Effects of Vitamin D on Satellite Cells: A Systematic Review of In Vivo Studies
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Selection Process
- Articles are written in English
- Published in the last ten years
- Intervention with vitamin D supplementation or vitamin D deficiency
- Assess the number or function of satellite cells
- Review articles
- In vitro studies
- Vitamin D supplementation in combination with other interventions (drugs/nutrients/exercise)
- The study does not state the markers of the satellite cell’s function
2.2. Data Extraction
2.3. Risk of Bias Assessment
3. Results
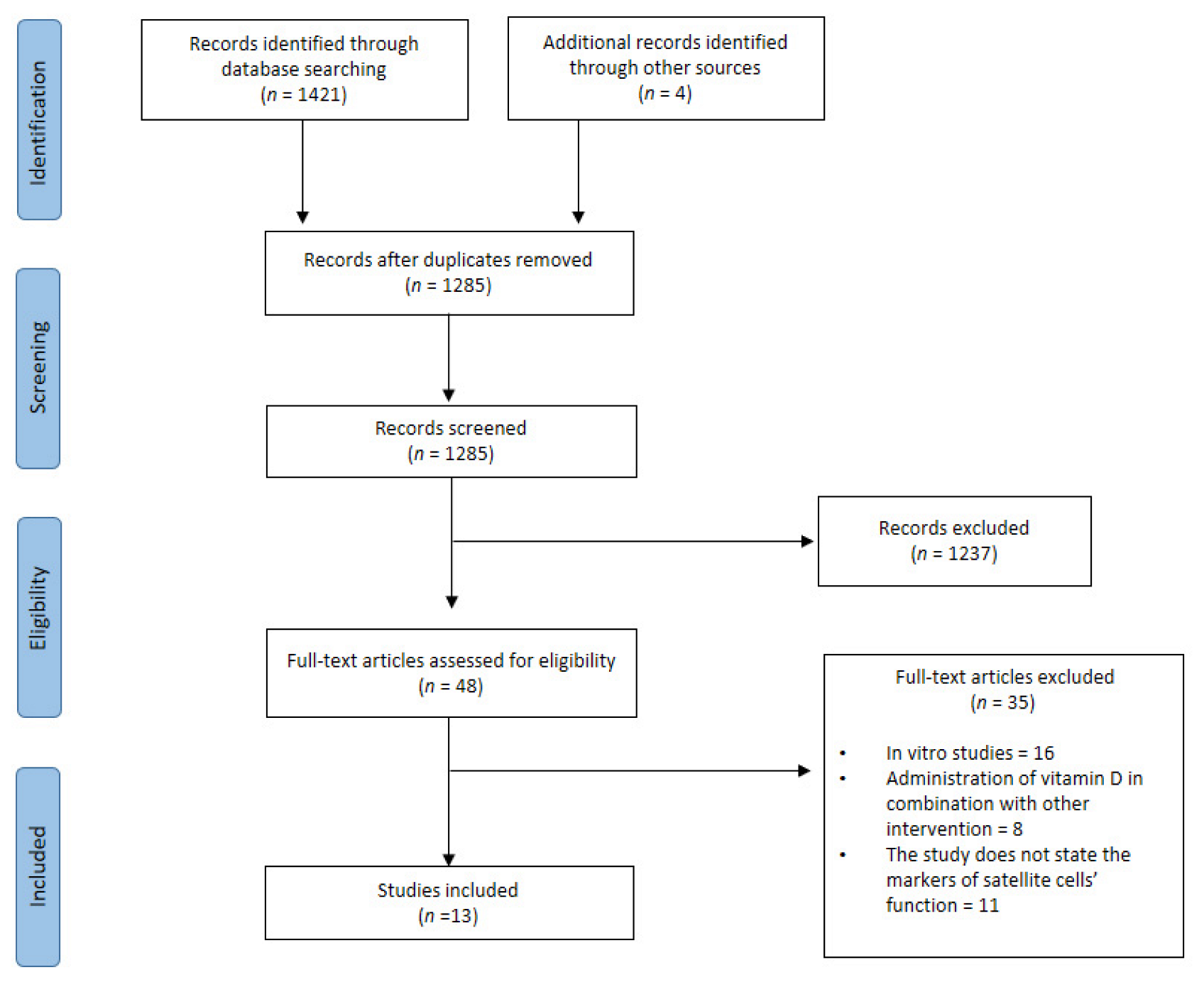
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias Assessment
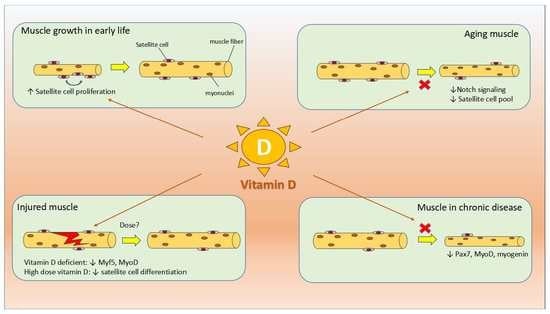
3.4. Effect of Vitamin D on Satellite Cells during Prenatal Development and Postnatal Growth
3.5. Effect of Vitamin D on Satellite Cell during Muscle Injury
3.6. Effect of Vitamin D on Satellite Cells during Aging and Diseased States
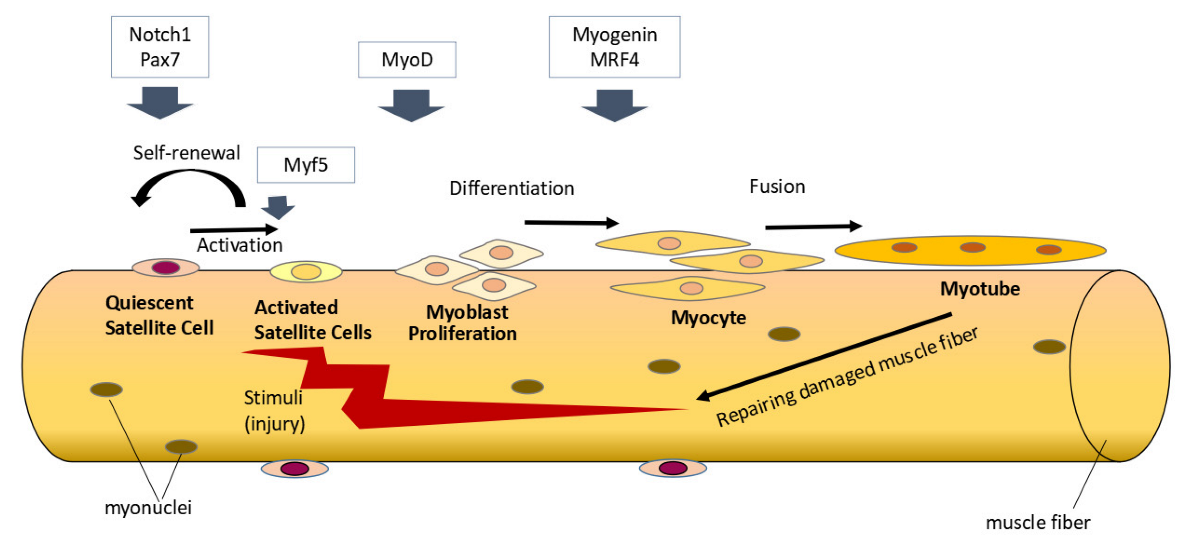
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latham, C.M.; Brightwell, C.R.; Keeble, A.R.; Munson, B.D.; Thomas, N.T.; Zagzoog, A.M.; Fry, C.S.; Fry, J.L. Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Front. Physiol. 2021, 12, 660498. [Google Scholar] [CrossRef] [PubMed]
- Ran, Z.; Declan, P.N. Vitamin D in health and disease: Current perspectives. Nutr. J. 2010, 9, 65. [Google Scholar] [CrossRef]
- Bollen, S.E.; Atherton, P.J. Myogenic, genomic and non-genomic influences of the vitamin D axis in skeletal muscle. Cell Biochem. Funct. 2021, 39, 48–59. [Google Scholar] [CrossRef]
- Lappe, J.M. The role of vitamin D in human health: A paradigm shift. Complement. Health Pract. Rev. 2011, 16, 58–72. [Google Scholar] [CrossRef]
- Holick, M. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol.-Ren. Physiol. 2005, 289, 8–28. [Google Scholar] [CrossRef]
- DeLuca, H.F. The metabolism and functions of vitamin D. Adv Exp Med Biol. 1986, 196, 361–375. [Google Scholar] [CrossRef]
