Does Human Papillomavirus Infection Influence the Frequency and Severity of Nutritional Disorders in Head and Neck Cancer?
Abstract
1. Introduction
1.1. Nutritional Disorders
1.2. Human Papillomavirus—HPV
2. Materials and Methods
3. Relationship between HPV Infection and Nutritional Disorders in Head and Neck Cancer
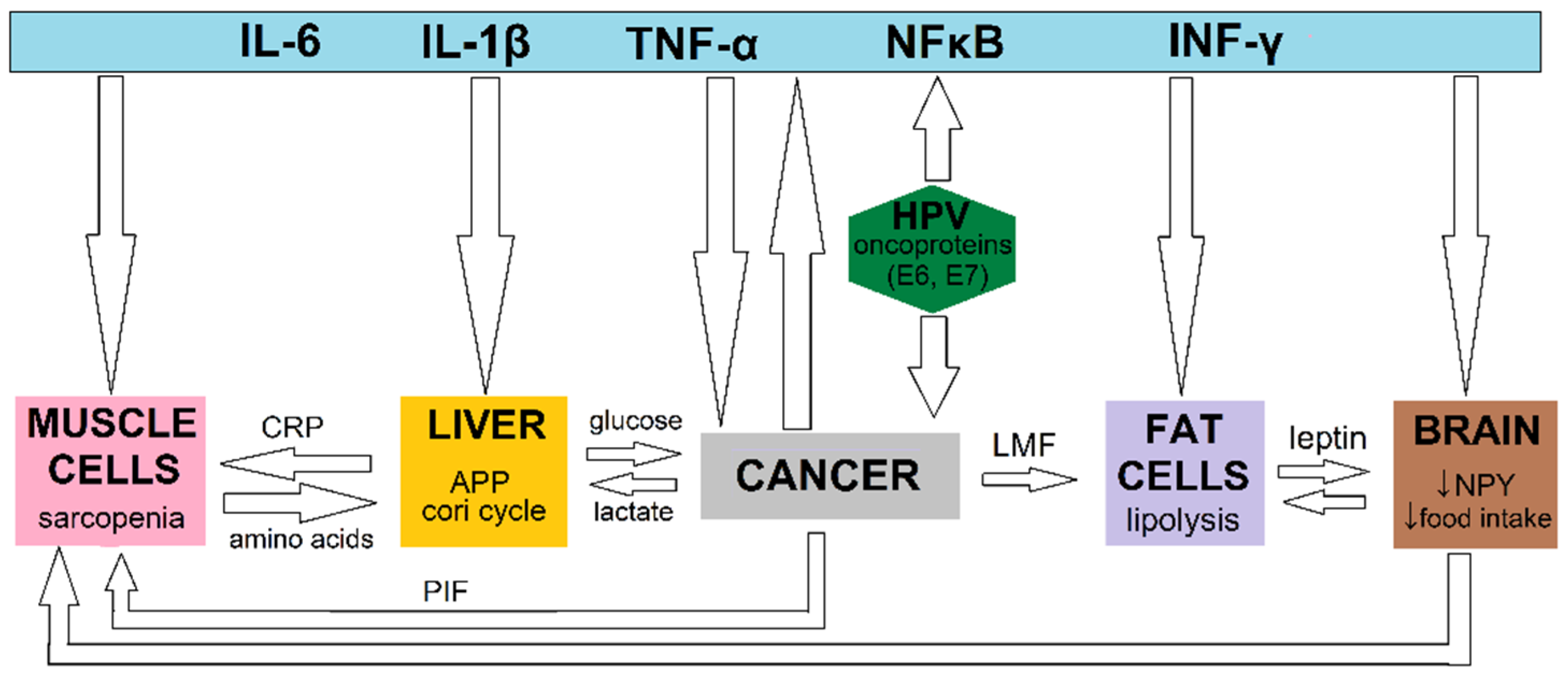
3.1. Basic Science Information and Pathophysiology
3.2. Clinical Papers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Couch, M.; Lai, V.; Cannon, T.; Guttridge, D.; Zanation, A.; George, J.; Hayes, D.N.; Zeisel, S.; Shores, C. Cancer cachexia syndrome in head and neck cancer patients: Part I. Diagnosis, impact on quality of life and survival, and treatment. Head Neck 2007, 29, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Richey, L.M.; George, J.R.; Couch, M.E.; Kanapkey, B.K.; Yin, X.; Cannon, T.; Stewart, P.W.; Weissler, M.C.; Shores, C.G. Defining cancer cachexia in head and neck squamous cell carcinoma. Clin. Cancer Res. 2007, 13, 6561–6567. [Google Scholar] [CrossRef]
- Cannon, T.; Shores, C.; Yin, X.; Dahlman, J.; Guttridge, D.; Lai, V.; George, J.; Buzkova, P.; Couch, M. Immunocompetent murine model of cancer cachexia for head and neck squamous cell carcinoma. Head Neck 2008, 30, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Gorenc, M.; Kozjek, N.R.; Strojan, P. Malnutrition and cachexia in patients with head and neck cancer treated with (chemo)radiotherapy. Rep. Pract. Oncol. Radiother. 2015, 20, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Silva, P.B.; Ramos, G.H.A.; Petterle, R.R.; Borba, V.Z.C. Sarcopenia as an early complication of patients with head and neck cancer with dysphagia. Eur. J. Cancer Care 2021, 30, e13343. [Google Scholar] [CrossRef]
- Takenaka, Y.; Takemoto, N.; Oya, R.; Inohara, H. Prognostic impact of sarcopenia in patients with head and neck cancer treated with surgery or radiation: A meta-analysis. PLoS ONE 2021, 16, e0259288. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- George, J.; Cannon, T.; Lai, V.; Richey, L.; Zanation, A.; Hayes, D.N.; Shores, C.; Guttridge, D.; Couch, M. Cancer cachexia syndrome in head and neck cancer patients: Part II. Pathophysiology. Head Neck 2007, 29, 497–507. [Google Scholar] [CrossRef]
- Couch, M.E.; Dittus, K.; Toth, M.J.; Willis, M.S.; Guttridge, D.C.; George, J.R.; Chang, E.Y.; Gourin, C.G.; Der-Torossian, H. Cancer cachexia update in head and neck cancer: Pathophysiology and treatment. Head Neck 2015, 37, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Kim, R.B.; Roh, J.-L.; Lee, S.-W.; Kim, S.-B.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Prevalence and clinical significance of cancer cachexia based on time from treatment in advanced-stage head and neck squamous cell carcinoma. Head Neck 2017, 39, 716–723. [Google Scholar] [CrossRef]
