Preeclampsia. Front. Cell. Infect. Microbiol. 2019, 9, 409. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Z.-H.; Yang, J.; Wei, Y.; Wang, X.-Y.; Zhao, Y.-Y. Gut microbiota dysbiosis in preeclampsia patients in the second and third trimesters. Chin. Med. J. 2020, 133, 1057–1065. [Google Scholar] [CrossRef]
- Liu, J.; An, N.; Ma, C.; Li, X.; Zhang, J.; Zhu, W.; Zhang, Y.; Li, J. Correlation analysis of intestinal flora with hypertension. Exp. Ther. Med. 2018, 16, 2325–2330. [Google Scholar] [CrossRef]
- Qin, Q.; Yan, S.; Yang, Y.; Chen, J.; Li, T.; Gao, X.; Yan, H.; Wang, Y.; Wang, J.; Wang, S.; et al. A Metagenome-Wide Association Study of the Gut Microbiome and Metabolic Syndrome. Front. Microbiol. 2021, 12, 682721. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Chen, J.; Ma, X.; Ni, Y.; Shen, Y.; Yu, H.; Panagiotou, G.; Bao, Y. A metagenome-wide association study of gut microbiome and visceral fat accumulation. Comput. Struct. Biotechnol. J. 2020, 18, 2596–2609. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Emoto, T.; Yamashita, T.; Watanabe, H.; Hayashi, T.; Tabata, T.; Hoshi, N.; Hatano, N.; Ozawa, G.; Sasaki, N.; et al. Bacteroides vulgatus and Bacteroides dorei Reduce Gut Microbial Lipopolysaccharide Production and Inhibit Atherosclerosis. Circulation 2018, 138, 2486–2498. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The GenusAlistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, Y.; Zhou, Q.; Wang, C.; Chen, L.; Di, W.; Zhang, Y. Short-chain fatty acids accompanying changes in the gut microbiome contribute to the development of hypertension in patients with preeclampsia. Clin. Sci. 2020, 134, 289–302. [Google Scholar] [CrossRef]
- Cheng, D.; Xie, M.Z. A review of a potential and promising probiotic candidate—Akkermansia muciniphila. J. Appl. Microbiol. 2021, 130, 1813–1822. [Google Scholar] [CrossRef]
- Macchione, I.G.; Lopetuso, L.R.; Ianiro, G.; Napoli, M.; Gibiino, G.; Rizzatti, G.; Petito, V.; Gasbarrini, A.; Scaldaferri, F. Akkermansia muciniphila: Key player in metabolic and gastrointestinal disorders. Eur. Rev. Med. Pharmacol. 2019, 23, 8075–8083. [Google Scholar]
- Roshanravan, N.; Bastani, S.; Tutunchi, H.; Kafil, B.; Nikpayam, O.; Mesri Alamdari, N.; Hadi, A.; Sotoudeh, S.; Ghaffari, S.; Ostadrahimi, A. A comprehensive systematic review of the effectiveness of Akkermansia muciniphila, a member of the gut microbiome, for the management of obesity and associated metabolic disorders. Arch. Physiol. Biochem. 2021, 15, 1–11. [Google Scholar] [CrossRef]
- Zeng, Z.; Guo, X.; Zhang, J.; Yuan, Q.; Chen, S. Lactobacillus paracasei modulates the gut microbiota and improves inflammation in type 2 diabetic rats. Food Funct. 2021, 12, 6809–6820. [Google Scholar] [CrossRef]
- Dam, V.; Dalmeijer, G.W.; Vermeer, C.; Drummen, N.E.; Knapen, M.H.; Van Der Schouw, Y.T.; Beulens, J.W. The association between vitamin K and the metabolic syndrome: A ten year follow-up study in adults. J. Clin. Endocrinol. Metab. 2015, 100, 2472–2479. [Google Scholar] [CrossRef]
