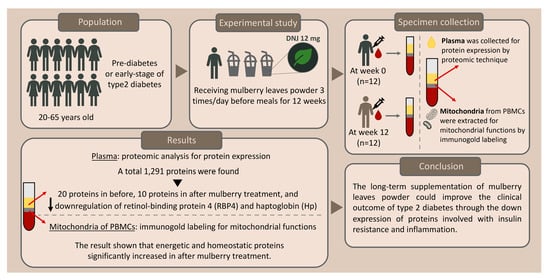
Mulberry-Derived 1-Deoxynojirimycin Prevents Type 2 Diabetes Mellitus Progression via Modulation of Retinol-Binding Protein 4 and Haptoglobin
Abstract
1. Introduction
2. Materials and Methods
2.1. Mulberry Leaf Powder Preparation and Determination of DNJ
2.2. Study Design
2.2.1. Ethical Statement and Participant Inclusion
2.2.2. Nutritional and Diet Control
2.2.3. Human Experimental Protocol
2.2.4. Specimen Collection and Plasma Preparation
2.3. Label-Free Quantitative Proteomics of Plasma Samples from Mulberry Leaf Treatment
2.3.1. Plasma Sample Extraction and Protein Size Separation
2.3.2. In-Gel Tryptic Digestion
2.3.3. Protein Identification
2.4. Extraction of Mitochondria from Peripheral Blood Mononuclear Cells before and after Mulberry Leaf Treatment
2.5. Mitochondrial Immunogold Labeling from PBMCs
2.6. Statistical Analysis
3. Results
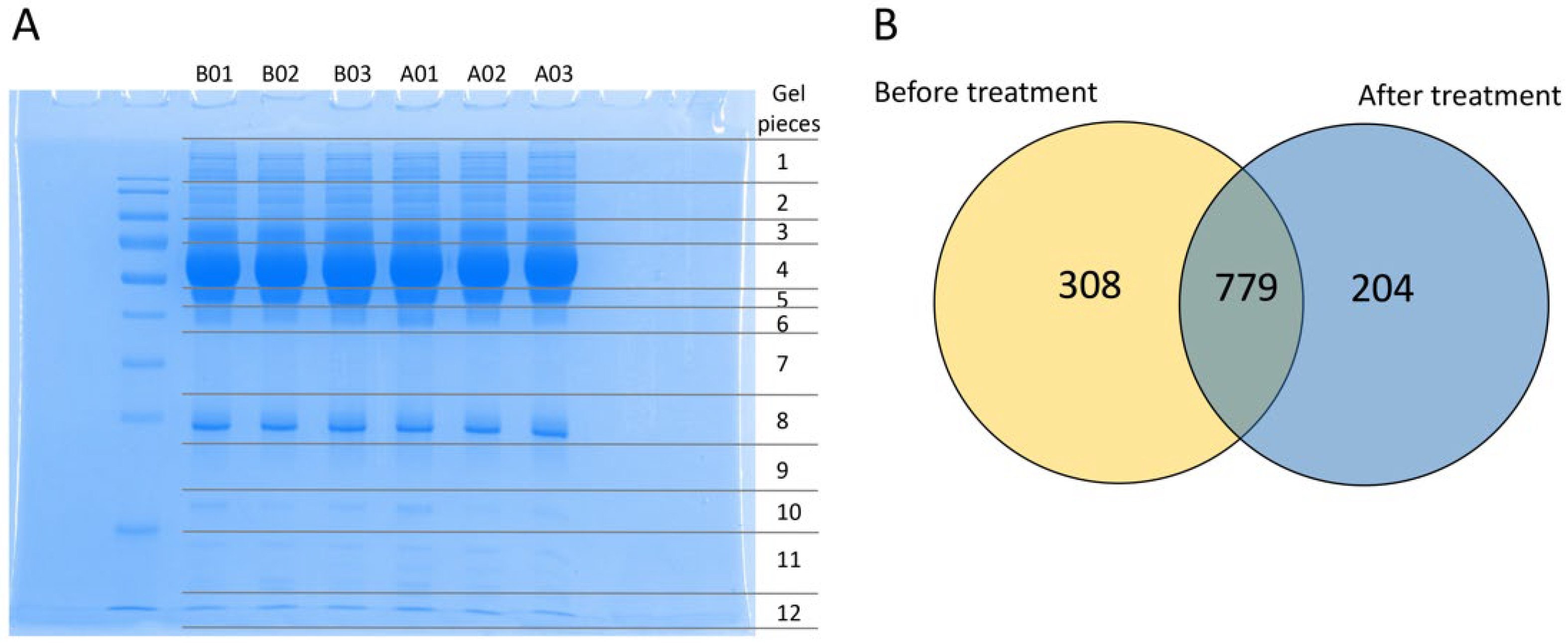
3.1. Identification of the Protein Profile in Plasma before and after Mulberry Leaf Treatment
3.2. Differentiation of Proteins, Their Function, and Associated Pathways
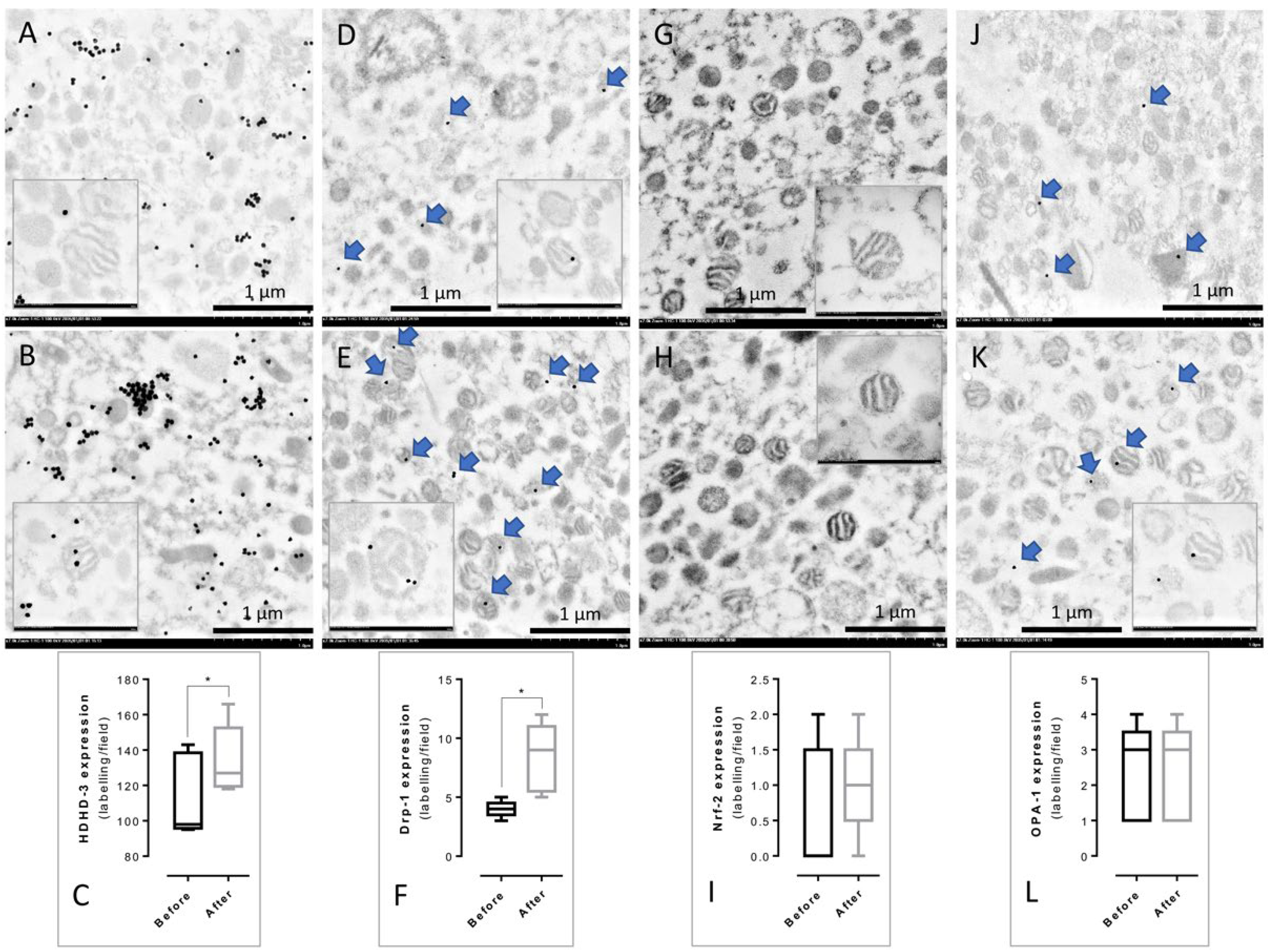
3.3. Immunogold Labeling of PBMCs’ Mitochondria from before and after Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Blaslov, K.; Naranda, F.S.; Kruljac, I.; Renar, I.P. Treatment approach to type 2 diabetes: Past, present and future. World J. Diabetes 2018, 9, 209–219. [Google Scholar] [CrossRef]
- Hameed, I.; Masoodi, S.R.; Mir, S.A.; Nabi, M.; Ghazanfar, K.; Ganai, B.A. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J. Diabetes 2015, 6, 598–612. [Google Scholar] [CrossRef]
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181. [Google Scholar] [CrossRef]
- Clements, R.S., Jr.; Bell, D.S. Complications of diabetes. Prevalence, detection, current treatment, and prognosis. Am. J. Med. 1985, 79, 2–7. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, C.; Wang, W.; Xu, B. Prevalence of chronic complications of type 2 diabetes mellitus in outpatients—A cross-sectional hospital based survey in urban China. Health Qual. Life Outcomes 2010, 8, 62. [Google Scholar] [CrossRef]
- Carracher, A.M.; Marathe, P.H.; Close, K.L. International Diabetes Federation 2017. J. Diabetes 2018, 10, 353–356. [Google Scholar] [CrossRef]
- American Diabetes, A. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef]