- Zittermann, A.; Gummert, J.F. Nonclassical vitamin D Actions. Nutrients 2010, 2, 408–425. [Google Scholar] [CrossRef]
- Ceglia, L. Vitamin D and its role in skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 628–633. [Google Scholar] [CrossRef]
- Wang, Y.X.; Rudnicki, M.A. Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Biol. 2012, 13, 127–133. [Google Scholar] [CrossRef]
- Chen, W.; Datzkiw, D.; Rudnicki, M.A. Satellite cells in ageing: Use it or lose it. Open Biol. 2020, 10, 200048. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, N.; Asakura, A. Molecular Regulation of Muscle Satellite Cell Self-Renewal. J. Stem. Cell Res. Ther. 2012, S11, e002. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.A.; Wang, Y.X.; Rudnicki, M.A. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 2015, 142, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Marg, A.; Escobar, H.; Gloy, S.; Kufeld, M.; Zacher, J.; Spuler, A.; Birchmeier, C.; Izsvák, Z.; Spuler, S. Human satellite cells have regenerative capacity and are genetically manipulable. J. Clin. Investig. 2014, 124, 4257–4265. [Google Scholar] [CrossRef]
- Almeida, C.F.; Fernandes, S.A.; Ribeiro, A.F., Jr.; Okamoto, O.K. Muscle Satellite Cells: Exploring the Basic Biology to Rule Them. Stem Cells Int. 2016, 2016, 1078686. [Google Scholar] [CrossRef]
- Olsson, K.; Saini, A.; Strömberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for Vitamin D receptor expression and direct effects of 1α,25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology 2016, 157, 98–111. [Google Scholar] [CrossRef]
- Braga, M.; Simmons, Z.; Norris, K.C.; Ferrini, M.G.; Artaza, J.N. Vitamin D induces myogenic differentiation in skeletal muscle derived stem cells. Endocr. Connect. 2017, 6, 139–150. [Google Scholar] [CrossRef]
- Srikuea, R.; Hirunsai, M.; Charoenphandhu, N. Regulation of vitamin D system in skeletal muscle and resident myogenic stem cell during development, maturation, and ageing. Sci. Rep. 2020, 10, 8239. [Google Scholar] [CrossRef]