- Stępień, A.; Koziarska-Rościszewska, M.; Rysz, J.; Stępień, M. Biological Role of Vitamin K—With Particular Emphasis on Cardiovascular and Renal Aspects. Nutrients 2022, 14, 262. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.J.; Komai, M.; Shirakawa, H. Beneficial Effects of Vitamin K Status on Glycemic Regulation and Diabetes Mellitus: A Mini-Review. Nutrients 2020, 12, 2485. [Google Scholar] [CrossRef] [PubMed]
- Sakakima, Y.; Hayakawa, A.; Nagasaka, T.; Nakao, A. Prevention of hepatocarcinogenesis with phosphatidylcholine and menaquinone-4: In vitro and in vivo experiments. J. Hepatol. 2007, 47, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ou, W.; Lin, D.; Lin, M.; Huang, X.; Ni, S.; Chen, S.; Yong, J.; O’Gara, M.C.; Tan, X.; et al. Increased Uric Acid, Gamma-Glutamyl Transpeptidase and Alkaline Phosphatase in Early-Pregnancy Associated with the Development of Gestational Hypertension and Preeclampsia. Front. Cardiovasc. Med. 2021, 8, 756140. [Google Scholar] [CrossRef] [PubMed]
- Anusha, T.; Sankaranarayana, T. Study of Serum Calcium, Magnesium, Uric Acid and Liver Enzymes in Pregnancy Induced Hypertension. J. Evol. Med. Dent. Sci. 2018, 7, 1347–1352. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K. Maternal Serum Uric Acid as a Predictor of Severity of Hypertensive Disorders of Pregnancy: A Prospective Cohort Study. Curr. Hypertens Rev. 2019, 15, 154–160. [Google Scholar] [CrossRef]
- Cundiff, D.K.; Agutter, P.S. Cardiovascular Disease Death before Age 65 in 168 Countries Correlated Statistically with Biometrics, Socioeconomic Status, Tobacco, Gender, Exercise, Macronutrients, and Vitamin, K. Cureus 2016, 8, e748. [Google Scholar] [CrossRef]
- Spijkers, L.J.A.; van den Akker, R.F.P.; Janssen, B.J.A.; Debets, J.J.; De Mey, J.G.R.; Stroes, E.S.G.; van den Born, B.-J.H.; Wijesinghe, D.S.; Chalfant, C.E.; MacAleese, L.; et al. Hypertension Is Associated with Marked Alterations in Sphingolipid Biology: A Potential Role for Ceramide. PLoS ONE 2011, 6, e21817. [Google Scholar] [CrossRef]
- Maldonado-Hernández, J.; Saldaña-Dávila, G.E.; Piña-Aguero, M.I.; Núñez-García, B.A.; López-Alarcón, M.G. Association between Plasmatic Ceramides Profile and AST/ALT Ratio: C14:0 Ceramide as Predictor of Hepatic Steatosis in Adolescents Independently of Obesity. Can. J. Gastroenterol. Hepatol. 2017, 2017, 3689375. [Google Scholar] [CrossRef]
- Chaurasia, B.; Summers, S.A. Ceramides—Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol. Metab. 2015, 26, 538–550. [Google Scholar] [CrossRef]
- Zici, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar]
- El Midaoui, A.; de Champlain, J. Prevention of hypertension, insulin resistance, and oxidative stress by alpha-lipoic acid. Hypertension 2002, 39, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Ergür, B.U.; Çilaker Micili, S.; Yilmaz, O.; Akokay, P. The effects of α-lipoic acid on aortic injury and hypertension in the rat remnant kidney (5/6 nephrectomy) model. Anatol. J. Cardiol. 2015, 15, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.M.; Mcknight, S.M.; Edwards, A.K.; Wu, G.; Satterfield, M.C. Obesity increases hepatic glycine dehydrogenase and aminomethyltransferase expression while dietary glycine supplementation reduces white adipose tissue in Zucker diabetic fatty rats. Amino Acids 2020, 52, 1413–1423. [Google Scholar] [CrossRef]