- Williams, R.; Karuranga, S.; Malanda, B.; Saeedi, P.; Basit, A.; Besancon, S.; Bommer, C.; Esteghamati, A.; Ogurtsova, K.; Zhang, P.; et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108072. [Google Scholar] [CrossRef]
- Afroz, A.; Ali, L.; Karim, M.N.; Alramadan, M.J.; Alam, K.; Magliano, D.J.; Billah, B. Glycaemic Control for People with Type 2 Diabetes Mellitus in Bangladesh—An urgent need for optimization of management plan. Sci. Rep. 2019, 9, 10248. [Google Scholar] [CrossRef]
- American Diabetes, A. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin. Diabetes 2022, 40, 10–38. [Google Scholar] [CrossRef]
- Frenchman, I.B. Treatment and treatment-related adverse events of type 2 diabetes mellitus in residents of long-term care facilities: A retrospective study. Curr. Ther. Res. Clin. Exp. 2003, 64, 1–9. [Google Scholar] [CrossRef][Green Version]
- Loke, Y.K.; Singh, S.; Furberg, C.D. Long-term use of thiazolidinediones and fractures in type 2 diabetes: A meta-analysis. Can. Med. Assoc. J. 2009, 180, 32. [Google Scholar] [CrossRef]
- Razavi-Nematollahi, L.; Ismail-Beigi, F. Adverse Effects of Glycemia-Lowering Medications in Type 2 Diabetes. Curr. Diabetes Rep. 2019, 19, 132. [Google Scholar] [CrossRef]
- Seidu, S.; Cos, X.; Brunton, S.; Harris, S.B.; Jansson, S.P.O.; Mata-Cases, M.; Neijens, A.M.J.; Topsever, P.; Khunti, K. 2022 update to the position statement by Primary Care Diabetes Europe: A disease state approach to the pharmacological management of type 2 diabetes in primary care. Prim. Care Diabetes 2022, 16, 223–244. [Google Scholar] [CrossRef]
- Chang, C.L.; Lin, Y.; Bartolome, A.P.; Chen, Y.C.; Chiu, S.C.; Yang, W.C. Herbal therapies for type 2 diabetes mellitus: Chemistry, biology, and potential application of selected plants and compounds. Evid. Based Complement. Altern. Med. 2013, 2013, 378657. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Lown, M.; Fuller, R.; Lightowler, H.; Fraser, A.; Gallagher, A.; Stuart, B.; Byrne, C.; Lewith, G. Mulberry-extract improves glucose tolerance and decreases insulin concentrations in normoglycaemic adults: Results of a randomised double-blind placebo-controlled study. PLoS ONE 2017, 12, e0172239. [Google Scholar] [CrossRef]