- Alliband, K.H.; Kozhevnikova, S.V.; Parr, T.; Jethwa, P.H.; Brameld, J.M. In Vitro Effects of Biologically Active Vitamin D on Myogenesis: A Systematic Review. Front. Physiol. 2021, 12, 736708. [Google Scholar] [CrossRef]
- Cornelison, D.D.W. Context matters: In vivo and in vitro influences on muscle satellite cell activity. J. Cell. Biochem. 2008, 105, 663–669. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef]
- Kuang, S.; Gillespie, M.A.; Rudnicki, M.A. Niche Regulation of Muscle Satellite Cell Self-Renewal and Differentiation. Cell Stem Cell 2008, 2, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, B.D.; Sacco, A.; Gilbert, P.M.; Blau, H.M. A home away from home: Challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation 2009, 78, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.D.; Nowomiejska, K.; Avitabile, T.; Rejdak, R.; Tripodi, S.; Porta, A.; Reibaldi, M.; Figus, M.; Posarelli, C.; Fiedorowicz, M. Effect of resveratrol on in vitro and in vivo models of diabetic retinophathy: A systematic review. Int. J. Mol. Sci. 2019, 20, 3503. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Hutton, K.C.; Vaughn, M.A.; Litta, G.; Turner, B.J.; Starkey, J.D. Effect of vitamin D status improvement with 25-hydroxycholecalciferol on skeletal muscle growth characteristics and satellite cell activity in broiler chickens. J. Anim. Sci. 2014, 92, 3291–3299. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Y.; Lv, G.; Zhuo, Y.; Lin, Y.; Feng, B.; Fang, Z.; Che, L.; Li, J.; Xu, S.; et al. Improving maternal vitamin D status promotes prenatal and postnatal skeletal muscle development of pig offspring. Nutrition 2016, 32, 1144–1152. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84969508548&doi=10.1016%2Fj.nut.2016.03.004&partnerID=40&md5=89bfc0901e2b97b308637964014ff6e7 (accessed on 26 September 2022). [CrossRef]