- Suzuki, H.; Asakawa, A.; Amitani, H.; Nakamura, N.; Inui, A. Cancer cachexia—Pathophysiology and management. J. Gastroenterol. 2013, 48, 574–594. [Google Scholar] [CrossRef] [PubMed]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachexia. Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, V.S.; Fitzgerald, L.W.; Bathe, O.F. Cancer-associated muscle wasting—Candidate mechanisms and molecular pathways. Int. J. Mol. Sci. 2020, 21, 9268. [Google Scholar] [CrossRef]
- Faraji, F.; Zaidi, M.; Fakhry, C.; Gaykalova, D.A. Molecular mechanisms of human papillomavirus-related carcinogenesis in head and neck cancer. Microbes Infect. 2017, 19, 464–475. [Google Scholar] [CrossRef]
- Garland, S.M.; Joura, E.A.; Ault, K.A.; Bosch, F.X.; Brown, D.R.; Castellsagué, X.; Ferenczy, A.; Ferris, D.G.; Giuliano, A.R.; Hernandez-Avila, M.; et al. Human Papillomavirus Genotypes from Vaginal and Vulvar Intraepithelial Neoplasia in Females 15-26 Years of Age. Obstet. Gynecol. 2018, 132, 261–270. [Google Scholar] [CrossRef]
- Krzowska-Firych, J.; Lucas, G.; Lucas, C.; Lucas, N.; Pietrzyk, Ł. An overview of Human Papillomavirus (HPV) as an etiological factor of the anal cancer. J. Infect. Public Health 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Pham, T.T.T.; Bi, X.; Hoang, H.T.T.; Ishizaki, A.; Nguyen, M.T.P.; Nguyen, C.H.; Nguyen, H.P.; Van Pham, T.; Ichimura, H. Human papillomavirus genotypes and hpv16 e6/e7 variants among patients with genital cancers in Vietnam. Jpn. J. Infect. Dis. 2018, 71, 419–426. [Google Scholar] [CrossRef]
- Powell, N.G.; Evans, M. Human papillomavirus-associated head and neck cancer: Oncogenic mechanisms, epidemiology and clinical behaviour. Diagn. Histopathol. 2015, 21, 49–64. [Google Scholar] [CrossRef]
- Bogani, G.; Lalli, L.; Sopracordevole, F.; Ciavattini, A.; Ghelardi, A.; Simoncini, T.; Plotti, F.; Casarin, J.; Serati, M.; Pinelli, C.; et al. Development of a Nomogram Predicting the Risk of Persistence/Recurrence of Cervical Dysplasia. Vaccines 2022, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Sopracordevole, F.; Di Donato, V.; Ciavattini, A.; Ghelardi, A.; Lopez, S.; Simoncini, T.; Plotti, F.; Casarin, J.; Serati, M.; et al. High-risk HPV−positive and -negative high-grade cervical dysplasia: Analysis of 5-year outcomes. Gynecol. Oncol. 2021, 161, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ghelardi, A.; Marrai, R.; Bogani, G.; Sopracordevole, F.; Bay, P.; Tonetti, A.; Lombardi, S.; Bertacca, G.; Joura, E.A. Surgical Treatment of Vulvar HSIL: Adjuvant HPV Vaccine Reduces Recurrent Disease. Vaccines 2021, 9, 83. [Google Scholar] [CrossRef]