- Zhang, L.; Su, S.; Zhu, Y.; Guo, J.; Guo, S.; Qian, D.; Ouyang, Z.; Duan, J.A. Mulberry leaf active components alleviate type 2 diabetes and its liver and kidney injury in db/db mice through insulin receptor and TGF-beta/Smads signaling pathway. Biomed. Pharmacother. 2019, 112, 108675. [Google Scholar] [CrossRef]
- Kumar, R.; Srivastava, D.; Singh, P.; Kumar, U.; Singh, V. Mulberry 1-deoxynojirimycin (DNJ): An exemplary compound for therapeutics. J. Hortic. Sci. Biotechnol. 2020, 95, 679–686. [Google Scholar]
- Gao, K.; Zheng, C.; Wang, T.; Zhao, H.; Wang, J.; Wang, Z.; Zhai, X.; Jia, Z.; Chen, J.; Zhou, Y.; et al. 1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing. Molecules 2016, 21, 1600. [Google Scholar] [CrossRef]
- Oku, T.; Yamada, M.; Nakamura, M.; Sadamori, N.; Nakamura, S. Inhibitory effects of extractives from leaves of Morus alba on human and rat small intestinal disaccharidase activity. Br. J. Nutr. 2006, 95, 933–938. [Google Scholar] [CrossRef]
- Asai, A.; Nakagawa, K.; Higuchi, O.; Kimura, T.; Kojima, Y.; Kariya, J.; Miyazawa, T.; Oikawa, S. Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on postprandial glycemic control in subjects with impaired glucose metabolism. J. Diabetes Investig. 2011, 2, 318–323. [Google Scholar] [CrossRef]
- Kimura, T.; Nakagawa, K.; Kubota, H.; Kojima, Y.; Goto, Y.; Yamagishi, K.; Oita, S.; Oikawa, S.; Miyazawa, T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J. Agric. Food Chem. 2007, 55, 5869–5874. [Google Scholar] [CrossRef]
- Banu, S.; Jabir, N.R.; Manjunath, N.C.; Khan, M.S.; Ashraf, G.M.; Kamal, M.A.; Tabrez, S. Reduction of post-prandial hyperglycemia by mulberry tea in type-2 diabetes patients. Saudi J. Biol. Sci. 2015, 22, 32–36. [Google Scholar] [CrossRef]
- Kojima, Y.; Kimura, T.; Nakagawa, K.; Asai, A.; Hasumi, K.; Oikawa, S.; Miyazawa, T. Effects of mulberry leaf extract rich in 1-deoxynojirimycin on blood lipid profiles in humans. J. Clin. Biochem. Nutr. 2010, 47, 155–161. [Google Scholar] [CrossRef]
- Thaipitakwong, T.; Supasyndh, O.; Rasmi, Y.; Aramwit, P. A randomized controlled study of dose-finding, efficacy, and safety of mulberry leaves on glycemic profiles in obese persons with borderline diabetes. Complement. Ther. Med. 2020, 49, 102292. [Google Scholar] [CrossRef]
- American Diabetes, A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef]
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell Proteom. 2005, 4, 1265–1272. [Google Scholar] [CrossRef]