| Variable | Control (N = 35) | PIH (N = 35) | p-Value |
|---|---|---|---|
| Age (year) | 31.3 ± 3.9 | 31.6 ± 4.2 | 0.221 |
| Gestational age (week) | 12.70 ± 0.86 | 13.15 ± 3.26 | 0.428 |
| Race | 0.221 | ||
| Han | 33 (94.3%) | 34 (97.1%) | |
| Others | 2 (5.7%) | 1 (2.9%) | |
| Occupation | 0.954 | ||
| Professionals | 9 (25.7%) | 8 (22.9%) | |
| Company employee | 14 (40.0%) | 15 (42.9%) | |
| Others | 12 (34.3%) | 12 (34.3%) | |
| Education | |||
| Junior college and below | 18 (51.4%) | 14 (40.0%) | |
| Undergraduate and above | 17 (48.6%) | 21 (60.0%) | |
| Monthly income | 0.334 | ||
| 10,000 and below | 18 (51.4%) | 22 (62.9%) | |
| 10,000 and above | 17 (48.6%) | 13 (37.1%) |
| Variable | Control (N = 35) | PIH (N = 35) | p-Value |
|---|---|---|---|
| Early waist (cm) | 79.47 ± 7.01 | 82.27 ± 9.08 | 0.041 |
| Early BMI (kg/m2) | 21.80 ± 2.20 | 23.27 ± 3.56 | 0.004 |
| Early SBP (mmHg) | 114.77 ± 9.54 | 125.06 ± 7.82 | <0.001 |
| Early DBP (mmHg) | 73.49 ± 8.46 | 81.74 ± 7.28 | <0.001 |
| HGB (g/L) | 122.71 ± 10.51 | 127.61 ± 7.27 | 0.026 |
| GLU (mmol/L) | 4.52 ± 0.39 | 4.72 ± 0.47 | 0.062 |
| ALB (g/L) | 45.84 ± 2.26 | 44.99 ± 2.48 | 0.150 |
| ALT (U/L) | 17.57 ± 10.54 | 22.74 ± 16.28 | 0.123 |
| AST (U/L) | 18.46 ± 5.48 | 21.34 ± 8.15 | 0.093 |
| CREA (umol/L) | 43.23 ± 8.16 | 44.09 ± 7.44 | 0.640 |
| UA (umol/L) | 197.89 ± 38.93 | 230.62 ± 54.07 | 0.006 |
| UREA (mmol/L) | 2.50 ± 0.55 | 2.62 ± 0.78 | 0.497 |
| TG (mmol/L) | 1.42 ± 0.40 | 1.67 ± 0.65 | 0.057 |
| TCHOL (mmol/L) | 4.74 ± 0.86 | 4.60 ± 0.79 | 0.500 |
| HDLCH (mmol/L) | 1.89 ± 0.39 | 1.90 ± 0.44 | 0.960 |
| LDLCH (mmol/L) | 2.74 ± 0.78 | 2.55 ± 0.71 | 0.313 |
| hsCRP (mg/L) | 3.86 ± 4.20 | 4.95 ± 5.01 | 0.324 |
| INS (mU/L) | 19.19 ± 31.74 | 45.28 ± 60.05 | 0.020 |
| GGT (U/L) | 13.57 ± 5.79 | 22.33 ± 16.94 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Chen, J.; Ma, S.; An, R.; Li, X.; Tan, H. The Association between Gut Microbiome and Pregnancy-Induced Hypertension: A Nested Case–Control Study. Nutrients 2022, 14, 4582. https://doi.org/10.3390/nu14214582
Lin H, Chen J, Ma S, An R, Li X, Tan H. The Association between Gut Microbiome and Pregnancy-Induced Hypertension: A Nested Case–Control Study. Nutrients. 2022; 14(21):4582. https://doi.org/10.3390/nu14214582
Chicago/Turabian StyleLin, Huijun, Junru Chen, Shujuan Ma, Rongjing An, Xingli Li, and Hongzhuan Tan. 2022. "The Association between Gut Microbiome and Pregnancy-Induced Hypertension: A Nested Case–Control Study" Nutrients 14, no. 21: 4582. https://doi.org/10.3390/nu14214582
APA StyleLin, H., Chen, J., Ma, S., An, R., Li, X., & Tan, H. (2022). The Association between Gut Microbiome and Pregnancy-Induced Hypertension: A Nested Case–Control Study. Nutrients, 14(21), 4582. https://doi.org/10.3390/nu14214582