- Di Donato, V.; Caruso, G.; Petrillo, M.; Kontopantelis, E.; Palaia, I.; Perniola, G.; Plotti, F.; Angioli, R.; Muzii, L.; Benedetti Panici, P.; et al. Adjuvant HPV Vaccination to Prevent Recurrent Cervical Dysplasia after Surgical Treatment: A Meta-Analysis. Vaccines 2021, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, V.; Caruso, G.; Bogani, G.; Cavallari, E.N.; Palaia, G.; Perniola, G.; Ralli, M.; Sorrenti, S.; Romeo, U.; Pernazza, A.; et al. HPV Vaccination after Primary Treatment of HPV−Related Disease across Different Organ Sites: A Multidisciplinary Comprehensive Review and Meta-Analysis. Vaccines 2022, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Westra, W.H.; Taube, J.M.; Poeta, M.L.; Begum, S.; Sidransky, D.; Koch, W.M. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2008, 14, 366–369. [Google Scholar] [CrossRef]
- Ochoa, I.S.; O’Regan, E.; Toner, M.; Kay, E.; Faul, P.; O’Keane, C.; O’Connor, R.; Mullen, D.; Nur, M.; O’Murchu, E.; et al. The Role of HPV in Determining Treatment, Survival, and Prognosis of Head and Neck Squamous Cell Carcinoma. Cancers 2022, 14, 4321. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Bansal, A.; Singh, M.; Rai, B. Human papillomavirus-associated cancers: A growing global problem. Int. J. Appl. Basic Med. Res. 2016, 6, 84. [Google Scholar] [CrossRef]
- McBride, A.A. Mechanisms and strategies of papillomavirus replication. Biol. Chem. 2017, 398, 919–927. [Google Scholar] [CrossRef]
- da Costa, R.M.G.; Aragão, S.; Moutinho, M.; Alvarado, A.; Carmo, D.; Casaca, F.; Silva, S.; Ribeiro, J.; Sousa, H.; Ferreira, R.; et al. HPV16 induces a wasting syndrome in transgenic mice: Amelioration by dietary polyphenols via NF-κB inhibition. Life Sci. 2017, 169, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bharti, A.C.; Varghese, P.; Saluja, D.; Das, B.C. Differential expression and activation of NF-kappaB family proteins during oral carcinogenesis: Role of high risk human papillomavirus infection. Int. J. Cancer 2006, 119, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Klingelhutz, A.J.; Roman, A. Cellular transformation by human papillomaviruses: Lessons learned by comparing high- and low-risk viruses. Virology 2012, 424, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Busquets, S.; Toledo, M.; López-Soriano, F.J. The role of cytokines in cancer cachexia. Curr. Opin. Support. Palliat. Care 2009, 3, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Kemp, T.J.; Hildesheim, A.; García-Piñeres, A.; Williams, M.C.; Shearer, G.M.; Rodriguez, A.C.; Schiffman, M.; Burk, R.; Freer, E.; Bonilla, J.; et al. Elevated systemic levels of inflammatory cytokines in older women with persistent cervical human papillomavirus infection. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Harrowfield, J.; Isenring, E.; Kiss, N.; Laing, E.; Lipson-Smith, R.; Britton, B. The Impact of Human Papillomavirus (HPV) Associated Oropharyngeal Squamous Cell Carcinoma (OPSCC) on Nutritional Outcomes. Nutrients 2021, 13, 514. [Google Scholar] [CrossRef]
- McIlwain, W.R.; Sood, A.J.; Nguyen, S.A.; Day, T.A. Initial symptoms in patients with HPV−positive and HPV−negative oropharyngeal cancer. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 441–447. [Google Scholar] [CrossRef]
- Vangelov, B.; Kotevski, D.P.; Williams, J.R.; Smee, R.I. The impact of HPV status on weight loss and feeding tube use in oropharyngeal carcinoma. Oral Oncol. 2018, 79, 33–39. [Google Scholar] [CrossRef]
- Anderson, N.J.; Jackson, J.E.; Wada, M.; Schneider, M.; Poulsen, M.; Rolfo, M.; Fahandej, M.; Gan, H.; Khoo, V. The changing landscape of head and neck cancer radiotherapy patients: Is high-risk, prolonged feeding tube use indicative of on-treatment weight loss? J. Med. Radiat. Sci. 2019, 66, 250–258. [Google Scholar] [CrossRef]
- Brown, T.E.; Wittholz, K.; Way, M.; Banks, M.D.; Hughes, B.G.M.; Lin, C.Y.; Kenny, L.M.; Bauer, J.D. Investigation of p16 status, chemotherapy regimen, and other nutrition markers for predicting gastrostomy in patients with head and neck cancer. Head Neck 2017, 39, 868–875. [Google Scholar] [CrossRef]
- Tamaki, A.; Manzoor, N.F.; Babajanian, E.; Ascha, M.; Rezaee, R.; Zender, C.A. Clinical Significance of Sarcopenia among Patients with Advanced Oropharyngeal Cancer. Otolaryngol.—Head Neck Surg. 2019, 160, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Stone, L.; Olson, B.; Mowery, A.; Krasnow, S.; Jiang, A.; Li, R.; Schindler, J.; Wax, M.K.; Andersen, P.; Marks, D.; et al. Association Between Sarcopenia and Mortality in Patients Undergoing Surgical Excision of Head and Neck Cancer. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Clayburgh, D.; Olson, B.; Edwards, J.; Stone, L.; Jiang, A.; Zhu, X.; Holland, J.; Li, R.; Andersen, P.; Krasnow, S.; et al. Association of Sarcopenia with Oncologic Outcomes of Primary Surgery or Definitive Radiotherapy among Patients with Localized Oropharyngeal Squamous Cell Carcinoma. JAMA Otolaryngol.—Head Neck Surg. 2020, 146, 714–722. [Google Scholar] [CrossRef]