- Krako Jakovljevic, N.; Pavlovic, K.; Jotic, A.; Lalic, K.; Stoiljkovic, M.; Lukic, L.; Milicic, T.; Macesic, M.; Stanarcic Gajovic, J.; Lalic, N.M. Targeting Mitochondria in Diabetes. Int. J. Mol. Sci. 2021, 22, 6642. [Google Scholar] [CrossRef]
- Kim, J.A.; Wei, Y.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Kwak, S.H.; Park, K.S.; Lee, K.U.; Lee, H.K. Mitochondrial metabolism and diabetes. J. Diabetes Investig. 2010, 1, 161–169. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Garcia, R.; Shulman, G.I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 2004, 350, 664–671. [Google Scholar] [CrossRef]
- Sharma, K. Mitochondrial hormesis and diabetic complications. Diabetes 2015, 64, 663–672. [Google Scholar] [CrossRef]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016, 27, 105–117. [Google Scholar] [CrossRef]
- Turaihi, A.H.; Bakker, W.; van Hinsbergh, V.W.M.; Serne, E.H.; Smulders, Y.M.; Niessen, H.W.M.; Eringa, E.C. Insulin Receptor Substrate 2 Controls Insulin-Mediated Vasoreactivity and Perivascular Adipose Tissue Function in Muscle. Front. Physiol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Hennige, A.M.; Burks, D.J.; Ozcan, U.; Kulkarni, R.N.; Ye, J.; Park, S.; Schubert, M.; Fisher, T.L.; Dow, M.A.; Leshan, R.; et al. Upregulation of insulin receptor substrate-2 in pancreatic beta cells prevents diabetes. J. Clin. Investig. 2003, 112, 1521–1532. [Google Scholar] [CrossRef]
- Lo Sasso, G.; Murzilli, S.; Salvatore, L.; D’Errico, I.; Petruzzelli, M.; Conca, P.; Jiang, Z.Y.; Calabresi, L.; Parini, P.; Moschetta, A. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010, 12, 187–193. [Google Scholar] [CrossRef]
- Jacobsen, J.N.; Steffensen, B.; Hakkinen, L.; Krogfelt, K.A.; Larjava, H.S. Skin wound healing in diabetic beta6 integrin-deficient mice. APMIS 2010, 118, 753–764. [Google Scholar] [CrossRef]
- Youle, R.J.; Narendra, D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Y.; Yang, Y.; Tang, H.; Si, Y.; Chen, Z.; Shi, Y.; Fang, H. Metformin Protects Against Diabetes-Induced Cognitive Dysfunction by Inhibiting Mitochondrial Fission Protein DRP1. Front. Pharmacol. 2022, 13, 832707. [Google Scholar] [CrossRef]
- Roth, R.A.; Liu, F.; Chin, J.E. Biochemical mechanisms of insulin resistance. Horm. Res. 1994, 41 (Suppl. S2), 51–55. [Google Scholar] [CrossRef]
