Dietary Quality and Relationships with Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) among United States Adults, Results from NHANES 2017–2018
Abstract
1. Introduction
2. Methods
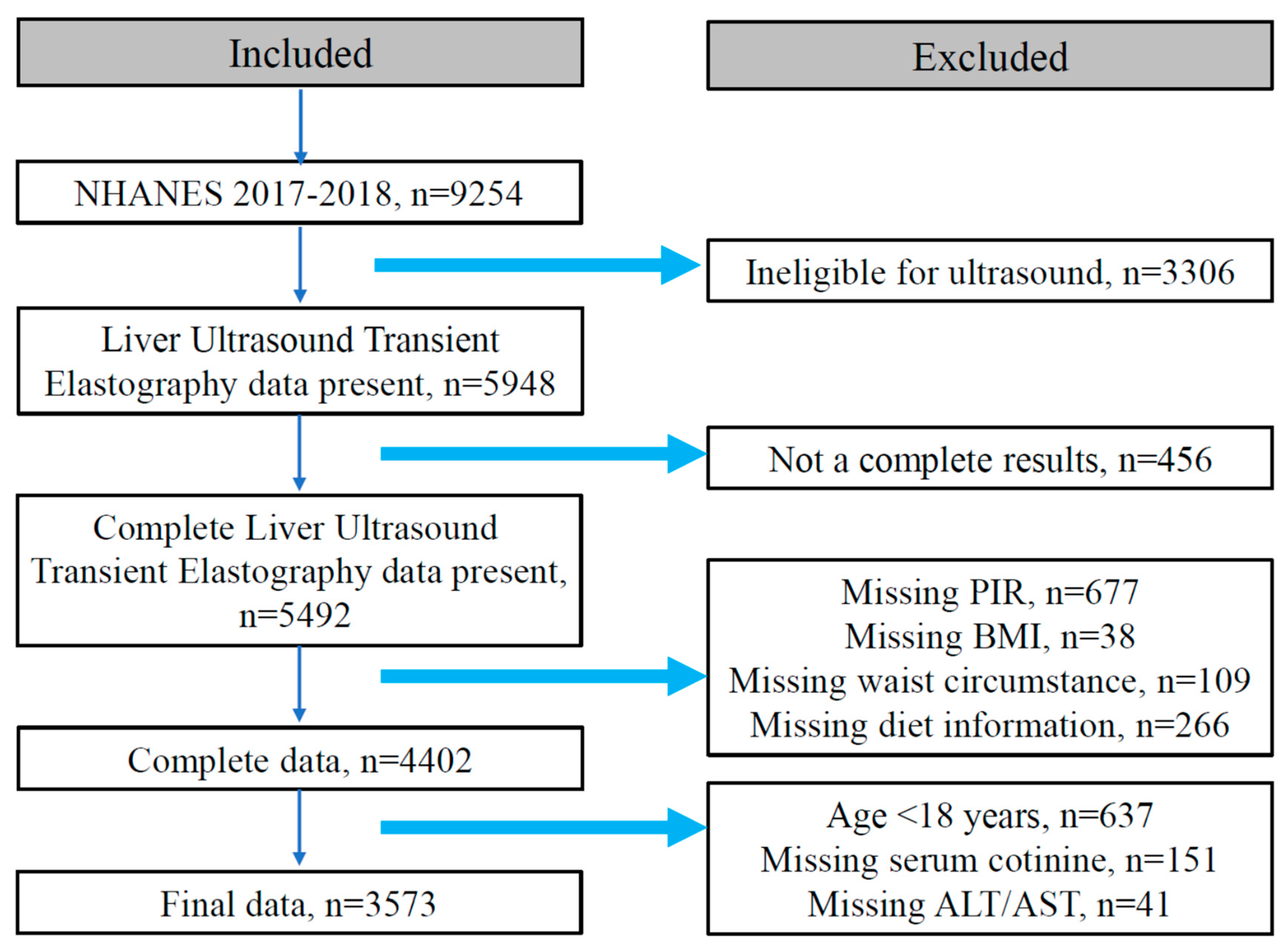
2.1. Study Design and Population
2.2. Dietary Assessment and Covariates
2.3. Diagnostic Criteria and Definitions
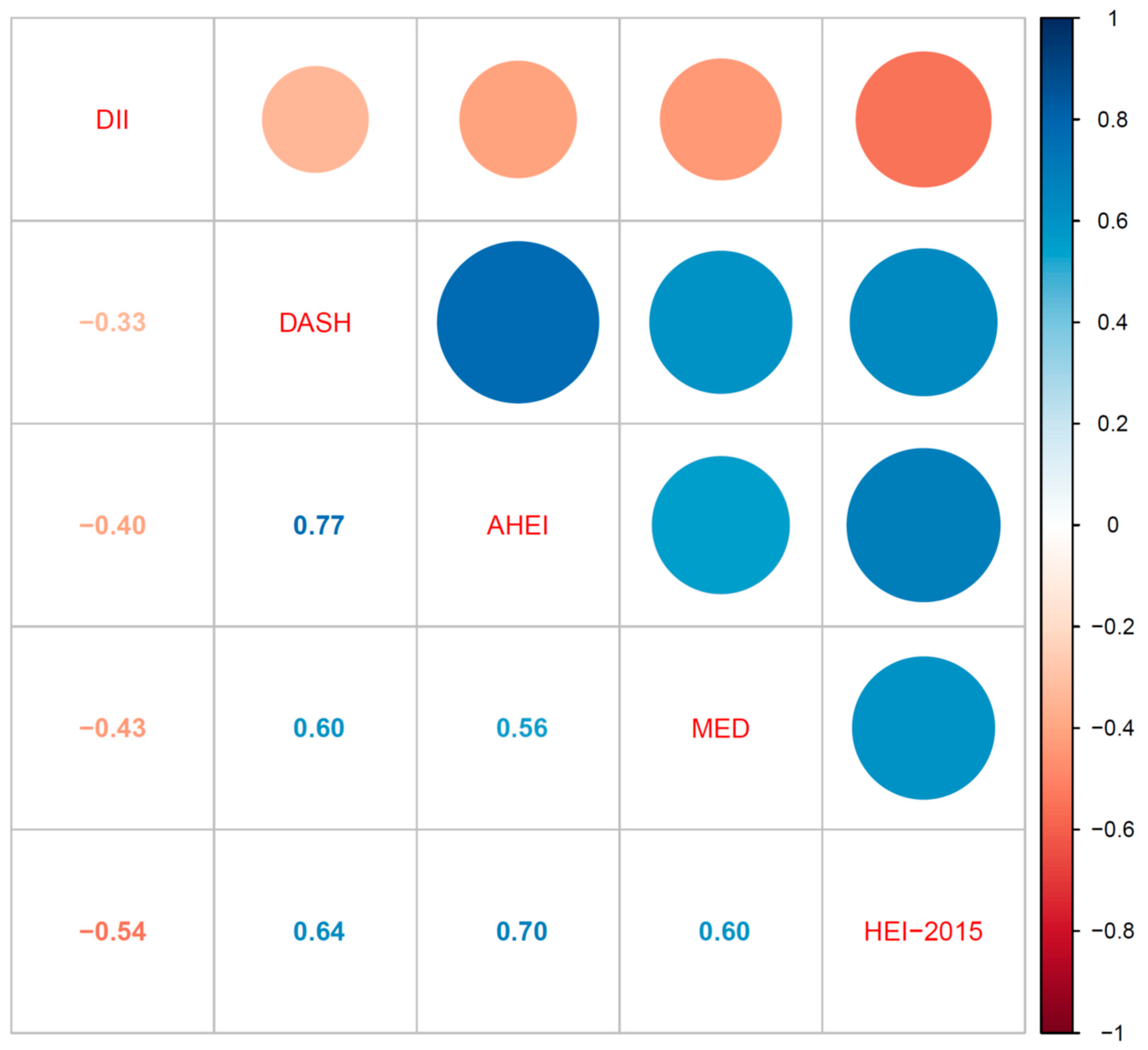
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Participants Based on MAFLD Phenotypes
3.2. The Relationship between Various Dietary Quality Indexes and CAP, LSM, ALT, or AST
3.3. The Prevalence of MAFLD Phenotypes across Five Dietary Quality Indexes Tertiles
3.4. Associations between Dietary Quality Indexes and MAFLD Phenotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Blond, E.; Disse, E.; Cuerq, C.; Drai, J.; Valette, P.J.; Laville, M.; Thivolet, C.; Simon, C.; Caussy, C. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease in severely obese people: Do they lead to over-referral. Diabetologia 2017, 60, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Perseghin, G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021, 41, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, X.; Li, G.; Wong, G.L.; Wong, V.W. Epidemiology and Clinical Outcomes of Metabolic (Dysfunction)-associated Fatty Liver Disease. J. Clin. Transl. Hepatol. 2021, 9, 972–982. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, Y.Y.; Chen, C.; Lu, Y.L.; Wang, N.J. Cardiovascular and renal burdens of metabolic associated fatty liver disease from serial US national surveys, 1999–2016. Chin. Med. J. 2021, 134, 1593–1601. [Google Scholar] [CrossRef]
- Kim, D.; Konyn, P.; Sandhu, K.K.; Dennis, B.B.; Cheung, A.C.; Ahmed, A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J. Hepatol. 2021, 75, 1284–1291. [Google Scholar] [CrossRef]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Song, C.; Lv, J.; Yu, C.; Zhu, M.; Yu, C.; Guo, Y.; Yang, L.; Chen, Y.; Chen, Z.; Jiang, T.; et al. Adherence to Healthy Lifestyle and Liver cancer in Chinese: A prospective cohort study of 0.5 million people. Br. J. Cancer 2022, 126, 815–821. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Berná, G.; Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 2020, 40 (Suppl. 1), 102–108. [Google Scholar] [CrossRef]