- Naik, M.; Ward, M.C.; Bledsoe, T.J.; Kumar, A.M.S.; Rybicki, L.A.; Saxton, J.P.; Burkey, B.B.; Greskovich, J.F.; Adelstein, D.J.; Koyfman, S.A. It is not just IMRT: Human papillomavirus related oropharynx squamous cell carcinoma is associated with better swallowing outcomes after definitive chemoradiotherapy. Oral Oncol. 2015, 51, 800–804. [Google Scholar] [CrossRef] [PubMed]
| Reference (Year) | Race | Study Design | Nutritional Status Measure | The Time Point of Evaluation | Results | Conclusions | |||
|---|---|---|---|---|---|---|---|---|---|
| Harrowfield et al. (2021) [36] | Caucasian | Prospective | WL (>5%; >10%) Malnutrition (B or C according to PG-SGA) | Last week as well as 1 and 3 mths after C-RT | 83 OPSCC; HPV+: 70 (84.3%); HPV−: 13(15.7%) | HPV+ patients had: (a) NS ↓ risk of >5% WL in last week C-RT (68.6% vs. 69.3%; p = 1.0000; OR = 0.97; 95% CI: 0.26–3.49; p = 0.9625). (b) NS ↑ risk of >5% WL 1 mth after C-RT (80% vs. 76.9%; p = 0.7238; OR = 1.20; 95% CI: 0.29–4.94; p = 0.8009). (c) ↑ risk of >5% WL 3 mths after C-RT (87.1% vs. 76.9%; p = 0.3900; OR = 2.03; 95% CI: 0.47–8.82; p = 0.3433). (d) NS ↓ risk of >10% WL in last week C-RT (27.1% vs. 30.7%; p = 0.7476; OR = 0.84; 95% CI: 0.23–3.05; p = 0.7887). (e) NS ↓ risk of >10% WL 1 mth after C-RT (52.8% vs. 53.8%; p = 1.0000; OR = 0.96; 95% CI: 0.29–3.15; p = 0.9477). (f) S ↑ risk of >10% WL 3 mths after C-RT (67% vs. 31%; p = 0.0266; OR = 4.60; 95% CI: 1.28–16.52; p = 0.0194). (g) NS ↑ rate and risk of malnutrition in last week of C-RT (88.6% vs. 84.6%; p = 0.6524; OR = 1.41; 95% CI: 0.26–7.54; p = 0.6885). (h) NS ↑ rate and risk of malnutrition 1 mth after C-RT (71.4% vs. 61.6%; p = 0.5184; OR = 1.56; 95% CI: 0.46–5.35; p = 0.4776). (i) S ↑ rate and NS ↑ risk of malnutrition 3 mths after C-RT (42.8% vs. 38.5%; p = 0.0266; OR = 1.20; 95% CI: 0.36–4.04; p = 0.7684). | |||
| >5% WL (last week of C-RT) (yes): HPV+: 48 (68.6%) HPV−: 9 (69.3%) | >5% WL (last week of C-RT) (no): HPV+: 22 (31.4%) HPV−: 4 (30.7%) | ||||||||
| >5% WL (1 mth after C-RT) (yes): HPV+: 56 (80%) HPV−: 10 (76.9%) | >5% WL (1 mth after C-RT) (no): HPV+: 14 (20%) HPV−: 3 (23.1%) | ||||||||
| >5% WL (3 mths after C-RT) (yes): HPV+: 61 (87.1%) HPV−: 10 (76.9%) | >5% WL (3 mths after C-RT) (no): HPV+: 9 (12.9%) HPV−: 3 (23.1%) | ||||||||
| >10% WL (last week of C-RT) (yes): HPV+: 19 (27.1%) HPV−: 4 (30.7%) | >10% WL (last week of C-RT) (no): HPV+: 51 (72.9%) HPV−: 9 (69.3%) | ||||||||
| >10% WL (1 mth after C-RT) (yes): HPV+: 37 (52.8%) HPV−: 7 (53.8%) | >10% WL (1 mth after C-RT) (no): HPV+: 33 (47.2%) HPV−: 6 (46.2%) | ||||||||
| >10% WL (3 mths after C-RT) (yes): HPV+: 47 (67%) HPV−: 4 (31%) | >10% WL (3 mths after C-RT) (no): HPV+: 23 (33%) HPV−: 9 (69%) | ||||||||