- Watson, P.A.; Nesterova, A.; Burant, C.F.; Klemm, D.J.; Reusch, J.E. Diabetes-related changes in cAMP response element-binding protein content enhance smooth muscle cell proliferation and migration. J. Biol. Chem. 2001, 276, 46142–46150. [Google Scholar] [CrossRef]
- Park, S.E.; Park, C.Y.; Sweeney, G. Biomarkers of insulin sensitivity and insulin resistance: Past, present and future. Crit. Rev. Clin. Lab. Sci. 2015, 52, 180–190. [Google Scholar] [CrossRef]
- Graham, T.E.; Yang, Q.; Bluher, M.; Hammarstedt, A.; Ciaraldi, T.P.; Henry, R.R.; Wason, C.J.; Oberbach, A.; Jansson, P.A.; Smith, U.; et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 2006, 354, 2552–2563. [Google Scholar] [CrossRef]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef]
- Rodrigues, K.F.; Pietrani, N.T.; Carvalho, L.M.L.; Bosco, A.A.; Sandrim, V.C.; Ferreira, C.N.; Gomes, K.B. Haptoglobin levels are influenced by Hp1–Hp2 polymorphism, obesity, inflammation, and hypertension in type 2 diabetes mellitus. Endocrinol. Diabetes Y Nutr. 2019, 66, 99–107. [Google Scholar]
- Kushi, R.; Hirota, Y.; Ogawa, W. Insulin resistance and exaggerated insulin sensitivity triggered by single-gene mutations in the insulin signaling pathway. Diabetol. Int. 2021, 12, 62–67. [Google Scholar] [CrossRef]
- Khan, M.W.; Terry, A.R.; Priyadarshini, M.; Ilievski, V.; Farooq, Z.; Guzman, G.; Cordoba-Chacon, J.; Ben-Sahra, I.; Wicksteed, B.; Layden, B.T. The hexokinase “HKDC1” interaction with the mitochondria is essential for liver cancer progression. Cell Death Dis. 2022, 13, 660. [Google Scholar] [CrossRef]


| Timepoint of Measurement | Treatment Group (n = 28) | #p-Values | Control Group (n = 26) | #p-Values | * p-Values | |
|---|---|---|---|---|---|---|
| Total calories (kcal/day) | Week 0 | 1707.75 ± 144.16 (1561.6–2001.8) | 0.409 | 1648.29 ± 173.75 (1535.7–1920.9) | 0.156 | 0.083 |
| Week 12 | 1777.41 ± 141.94 (1479.7–1804.4) | 1626.62 ± 152.42 (1458.0–1780.2) | ||||
| Carbohydrate (g/day) | Week 0 | 169.13 ± 56.71 (88.1–215.4) | 0.696 | 163.44 ± 69.08 (123.7–197.8) | 0.534 | 0.482 |
| Week 12 | 165.94 ± 52.52 (90.4–189.0) | 156.78 ± 41.39 (110.6–192.3) | ||||
| Fat (g/day) | Week 0 | 52.33 ± 14.10 (33.8–80.2) | 0.224 | 57.29 ± 16.27 (39.9–101.5) | 0.732 | 0.152 |
| Week 12 | 55.98 ± 9.27 (30.5–90.9) | 56.13 ± 8.54 (48.1–104.2) | ||||
| Protein (g/day) | Week 0 | 64.16 ± 17.66 (47.2–88.6) | 0.325 | 63.86 ± 14.40 (51.8–96.6) | 0.077 | 0.748 |