- Harrison, S.; Couture, P.; Lamarche, B. Diet Quality, Saturated Fat and Metabolic Syndrome. Nutrients 2020, 12, 3232. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Nephew, L.D.; Vuppalanchi, R.; Gawrieh, S.; Mladenovic, A.; Pike, F.; Samala, N.; Chalasani, N. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology 2022, 75, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet: A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Bogensberger, B.; Hoffmann, G. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: An Updated Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2018, 118, 74–100.e11. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Patel, C.J.; Pho, N.; McDuffie, M.; Easton-Marks, J.; Kothari, C.; Kohane, I.S.; Avillach, P. A database of human exposomes and phenomes from the US National Health and Nutrition Examination Survey. Sci. Data 2016, 3, 160096. [Google Scholar] [CrossRef]
- Stebbins, R.C.; Noppert, G.A.; Aiello, A.E.; Cordoba, E.; Ward, J.B.; Feinstein, L. Persistent socioeconomic and racial and ethnic disparities in pathogen burden in the United States, 1999–2014. Epidemiol. Infect. 2019, 147, e301. [Google Scholar] [CrossRef]
- Kyu, H.H.; Bachman, V.F.; Alexander, L.T.; Mumford, J.E.; Afshin, A.; Estep, K.; Veerman, J.L.; Delwiche, K.; Iannarone, M.L.; Moyer, M.L.; et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: Systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ 2016, 354, i3857. [Google Scholar] [CrossRef]
- Reja, D.; Makar, M.; Visaria, A.; Karanfilian, B.; Rustgi, V. Blood lead level is associated with advanced liver fibrosis in patients with non-alcoholic fatty liver disease: A nationwide survey (NHANES 2011–2016). Ann. Hepatol. 2020, 19, 404–410. [Google Scholar] [CrossRef]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Vuppalanchi, R.; Van Natta, M.L.; Hallinan, E.; Kowdley, K.V.; Abdelmalek, M.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Brandman, D.; et al. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 156–163.e2. [Google Scholar] [CrossRef] [PubMed]
- Cassinotto, C.; Boursier, J.; de Lédinghen, V.; Lebigot, J.; Lapuyade, B.; Cales, P.; Hiriart, J.B.; Michalak, S.; Bail, B.L.; Cartier, V.; et al. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 2016, 63, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Fasoli, L.; Castillo, M.J.; Amaro-Gahete, F.J. Dietary Inflammatory Index and S-Klotho Plasma Levels in Middle-Aged Adults. Nutrients 2020, 12, 281. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef]