- Oku, Y.; Tanabe, R.; Nakaoka, K.; Yamada, A.; Noda, S.; Hoshino, A.; Haraikawa, M.; Goseki-Sone, M. Influences of dietary vitamin D restriction on bone strength, body composition and muscle in rats fed a high-fat diet: Involvement of mRNA expression of MyoD in skeletal muscle. J. Nutr. Biochem. 2016, 32, 85–90. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84962343810&doi=10.1016%2Fj.jnutbio.2016.01.013&partnerID=40&md5=1a5a871372bdedafd1caf0e05540d800 (accessed on 26 September 2022). [CrossRef]
- Thayer, M.T.; Nelssen, J.L.; Langemeier, A.J.; Morton, J.M.; Gonzalez, J.M.; Kruger, S.R.; Ou, Z.; Makowski, A.J.; Bergstrom, J.R. The effects of maternal dietary supplementation of cholecalciferol (vitamin D3) and 25(OH)D3 on sow and progeny performance. Transl. Anim. Sci. 2019, 3, 693–708. [Google Scholar] [CrossRef]
- Reis, N.G.; Assis, A.P.; Lautherbach, N.; Gonçalves, D.A.; Silveira, W.A.; Morgan, H.J.N.; Valentim, R.R.; Almeida, L.F.; Heck, L.C.; Zanon, N.M.; et al. Maternal vitamin D deficiency affects the morphology and function of glycolytic muscle in adult offspring rats. J. Cachexia. Sarcopenia Muscle 2022, 13, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Stratos, I.; Li, Z.; Herlyn, P.; Rotter, R.; Behrendt, A.K.; Mittlmeier, T.; Vollmar, B. Vitamin D increases cellular turnover and functionally restores the skeletal muscle after crush injury in rats. Am. J. Pathol. 2013, 182, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Srikuea, R.; Hirunsai, M. Effects of intramuscular administration of 1α,25(OH)2D3 during skeletal muscle regeneration on regenerative capacity, muscular fibrosis, and angiogenesis. J. Appl. Physiol. 2016, 120, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ren, B.; Chen, H.; Goltzman, D.; Yan, J.; Miao, D. 1,25-Dihydroxyvitamin D deficiency induces sarcopenia by inducing skeletal muscle cell senescence. Am. J. Transl. Res. 2021, 13, 12638–12649. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34956479%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC8661220 (accessed on 26 September 2022).