| Malnutrition (last week of C-RT) (yes): HPV+: 62 (88.6%) HPV−: 11 (84.6%) | Malnutrition (last week of C-RT) (no): HPV+: 8 (11.4%) HPV−: 2 (15.4%) | ||||||||
| Malnutrition (1 mth after C-RT) (yes): HPV+: 50 (71.4%) HPV−: 8 (61.6%) | Malnutrition (1 mth after C-RT) (no): HPV+: 20 (28.6%) HPV−: 5 (38.4%) | ||||||||
| Malnutrition (3 mths after C-RT) (yes): HPV+: 30 (42.8%) HPV−: 5 (38.5%) | Malnutrition (3 mths after C-RT) (no): HPV+: 40 (57.2%) HPV−: 8 (61.5%) | ||||||||
| Olson et al. (2020) [43] | Caucasian | Retrospective | Sarcopenia (defined a priori as a skeletal muscle index of less than 52.4 for men and 38.5 for women) | Pretreatment (surgery/RT) | 245 OPSCC; HPV+: 197 (87.2%); HPV−: 28 (12.8%) | HPV+ patients had NS ↓ risk of sarcopenia (54.3% vs. 64.3%; p = 0.4170; OR = 0.66, 95% CI: 0.29–1.50; p = 0.3228). | |||
| Sarcopenia (yes): 135 HPV+: 107 (54.3%) HPV−: 18 (64.3%) | Sarcopenia (no): 110 HPV+: 90 (45.7%) HPV−: 10 (35.7%) | ||||||||
| Anderson et al. (2019) [39] | Caucasian | Retrospective | WL (% weight change between commencement and final week of RT). WL during FT use (as above) | Weight change between commence men and the final week of RT(6–7 weeks) | 101 OPSCC; HPV+: 59 (58.4%); HPV−: 42(41.6%) | In the LIRi group: (a) compared to HRi and HIRi groups a S ↑ proportion of HPV+ patients was noted (81% vs. 71% and 52%, respectively; p = 0.008). (b) total WL was S ↑ compared to the HRi (8.2% vs. 4.8%; p = 0.002;) and HIRi (8.2% vs.5.2%; p = 0.006;) groups. (c) percent of WL during FT use was S ↑ compared to the HRi (HRi: 8.8% vs. 4.6%; p < 0.001) and HIRi (8.8% vs.5.3%; p = 0.002) group. (d) compared to HRi and HIRi groups a S ↑ proportion of HPV+ patients was noted (81% vs. 71% and 52%, respectively; p = 0.008). (e) total WL was S ↑ compared to the HRi (8.2% vs. 4.8%; p = 0.002;) and HIRi (8.2% vs.5.2%; p = 0.006;) groups. (f) percent of WL during FT use was S ↑ compared to the HRi (HRi: 8.8% vs. 4.6%; p < 0.001) and HIRi (8.8% vs.5.3%; p = 0.002) group. | |||
| % of WL: | |||||||||
| LIRi: 8.2% | HRi: 4.8% | HIRi: 5.2% | |||||||
| % of WL during FT use: | |||||||||
| LIRi: 8.8% | HRi: 4.6% | HIRi: 5.3% | |||||||
| Tamaki et al.(2019) [41] | Caucasian | Retrospective | BMI (pretreatment) Sarcopenia Male: SMI < 43 cm2/m2 and BMI < 20.0 kg/m2 (underweight) or 20.0–24.9 kg/m2 (normal weight) SMI <41 cm2/m2 and BMI = 25.0–29.9 kg/m2 (overweight) and BMI = 30.0 kg/m2 (obese) Female: SMI < 41 cm2/m2 and all BMI categories | Pretreatment (surgery, C-RT and/or adjuvant treatment) | 113 OPC; HPV+: 85 (75.9%); HPV−: 27 (24.1%) | HPV+ patients had: (a) S ↑ pretreatment BMI (28.2 vs. 24.2 kg/m2; p = 0.001). (b) NS ↓ risk of sarcopenia (27.0% vs. 32.1%; p = 0.6332; OR = 0.78; 95% Cl: 0.31–1.98; p = 0.7832). | |||