- Mellen, P.B.; Gao, S.K.; Vitolins, M.Z.; Goff, D.C., Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch. Intern. Med. 2008, 168, 308–314. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Yoo, E.R.; Kim, D.; Vazquez-Montesino, L.M.; Escober, J.A.; Li, A.A.; Tighe, S.P.; Fernandes, C.T.; Cholankeril, G.; Ahmed, A. Diet quality and its association with nonalcoholic fatty liver disease and all-cause and cause-specific mortality. Liver Int. 2020, 40, 815–824. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Doustmohammadian, A.; Nouri Saeidlou, S.; Esfandyari, S.; Gholizadeh, E.; Maadi, M.; Motamed, N.; Ajdarkosh, H.; Khoonsari, M.; Clark, C.; Zamani, F. Dietary Acid Load (DAL), Glycated Hemoglobin A1c (HbA1c), and Metabolic Syndrome (MeS) Mediate the Association of the Adherence to the Dietary Approaches to Stopping Hypertension (DASH) and Mediterranean Diet (MeD) With Nonalcoholic Fatty Liver Disease. Front. Nutr. 2022, 9, 921415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Heredia, N.I.; Balakrishnan, M.; Thrift, A.P. Prevalence and factors associated with NAFLD detected by vibration controlled transient elastography among US adults: Results from NHANES 2017–2018. PLoS ONE 2021, 16, e0252164. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Paik, J.M.; Al Shabeeb, R.; Golabi, P.; Younossi, I.; Henry, L. Are there outcome differences between NAFLD and metabolic-associated fatty liver disease. Hepatology 2022, 76, 1423–1437. [Google Scholar] [CrossRef]
- Maskarinec, G.; Lim, U.; Jacobs, S.; Monroe, K.R.; Ernst, T.; Buchthal, S.D.; Shepherd, J.A.; Wilkens, L.R.; Le Marchand, L.; Boushey, C.J. Erratum: Diet Quality Predicts Visceral Adiposity and Liver Fatness: The Multiethnic Cohort Study. Obesity 2018, 26, 239. [Google Scholar] [CrossRef]
- Hébert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)-Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Phillips, C.M.; Chen, L.W.; Heude, B.; Bernard, J.Y.; Harvey, N.C.; Duijts, L.; Mensink-Bout, S.M.; Polanska, K.; Mancano, G.; Suderman, M.; et al. Dietary Inflammatory Index and Non-Communicable Disease Risk: A Narrative Review. Nutrients 2019, 11, 1873. [Google Scholar] [CrossRef]