| Week 12 | 70.39 ± 12.19 (50.1–90.1) | 68.34 ± 9.17 (45.6–111.4) |
| Time Points of Measurement | Treatment Group (n = 28) | #p | Control Group (n = 26) | #p | * p | |
|---|---|---|---|---|---|---|
| Weight (kg) | Week 0 | 78.06 ± 13.98 | 81.31 ± 20.79 | |||
| Week 12 | 77.43 ± 14.30 | 0.075 | 80.92 ± 14.06 | 0.098 | 0.352 | |
| BMI (kg/m2) | Week 0 | 30.06 ± 4.06 | 31.01 ± 5.85 | |||
| Week 12 | 29.80 ± 4.07 | 0.075 | 30.82 ± 3.86 | 0.094 | 0.272 | |
| Waist circumference (cm) | Week 0 | 100.52 ± 11.21 | 102.92 ± 15.67 | |||
| Week 12 | 100.77 ± 11.32 | 0.586 | 101.98 ± 11.08 | 0.459 | 0.693 | |
| PPG-30 (mg/dL) | Week 0 | 173.89 ± 17.52 | 172.04 ± 21.48 | |||
| Week 12 | 163.14 ± 20.63 | 0.018 | 166.96 ± 19.78 | 0.210 | 0.491 | |
| PPG-60 (mg/dL) | Week 0 | 186.61 ± 29.95 | 191.46 ± 31.96 | |||
| Week 12 | 182.14 ± 24.81 | 0.528 | 190.65 ± 34.30 | 0.865 | 0.358 | |
| PPG-90 (mg/dL) | Week 0 | 171.21 ± 33.19 | 177.46 ± 40.25 | |||
| Week 12 | 169.29 ± 29.92 | 0.734 | 174.04 ± 45.20 | 0.482 | 0.648 | |
| PPG-120 (mg/dL) | Week 0 | 141.14 ± 32.97 | 155.12 ± 37.53 | |||
| Week 12 | 163.14 ± 20.63 | 0.251 | 166.96 ± 19.78 | 0.626 | 0.173 | |
| FPI (µIU/mL) | Week 0 | 12.55 ± 7.85 | 14.39 ± 6.02 | |||
| Week 12 | 12.41 ± 6.66 | 0.188 | 14.05 ± 7.32 | 0.643 | 0.399 | |
| HOMA-IR | Week 0 | 3.60 ± 2.18 | 3.84 ± 1.72 | |||
| Week 12 | 3.18 ± 1.78 | 0.057 | 3.78 ± 2.18 | 0.781 | 0.278 | |
| TC (mg/dL) | Week 0 | 194.99 ± 35.53 | 200.66 ± 37.93 | |||
| Week 12 | 181.58 ± 26.64 | 0.002 | 199.49 ± 33.76 | 0.786 | 0.034 | |
| TG (mg/dL) | Week 0 | 131.63 ± 45.22 | 145.03 ± 59.99 | |||
| Week 12 | 123.52 ± 35.87 | 0.148 | 138.10 ± 51.38 | 0.583 | 0.193 | |
| HDL-C (mg/dL) | Week 0 | 54.89 ± 14.35 | 53.56 ± 9.82 | |||
| Week 12 | 52.48 ± 10.83 | 0.098 | 54.63 ± 8.66 | 0.302 | 0.424 | |
| LDL-C (mg/dL) | Week 0 | 139.06 ± 33.80 | 137.46 ± 34.25 | |||
| Week 12 | 131.66 ± 29.54 | 0.090 | 137.25 ± 29.81 | 0.959 | 0.492 | |
| A1C (%) | Week 0 | 5.8 ± 0.4 | 5.7 ± 0.3 | |||
| Week 12 | 5.7 ± 0.3 | 0.011 | 5.8 ± 0.4 | 0.100 | 0.551 | |
| Creatinine (mg/dL) | Week 0 | 0.81 ± 0.17 | 0.72 ± 0.18 | |||
| Week 12 | 0.76 ± 0.22 | 0.149 | 0.70 ± 0.17 | 0.191 | 0.244 | |
| eGFR | Week 0 | 92.79 ± 14.86 | 99.75 ± 17.39 | |||
| Week 12 | 88.33 ± 24.32 | 0.255 | 100.75 ± 16.48 | 0.467 | 0.034 | |
| AST (U/L) | Week 0 | 21.92 ± 4.84 | 19.39 ± 5.19 | |||