| Sarcopenia (yes): 32 HPV+: 23 (27%) HPV−: 9 (32.1%) | Sarcopenia (no): 81 HPV+: 62 (73%) HPV−: 19 (67.9%) | ||||||||
| Stone et al. (2019) [42] | Caucasian | Retrospective | Sarcopenia (defined as L3 skeletal muscle index below 52.4 cm2/m2 for men and below 38.5 cm2/m2 for women | Pretreatment (surgery) | 260 HNC; HPV+: 92 (71.3%); HPV−: 37(28.7%) | HPV+ patients had S ↓ risk of sarcopenia (47.8% vs. 67.6%; p = 0.0517; OR = 0.44; 95% Cl:0.20–0.98; p = 0.0445). | |||
| Sarcopenia (yes): 144 HPV+: 44 (47.8%) HPV−: 25 (67.6%) | Sarcopenia (no): 116 HPV+: 48 (52.2%) HPV−: 12 (32.4%) | ||||||||
| Vangelov et al. (2018) [38] | Caucasian | Retrospective | CWL (defined as ≥5% WL during treatment) FTD (PFT, RFT) | In the 1st and in 6th week of RT | 100 OPSCC; HPV+: 68 (87.2%); HPV−: 10 (12.8%) | HPV+ patients had: (a) S ↑ risk of CWL (92.6% vs. 60%; p = 0.011; OR = 8.4, 95% CI: 1.77–39.93; p = 0.0075). (b) ↑ risk of FT used (PFT and RFT) (63.2% vs. 60%; p = 1.000; OR = 1.15, 95% CI: 0.29–4.46; p = 0.8434). (c) NS ↓ risk of PFT (39.0% vs. 55.6%; p = 0.4641; OR = 0.51, 95% CI: 0.12–2.20; p = 0.3678). (d) NS ↑ risk of RFT (51.9% vs. 20%; p = 0.3525; OR = 4.32, 95% CI: 0.45–41.31; p = 0.2040). (e) NS ↓ risk of CWL during PFT (41.7% vs. 60%; p = 0.6384; OR = 0.48, 95% CI: 0.07–3.21; p = 0.4460). (f) NS ↑risk of CWL during RFT (56.2% vs. 33.3%; p = 0.5825; OR = 2.57, 95% CI: 0.22–30.34; p = 0.4531). | |||
| CWL (yes): 86 HPV+: 63 (92.6%) HPV−: 6 (60%) | CWL (no): 14 HPV+: 5 (7.4%) HPV−: 4 (40%) | ||||||||
| FTD (PFT, RFT) (yes): 49 HPV+: 43 (63.2%) HPV−: 6 (60%) | FTD (PFT, RFT) (no): 29 HPV+: 25 (36.8%) HPV−: 4 (40%) | ||||||||
| PFT (yes): 21 HPV+: 16 (39%) HPV−: 5 (55.6%) | PFT (no): 29 HPV+: 25 (61%) HPV−: 4 (44.4%) | ||||||||
| RFT (yes): 28 HPV+: 27 (51.9%) HPV−: 1 (20%) | RFT (no): 29 HPV+: 25 (48.1%) HPV−: 4 (80%) | ||||||||
| PFT + CWL (yes): 18 HPV+: 15 (41.7%) HPV−: 3 (60%) | PFT + CWL (no): 23 HPV+: 21 (58.3%) HPV−: 2 (40%) | ||||||||
| RFT + CWL (yes): 28 HPV+: 27 (56.2%) HPV−: 1 (33.3%) | RFT + CWL (no): 23 HPV+: 21 (43.8%) HPV−: 2 (66.7%) | ||||||||
| Brown et al. (2017) [40] | Caucasian | Retrospective | High-risk category: proactive gastrostomy placement before treatment: C-RT or severe malnutrition (defined as >10% WL in 6 mths, BMI < 20 with unintentional WL 5–10% in 6 mths; PG-SGA C) | From baseline at diagnosis to the end of treatment (surgery/C-RT/RT) | 269 HNC; p16+: 59 (36.2%); p16-: 104 (63.8%) | HPV+ patients had S ↑ risk of gastrostomy (76.3% vs. 31.7%; p < 0.0001; OR = 4.4; 95% CI: 1.01–19.31; p = 0.049). | |||