- Mazidi, M.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Mikhailidis, D.P.; Kengne, A.P.; Banach, M. Dietary inflammatory index and cardiometabolic risk in US adults. Atherosclerosis 2018, 276, 23–27. [Google Scholar] [CrossRef]
- Garcia-Arellano, A.; Martínez-González, M.A.; Ramallal, R.; Salas-Salvadó, J.; Hébert, J.R.; Corella, D.; Shivappa, N.; Forga, L.; Schröder, H.; Muñoz-Bravo, C.; et al. Dietary inflammatory index and all-cause mortality in large cohorts: The SUN and PREDIMED studies. Clin. Nutr. 2019, 38, 1221–1231. [Google Scholar] [CrossRef]
- Koopman, K.E.; Caan, M.W.; Nederveen, A.J.; Pels, A.; Ackermans, M.T.; Fliers, E.; la Fleur, S.E.; Serlie, M.J. Hypercaloric diets with increased meal frequency, but not meal size, increase intrahepatic triglycerides: A randomized controlled trial. Hepatology 2014, 60, 545–553. [Google Scholar] [CrossRef]
- Pafili, K.; Roden, M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol. Metab. 2021, 50, 101122. [Google Scholar] [CrossRef]
- Hernandez-Rodas, M.C.; Valenzuela, R.; Videla, L.A. Relevant Aspects of Nutritional and Dietary Interventions in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2015, 16, 25168–25198. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montes de Oca, A.; Julián, M.T.; Ramos, A.; Puig-Domingo, M.; Alonso, N. Microbiota, Fiber, and NAFLD: Is There Any Connection. Nutrients 2020, 12, 3100. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ma, L.J.; Yang, Y.; Xiao, Z.; Wan, J.B. n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, S116–S129. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Ma, J.; Hennein, R.; Liu, C.; Long, M.T.; Hoffmann, U.; Jacques, P.F.; Lichtenstein, A.H.; Hu, F.B.; Levy, D. Improved Diet Quality Associates With Reduction in Liver Fat, Particularly in Individuals With High Genetic Risk Scores for Nonalcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 107–117. [Google Scholar] [CrossRef]
- Shan, Z.; Rehm, C.D.; Rogers, G.; Ruan, M.; Wang, D.D.; Hu, F.B.; Mozaffarian, D.; Zhang, F.F.; Bhupathiraju, S.N. Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality Among US Adults, 1999–2016. JAMA 2019, 322, 1178–1187. [Google Scholar] [CrossRef]
| Variables | Non-MAFLD Weighted Mean or Percentage | MAFLD without Clinical Fibrosis Weighted Mean or Percentage | MAFLD with Clinical Fibrosis Weighted Mean or Percentage | p Value |
|---|---|---|---|---|