- Domingues-Faria, C.; Chanet, A.; Salles, J.; Berry, A.; Giraudet, C.; Patrac, V.; Denis, P.; Bouton, K.; Goncalves-Mendes, N.; Vasson, M.-P.; et al. Vitamin D deficiency down-regulates Notch pathway contributing to skeletal muscle atrophy in old wistar rats. Nutr. Metab. 2014, 11, 47. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84908096067&doi=10.1186%2F1743-7075-11-47&partnerID=40&md5=1e7f23866adf31b8e2e035f7b8a2e8ee (accessed on 26 September 2022). [CrossRef]
- Kinoshita, H.; Miyakoshi, N.; Kasukawa, Y.; Sakai, S.; Shiraishi, A.; Segawa, T.; Ohuchi, K.; Fujii, M.; Sato, C.; Shimada, Y. Effects of eldecalcitol on bone and skeletal muscles in glucocorticoid-treated rats. J. Bone Miner. Metab. 2016, 34, 171–178. [Google Scholar] [CrossRef]
- Tamura, Y.; Fujito, H.; Kawao, N.; Kaji, H. Vitamin D deficiency aggravates diabetes-induced muscle wasting in female mice. Diabetol. Int. 2017, 8, 52–58. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85013162252&doi=10.1007%2Fs13340-016-0278-7&partnerID=40&md5=e79620fa5e240c9dbb768b3918e7f6dd (accessed on 26 September 2022). [CrossRef]
- Nakaoka, K.; Yamada, A.; Noda, S.; Goseki-Sone, M. Influence of dietary vitamin D deficiency on bone strength, body composition, and muscle in ovariectomized rats fed a high-fat diet. Nutrition. 2019, 60, 87–93. [Google Scholar] [CrossRef]
- Cheung, W.W.; Hao, S.; Wang, Z.; Ding, W.; Zheng, R.; Gonzalez, A.; Zhan, J.Y.; Zhou, P.; Li, S.; Esparza, M.C.; et al. Vitamin D repletion ameliorates adipose tissue browning and muscle wasting in infantile nephropathic cystinosis-associated cachexia. J. Cachexia. Sarcopenia Muscle 2020, 11, 120–134. [Google Scholar] [CrossRef]
- Macleod, M.R.; Lawson McLean, A.; Kyriakopoulou, A.; Serghiou, S.; de Wilde, A.; Sherratt, N.; Hirst, T.; Hemblade, R.; Bahor, Z.; Nunes-Fonseca, C.; et al. Risk of Bias in Reports of In Vivo Research: A Focus for Improvement. PLoS biology. 2015, 13, e1002273. [Google Scholar] [CrossRef]
- Roman, W.; Gomes, E.R. Nuclear positioning in skeletal muscle. Semin. Cell Dev. Biol. 2018, 82, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Echigoya, Y.; Maruyama, R.; Lim, K.R.Q.; Fukada, S.I.; Yokota, T. Impaired regenerative capacity and lower revertant fibre expansion in dystrophin-deficient mdx muscles on DBA/2 background. Sci. Rep. 2016, 6, 38371. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.N.; Tajbakhsh, S.; Mouly, V.; Cossu, G.; Buckingham, M.; Butler-Browne, G.S. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J. Cell Sci. 1999, 112, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S.; Golding, J.P.; Nagata, Y.; Hudon, V.; Partridge, T.A.; Beauchamp, J.R. Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J. Cell Biol. 2004, 166, 347–357. [Google Scholar] [CrossRef]
- Cornelison, D.D.W.; Wold, B.J. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 1997, 191, 270–283. [Google Scholar] [CrossRef]
- Gioftsidi, S.; Relaix, F.; Mourikis, P. The Notch signaling network in muscle stem cells during development, homeostasis, and disease. Skelet. Muscle 2022, 12, 9. [Google Scholar] [CrossRef]
- Fujimaki, S.; Ono, Y. Notch signaling in the regulation of skeletal muscle stem cells. J. Phys. Fit. Sport. Med. 2018, 7, 213–219. [Google Scholar] [CrossRef]
- Von Maltzahn, J.; Jones, A.E.; Parks, R.J.; Rudnicki, M.A. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc. Natl. Acad. Sci. USA 2013, 110, 16474–16479. [Google Scholar] [CrossRef]