| Week 12 | 21.83 ± 6.17 | 0.927 | 22.27 ± 8.19 | 0.113 | 0.825 | |
| ALT (U/L) | Week 0 | 23.21 ± 7.78 | 19.24 ± 7.05 | |||
| Week 12 | 25.24 ± 13.09 | 0.345 | 23.57 ± 17.49 | 0.114 | 0.692 |
| Groups | Accession No. | Gene | Protein Name | Protein Function | Pathways | Score | Coverage (%) |
|---|---|---|---|---|---|---|---|
| Before Treatment | P85B_HUMAN | PIK3R2 | Phosphatidylinositol 3-kinase regulatory subunit beta | Regulation of glucose metabolism | 1 | 43 | 8.1 |
| INSR_HUMAN | INSR | Insulin receptor | Receptor of insulin | 49 | 8.1 | ||
| HKDC1_HUMAN | HKDC1 | Putative hexokinase HKDC1 | Intermediate of glucose uptake in peripheral tissues | 38 | 16.5 | ||
| TNR1A_HUMAN | TNFRSF1A | Tumor necrosis factor receptor superfamily member 1A | Receptor of TNF-α | 2 | 41 | 16.7 | |
| MP2K6_HUMAN | MAP2K6 | Dual-specificity mitogen-activated protein kinase kinase 6 | Mediator of MAPK and JNK activation | 36 | 15.9 | ||
| ANGT_HUMAN | AGT | Angiotensinogen | 131 | 10.5 | |||
| A1AG2_HUMAN | ORM2 | Alpha-1-acid glycoprotein 2 | Positive acute phase reactant | 59 | 22.9 | ||
| FETUA_HUMAN | AHSG | Alpha-2-HS-glycoprotein | 55 | 15.3 | |||
| IKKA_HUMAN | CHUK | Inhibitor of nuclear factor kappa-B kinase subunit alpha | Mediator of NF-kB activation | 2 and 3 | 42 | 11.7 | |
| NEMO_HUMAN | IKBKG | NF-kappa-B essential modulator | 36 | 11 | |||
| Before treatment | NOD2_HUMAN | NOD2 | Nucleotide-binding oligomerization domain containing protein 2 | Immune response | 41 | 5.8 | |
| FCN3_HUMAN | FCN3 | Ficolin-3 | Immune response | 3 | 41 | 7 | |
| TEC_HUMAN | TEC | Tyrosine-protein kinase Tec | 66 | 19.2 | |||
| CO2A1_HUMAN | COL2A1 | Collagen alpha 1(II) chain | Structural and ECM constituents | 4 | 34 | 9.8 | |
| CO4A3_HUMAN | COL4A3 | Collagen alpha 3(IV) chain | 53 | 9.6 | |||
| CO6A3_HUMAN | COL6A3 | Collagen alpha 3(VI) chain | 75 | 4.8 | |||
| TRI46_HUMAN | TRIM46 | Tripartite motif-containing protein 46 | Cell interaction and communication | 49 | 19.1 | ||
| EMIL2_HUMAN | EMILIN2 | EMILIN-2 | 35 | 5.3 | |||
| ATS12_HUMAN | ADAMTS12 | A disintegrin and metalloproteinase with thrombospondin motifs 12 | 54 | 7.0 | |||
| ZO1_HUMAN | TJP1 | Tight junction protein ZO-1 | 49 | 5.3 | |||
| After treatment | IRS2_HUMAN | IRS2 | Insulin receptor substrate 2 | Mediator of insulin action | 1 | 54 | 6.7 |