| Gender | <0.001 | |||
| Male | 43.99 | 53.57 | 54.65 | |
| Female | 56.01 | 46.43 | 43.35 | |
| Ethnicity | <0.001 | |||
| Non-Hispanic White | 66.75 | 64.24 | 65.44 | |
| Non-Hispanic Black | 11.20 | 8.16 | 9.50 | |
| Other Hispanic | 6.41 | 5.73 | 5.99 | |
| Mexican American | 5.90 | 11.34 | 10.28 | |
| Other | 9.75 | 10.54 | 8.78 | |
| Education | 0.562 | |||
| <High school | 9.53 | 10.03 | 11.09 | |
| ≥High school | 90.47 | 89.97 | 88.91 | |
| Diabetes | <0.001 | |||
| No | 96.01 | 85.42 | 61.95 | |
| Yes | 3.99 | 14.58 | 38.05 | |
| PA level | <0.001 | |||
| Low | 22.75 | 33.74 | 35.92 | |
| Moderate | 56.36 | 47.96 | 45.85 | |
| High | 20.89 | 18.29 | 18.23 | |
| PIR | 0.680 | |||
| Low | 20.97 | 18.85 | 19.50 | |
| Middle | 33.46 | 36.65 | 37.93 | |
| High | 45.57 | 44.50 | 42.57 | |
| Smoking Status | 0.338 | |||
| Low | 35.94 | 39.85 | 41.20 | |
| Moderate | 37.85 | 37.655 | 36.44 | |
| High | 26.21 | 22.50 | 22.35 | |
| Drink Status | 0.093 | |||
| Never | 61.46 | 66.78 | 68.69 | |
| Moderate | 35.61 | 29.11 | 28.39 | |
| Heavy | 2.93 | 4.11 | 2.92 | |
| Age (years) | 42.70 (41.12–44.28) | 50.13 (48.53–51.73) | 52.01 (49.14–54.89) | <0.001 |
| CAP (dB/m) | 209.20 (207.53–210.87) | 301.39 (298.77–304.01) | 328.23 (321.77–224.69) | <0.001 |
| LSM (kPa) | 4.84 (4.65–5.03) | 4.66 (4.58–4.73) | 10.19 (9.16–11.22) | <0.001 |
| BMI (kg/m2) | 25.51 (24.98–26.03) | 31.68 (30.75–32.02) | 36.21 (35.01–37.41) | <0.001 |
| WC (cm) | 89.26 (88.03–90.49) | 106.13 (104.67–107.60) | 117.01 (114.80–119.23) | <0.001 |
| Cotinine (ng/mL) | 62.03 (47.38–76.69) | 46.11 (39.75–52.46) | 51.45 (31.31–71.59) | 0.019 |
| ALT (U/L) | 19.70 (18.57–20.83) | 24.34 (23.0–25.69) | 32.38 (29.12–35.64) | <0.001 |
| AST (U/L) | 21.69 (20.54–2.84) | 21.45 (20.67–22.22) | 26.61 (24.58–28.64) | 0.007 |
| GGT (IU/L) | 23.53 (22.08–24.99) | 29.83 (27.75–31.90) | 46.68 (40.06–53.30) | <0.001 |
| GHB (%) | 5.36 (5.32–5.39) | 5.71 (5.63–5.79) | 6.23 (6.13–6.33) | <0.001 |
| GLU (mg/dL) | 100.19 (99.21–101.18) | 112.30 (108.83–115.78) | 131.79 (123.15–140.43) | <0.001 |
| Insulin (uU/mL) | 4.04 (3.51–4.57) | 7.29 (6.41–8.17) | 11.69 (9.55–13.82) | <0.001 |
| TG (mg/dL) | 105.52 (100.79–11.026) | 162.81 (154.69–170.93) | 195.37 (179.45–211.29) | <0.001 |
| HDL (mg/dL) | 59.34 (57.78–60.89) | 50.27 (49.14–51.41) | 47.00 (45.30–48.71) | <0.001 |