- An, Y.; Wang, G.; Diao, Y.; Long, Y.; Fu, X.; Weng, M.; Zhou, L.; Sun, K.; Cheung, T.H.; Ip, N.Y.; et al. A Molecular Switch Regulating Cell Fate Choice between Muscle Progenitor Cells and Brown Adipocytes. Dev. Cell 2017, 41, 382–391.e5. [Google Scholar] [CrossRef]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 is required for the specification of myogenic satellite cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef]
- Davis, T.A.; Fiorotto, M.L. Regulation of muscle growth in neonates. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 78–85. [Google Scholar] [CrossRef]
- Kawano, F.; Takeno, Y.; Nakai, N.; Higo, Y.; Terada, M.; Ohira, T.; Nonaka, I.; Ohira, Y. Essential role of satellite cells in the growth of rat soleus muscle fibers. Am. J. Physiol.-Cell Physiol. 2008, 295, C458–C467. [Google Scholar] [CrossRef] [PubMed]
- Bendik, I.; Friedel, A.; Roos, F.F.; Weber, P.; Eggersdorfer, M. Vitamin D: A critical and essential micronutrient for human health. Front. Physiol. 2014, 5, 248. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; Summan, M.; Gao, X.; Chapman, R.; Hulderman, T.; Simeonova, P.P. Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J. Physiol. 2007, 582, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Hosoyama, T.; Iida, H.; Kawai-Takaishi, M.; Watanabe, K. Vitamin d inhibits myogenic cell fusion and expression of fusogenic genes. Nutrients 2020, 12, 2192. [Google Scholar] [CrossRef]
- Ryan, K.J.P.; Daniel, Z.C.T.R.; Craggs, L.J.L.; Parr, T.; Brameld, J.M. Dose-dependent effects of vitamin D on transdifferentiation of skeletal muscle cells to adipose cells. J. Endocrinol. 2013, 217, 45–58. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84877107028&doi=10.1530%2FJOE-12-0234&partnerID=40&md5=78beefb1f8149ca108fa2407dcd433d8 (accessed on 26 September 2022). [CrossRef]
- Owens, D.J.; Sharples, A.P.; Polydorou, I.; Alwan, N.; Donovan, T.; Tang, J.; Fraser, W.D.; Cooper, R.G.; Morton, J.P.; Stewart, C.; et al. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am. J. Physiol.-Endocrinol. Metab. 2015, 309, E1019–E1031. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84951834947&doi=10.1152%2Fajpendo.00375.2015&partnerID=40&md5=04cafdcd8c67023c6dc1de3e3172bdaa (accessed on 26 September 2022). [CrossRef]
- Parker, M.H. The altered fate of aging satellite cells is determined by signaling and epigenetic changes. Front. Genet. 2015, 5, 59. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. Available online: http://www.nature.com/nature/journal/v433/n7027/abs/nature03260.html (accessed on 26 September 2022). [CrossRef]
- Gopinath, S.D.; Webb, A.E.; Brunet, A.; Rando, T.A. FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal. Stem Cell Reports 2014, 2, 414–426. [Google Scholar] [CrossRef]
- Ono, Y.; Calhabeu, F.; Morgan, J.E.; Katagiri, T.; Amthor, H.; Zammit, P.S. BMP signalling permits population expansion by preventing premature myogenic differentiation in muscle satellite cells. Cell Death Differ. 2011, 18, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Stantzou, A.; Schirwis, E.; Swist, S.; Alonso-Martin, S.; Polydorou, I.; Zarrouki, F.; Mouisel, E.; Beley, C.; Julien, A.; Le Grand, F.; et al. BMP signaling regulates satellite cell-dependent postnatal muscle growth. Dev. 2017, 144, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Yablonka-Reuveni, Z.; Seger, R.; Rivera, A.J. Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J. Histochem. Cytochem. 1999, 47, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, J.V.; Jones, K.M.; Basson, M.A.; Brack, A.S. The aged niche disrupts muscle stem cell quiescence. Nature 2012, 490, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Doles, J.D. Disease-associated metabolic alterations that impact satellite cells and muscle regeneration: Perspectives and therapeutic outlook. Nutr. Metab. 2021, 18, 33. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85103197913&doi=10.1186%2Fs12986-021-00565-0&partnerID=40&md5=6ad7f3606e17cf4cc5e78ffcaedb95e1 (accessed on 26 September 2022). [CrossRef]
- McKenna, C.F.; Fry, C.S. Altered satellite cell dynamics accompany skeletal muscle atrophy during chronic illness, disuse, and aging. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 447–452. [Google Scholar] [CrossRef]
- Han, L.; Wang, G.; Zhou, S.; Situ, C.; He, Z.; Li, Y.; Qiu, Y.; Huang, Y.; Xu, A.; Ong, M.T.Y.; et al. Muscle satellite cells are impaired in type 2 diabetic mice by elevated extracellular adenosine. Cell Rep. 2022, 39, 110884. [Google Scholar] [CrossRef]
- Furuichi, Y.; Kawabata, Y.; Aoki, M.; Mita, Y.; Fujii, N.L.; Manabe, Y. Excess Glucose Impedes the Proliferation of Skeletal Muscle Satellite Cells Under Adherent Culture Conditions. Front. Cell Dev. Biol. 2021, 9, 640399. [Google Scholar] [CrossRef]
| References | Species | Vitamin D form, Dose, Duration | Importance |
|---|---|---|---|
| Animals in the period of prenatal development and postnatal growth | |||
| Hutton et al., 2014 [27] | Male, Ross 708 broiler chicks (n total = 150) | 2240 IU vitamin D3 per kg diet + 2760 IU of 25(OH)D3 per kg diet for 49 days | ↑ mitotically active satellite cells (Pax7+; BrdU+), tended to ↑ density of Pax7+ satellite cells and Myf-5 + satellite cells in pectoralis mayor muscle |
| Zhou et al., 2016 [28] | White gilts (n = 10 per group) | 50 µg/kg vitamin D3 + 50 µg/kg 25(OH)D3 given from mating to weaning | ↑ MyoD1 and ↑ Myogenin mRNA expression in newborn and weaning piglets |
| Oku et al., 2016 [29] | Male Sprague Dawley rats (n = 6 per group) | Diet with vitamin D restriction for 28 days | ↓ MyoD mRNA expression |
| Thayer et al., 2019 [30] | Sows and their progeny (n total = 69) | 1500 IU/kg vitamin D3; 500 IU/kg vitamin D3 + 25 μg/kg 25(OH)D3; or 1500 IU/kg vitamin D3 + 50 μg/kg 25(OH)D3, during gestation and lactation | No difference in satellite cells number per fiber in pig’s muscle at birth |
| Reis et al., 2022 [31] | Male and female Wistar Hannover rats (n total = 20) | Maternal vitamin D deficiency | Following 180 days, only in Vitamin D deficiency male adult offspring, there are ↑ local calcitriol, ↑ CYP27B1, ↑ number of satellite cells, ↑ MyoD and ↑myogenin protein expression |
| Animals with muscle injury | |||
| Stratos et al., 2013 [32] | Male Wistar rats (n total = 56) | 332,000 IU/kg body weight vitamin D3 single dose subcutaneous injection after muscle injury | ↑ non myogenic cell proliferation, ↓ apoptosis; Not significant influence the number of satellite cells, ↑ extracellular matrix protein |
| Srikuea et al., 2016 [33] | Male C57BL/6 mice (n = 6 per group) | 1.25(OH)2D3 1 g/kg relative to tibialis anterior muscle wet weight (physiological dose) or 1.25(OH)2D3 1 g/kg relative to mouse body weight (supraphysiological dose), intramuscular injection at day 4–7 post injury | ↑ Vdr expresion in both doses; 1.25(OH)2D3 at a supraphysiological dose: ↓ satellite cell differentiation, delayed regenerative muscle fiber formation, and increased muscular fibrosis |