| ↑ risk of gastrostomy: 88 p16+: 45 (76.3%) p16-: 33 (31.7%) | ↓risk of gastrostomy:181 p16+: 14 (23.7%) p16-: 71 (68.3%) | ||||||||
| Naik et al. (2015) [44] | Caucasian | Retrospective | Diet changes (significant restrictions in the types of foods eaten, and/or requiring nutritional supplementation for weight maintenance) FTD Swallowing disorders (defined as FTD or limited diet) | 3, 6, 12, and 24 mths after C-RT | 147 OPSCC; HPV+: 130 (88.4%); HPV−: 17 (11.6%) | HPV+ patients had: (a) S ↓incidences and risk of restricted diet (8.6% vs. 33.3%; p = 0.014; OR = 0.19, 95% CI: 0.05–0.06; p = 0.0082) (b) S ↓ risk of FTD (1.6% vs. 12.5%; p = 0.06; OR = 0.11, 95% CI: 0.01–0.85; p = 0.0338) at last follow-up (2 years after treatment start). (c) S reduced risk of late (24 mths) swallowing impairment after C-RT (HR = 0.19; 95% CI: 0.05–0.65; p = 0.008). (d) were S more likely to resume a normal diet at 55 mths of follow-up (87% vs. 65%; p = 0.0291; OR = 3.62, 95% CI: 1.19–11.09 p = 0.0239). | |||
| Restricted diet (24 mths after C-RT) (yes): HPV+: 11 (8.6%) HPV−:5 (33.3%) | Restricted diet (24 mths after C-RT) (no): HPV+:117 (91.4%) HPV−:10 (67.7%) | ||||||||
| FTD (24 mths after C-RT) (yes): HPV+: 2 (1.6%) HPV−: 2 (12.5%) | FTD (24 mths after C-RT) (no): HPV+: 127 (98.6%) HPV−: 14 (87.5%) | ||||||||
| Resume a normal diet (at 55 mths of follow-up) (yes): HPV+: 113 (87%) HPV−: 11 (65%) | Resume a normal diet (at 55 mths of follow-up) (no): HPV+: 17 (13%) HPV−: 6 (35%) | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, M.; Mlak, R.; Kot, A.; Rahnama-Hezavah, M.; Małecka-Massalska, T. Does Human Papillomavirus Infection Influence the Frequency and Severity of Nutritional Disorders in Head and Neck Cancer? Nutrients 2022, 14, 4528. https://doi.org/10.3390/nu14214528
Mazurek M, Mlak R, Kot A, Rahnama-Hezavah M, Małecka-Massalska T. Does Human Papillomavirus Infection Influence the Frequency and Severity of Nutritional Disorders in Head and Neck Cancer? Nutrients. 2022; 14(21):4528. https://doi.org/10.3390/nu14214528
Chicago/Turabian StyleMazurek, Marcin, Radosław Mlak, Agata Kot, Mansur Rahnama-Hezavah, and Teresa Małecka-Massalska. 2022. "Does Human Papillomavirus Infection Influence the Frequency and Severity of Nutritional Disorders in Head and Neck Cancer?" Nutrients 14, no. 21: 4528. https://doi.org/10.3390/nu14214528
APA StyleMazurek, M., Mlak, R., Kot, A., Rahnama-Hezavah, M., & Małecka-Massalska, T. (2022). Does Human Papillomavirus Infection Influence the Frequency and Severity of Nutritional Disorders in Head and Neck Cancer? Nutrients, 14(21), 4528. https://doi.org/10.3390/nu14214528