| NR1H3_HUMAN | NR1H3 | Oxysterols receptor LXR-alpha | Nuclear receptor in regulation of lipid metabolism | 35 | 23 | ||
| SOS1_HUMAN | SOS1 | Son of sevenless homolog 1 | Signaling pathway (G-protein-coupled receptor) | 1 and 3 | 47 | 6.3 | |
| SOS2_HUMAN | SOS2 | Son of sevenless homolog 2 | 38 | 8.6 | |||
| MEFV_HUMAN | MEFV | Pyrin | Inflammasome and inflammatory response | 3 | 49 | 8.3 | |
| NALP7_HUMAN | NLRP7 | NACHT, LRR and PYD domains containing protein 7 | 40 | 6.3 | |||
| DNM1L_HUMAN | DNM1L | Dynamin-1-like protein | Cellular process | 44 | 12.2 | ||
| COMP_HUMAN | COMP | Cartilage oligomeric matrix protein | Cellular process | 2 | 46 | 13.7 | |
| ITB6_HUMAN | ITGB6 | Integrin beta 6 | Cell interaction and communication | 41 | 15.2 | ||
| CBP_HUMAN | CREBBP | CREB-binding protein | Signaling pathway (Notch) | 51 | 5.6 | ||
| Down- regulation | RET4_HUMAN | RBP4 | Retinol-binding protein 4 | Adipokine | 1 | 133 | 24.9 |
| HPT_HUMAN | HP | Haptoglobin | Positive acute phase reactant | 2 | 1260 | 53.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fongsodsri, K.; Thaipitakwong, T.; Rujimongkon, K.; Kanjanapruthipong, T.; Ampawong, S.; Reamtong, O.; Aramwit, P. Mulberry-Derived 1-Deoxynojirimycin Prevents Type 2 Diabetes Mellitus Progression via Modulation of Retinol-Binding Protein 4 and Haptoglobin. Nutrients 2022, 14, 4538. https://doi.org/10.3390/nu14214538
Fongsodsri K, Thaipitakwong T, Rujimongkon K, Kanjanapruthipong T, Ampawong S, Reamtong O, Aramwit P. Mulberry-Derived 1-Deoxynojirimycin Prevents Type 2 Diabetes Mellitus Progression via Modulation of Retinol-Binding Protein 4 and Haptoglobin. Nutrients. 2022; 14(21):4538. https://doi.org/10.3390/nu14214538
Chicago/Turabian StyleFongsodsri, Kamonpan, Thanchanit Thaipitakwong, Kitiya Rujimongkon, Tapanee Kanjanapruthipong, Sumate Ampawong, Onrapak Reamtong, and Pornanong Aramwit. 2022. "Mulberry-Derived 1-Deoxynojirimycin Prevents Type 2 Diabetes Mellitus Progression via Modulation of Retinol-Binding Protein 4 and Haptoglobin" Nutrients 14, no. 21: 4538. https://doi.org/10.3390/nu14214538
APA StyleFongsodsri, K., Thaipitakwong, T., Rujimongkon, K., Kanjanapruthipong, T., Ampawong, S., Reamtong, O., & Aramwit, P. (2022). Mulberry-Derived 1-Deoxynojirimycin Prevents Type 2 Diabetes Mellitus Progression via Modulation of Retinol-Binding Protein 4 and Haptoglobin. Nutrients, 14(21), 4538. https://doi.org/10.3390/nu14214538