| Yu et al., 2021 [34] | Male Cyp27b1 knockout (KO) mice | Vitamin D deficiency in Cyp27b1 KO mice | ↓ MyoD, ↓Myf5, ↓MyHC in tibialis anterior muscle of Cyp27b1 KO after injected with BaCl2 |
| Aging or diseased animal | |||
| Faria et al., 2014 [35] | Male old Wistar rats (n = 10 per group) | AIN-93 M maintenance diet or a modified AIN-93 M diet with no vitamin D (to induce vitamin D deficiency) for 9 months | In vitamin D deficient group: ↓ Tibialis anterior weight, ↓ mRNA expression of marker of proliferation (Bmp4, Fgf-2, PCNA) ↓ Notch signaling activity |
| Kinoshita et al., 2015 [36] | Glucocorticoid-treated female Wistar rats (n = 5–9 per group) | Eldecalcitol (activated vitamin D3 analogue) for 2 or 4 weeks | ↑ Pax7 and ↑ Myogenin mRNA expression during 2 weeks of treatment |
| Tamura et al., 2016 [37] | Diabetic C57BL/6 mice | Vitamin D-deficient diet for 6 weeks | ↓ Pax7 mRNA expression |
| Nakaoka et al., 2019 [38] | Ovariectomized female Sprague–Dawley rats (n = 6 per group) | Vitamin D restriction for 28 days | ↓ Myf-5 and ↓Myogenin mRNA expression |
| Cheung et al., 2020 [39] | C57BL/6 Ctns-/- mice (mouse model of infantile nephropathic cystinosis with Vitamin D insufficiency) | Supplementation with 25(OH)D + 1.25(OH)2D3 (75 μg/kg/ day + 60 ng/kg/day, respectively), for 6 weeks | Ameliorate the decreased gene expression of Pax7, and MyoD |
| References | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Hutton et al. [27] |  |  |  |  |  |  |  |  |  |  |
| Zhou et al. [28] |  |  |  |  |  |  |  |  |  |  |
| Oku et al. [29] |  |  |  |  |  |  |  |  |  |  |
| Thayer et al. [30] |  |  |  |  |  |  |  |  |  |  |
| Reis et al. [31] |  |  |  |  |  |  |  |  |  |  |
| Stratos et al. [32] |  |  |  |  |  |  |  |  |  |  |
| Srikuea et al. [33] |  |  |  |  |  |  |  |  |  |  |
| Yu et al. [34] |  |  |  |  |  |  |  |  |  |  |
| Faria et al. [35] |  |  |  |  |  |  |  |  |  |  |
| Kinoshita et al. [36] |  |  |  |  |  |  |  |  |  |  |
| Tamura et al. [37] |  |  |  |  |  |  |  |  |  |  |
| Nakaoka et al. [38] |  |  |  |  |  |  |  |  |  |  |
| Cheung et al. [39] |  |  |  |  |  |  |  |  |  |  |
 Low risk of bias Low risk of bias |  No information/not applicable No information/not applicable | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaqih, M.S.; Tarawan, V.M.; Sylviana, N.; Goenawan, H.; Lesmana, R.; Susianti, S. Effects of Vitamin D on Satellite Cells: A Systematic Review of In Vivo Studies. Nutrients 2022, 14, 4558. https://doi.org/10.3390/nu14214558
Alfaqih MS, Tarawan VM, Sylviana N, Goenawan H, Lesmana R, Susianti S. Effects of Vitamin D on Satellite Cells: A Systematic Review of In Vivo Studies. Nutrients. 2022; 14(21):4558. https://doi.org/10.3390/nu14214558
Chicago/Turabian StyleAlfaqih, Muhammad Subhan, Vita Murniati Tarawan, Nova Sylviana, Hanna Goenawan, Ronny Lesmana, and Susianti Susianti. 2022. "Effects of Vitamin D on Satellite Cells: A Systematic Review of In Vivo Studies" Nutrients 14, no. 21: 4558. https://doi.org/10.3390/nu14214558
APA StyleAlfaqih, M. S., Tarawan, V. M., Sylviana, N., Goenawan, H., Lesmana, R., & Susianti, S. (2022). Effects of Vitamin D on Satellite Cells: A Systematic Review of In Vivo Studies. Nutrients, 14(21), 4558. https://doi.org/10.3390/nu14214558