| HSCRP (mg/L) | 2.54 (2.18–2.91) | 4.22 (3.74–4.70) | 5.60 (4.76–6.45) | <0.001 |
| DII | 1.36 (1.18–1.55) | 1.50 (1.31–1.68) | 1.64 (1.43–1.86) | 0.032 |
| HEI-2015 | 50.54 (48.40–52.69) | 48.47 (47.10–49.85) | 46.46 (44.84–48.08) | 0.005 |
| AHEI | 49.00 (47.40–50.61) | 47.01 (45.95–48.08) | 45.24 (44.16–46.33) | <0.001 |
| DASH | 26.12 (25.56–26.68) | 25.28 (24.90–25.65) | 24.66 (24.18–25.14) | <0.001 |
| MED | 6.06 (5.91–6.21) | 5.95 (5.84–6.06) | 5.91 (5.79–6.07) | 0.107 |
| Variables | CAP | LSM | ALT | AST | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficients | p Value | Coefficients | p Value | Coefficients | p Value | Coefficients | p Value | |
| DII | 4.624 | 0.009 | 0.012 | 0.903 | 0.125 | 0.674 | –0.128 | 0.471 |
| HEI-2015 | –0.520 | 0.013 | –0.020 | 0.026 | 0.006 | 0.864 | 0.051 | 0.031 |
| AHEI | –0.605 | 0.019 | –0.018 | 0.061 | –0.030 | 0.454 | –0.002 | 0.875 |
| DASH | –2.112 | 0.003 | –0.034 | 0.320 | –0.063 | 0.531 | 0.053 | 0.235 |
| MED | –4.141 | 0.077 | –0.062 | 0.485 | 0.389 | 0.450 | 0.337 | 0.381 |
| Tertiles | Non-MAFLD (47.05%) | MAFLD without Clinical Fibrosis (36.67%) | MAFLD with Clinical Fibrosis (16.28%) | p Value | p for Trend |
|---|---|---|---|---|---|
| DII T1 | 49.84 | 35.85 | 14.30 | 0.494 | 0.016 |
| T2 | 46.21 | 37.44 | 16.35 | ||
| T3 | 44.84 | 36.77 | 18.39 | ||
| HEI-2015 T1 | 42.13 | 37.72 | 20.14 | 0.002 | 0.001 |
| T2 | 46.15 | 38.61 | 15.24 | ||
| T3 | 53.32 | 33.40 | 13.28 | ||
| AHEI T1 | 42.47 | 36.93 | 20.60 | <0.001 | <0.001 |
| T2 | 46.46 | 37.93 | 15.62 | ||
| T3 | 52.51 | 35.15 | 12.34 | ||
| DASH T1 | 40.65 | 39.64 | 19.71 | <0.001 | <0.001 |
| T2 | 46.30 | 37.02 | 16.69 | ||
| T3 | 52.56 | 34.11 | 13.34 | ||
| MED T1 | 43.44 | 38.95 | 17.61 | 0.329 | 0.008 |
| T2 | 47.65 | 36.44 | 15.92 | ||
| T3 | 50.06 | 34.59 | 15.35 |
| Dietary Quality Indexes | Multivariate Logistic Regression of MAFLD | Multivariate Ordinal Logistic Regression of MAFLD Phenotypes | ||
|---|---|---|---|---|
| OR (95%CI) | p Value | OR (95%CI) | p Value | |
| DII | ||||
| Continuous scales | 1.146 (1.041–1.260) | 0.013 | 1.144 (1.069–1.225) | <0.001 |
| T1 (Reference) | 1.000 | 1.000 | ||
| T2 | 1.320 (0.982–1.774) | 0.061 | 1.300 (1.058–1.593) | 0.012 |
| T3 | 1.568 (0.984–2.484) | 0.056 | 1.561 (1.122–2.172) | 0.008 |
| HEI-2015 | ||||
| Continuous scales | 0.974 (0.968–0.990) | 0.003 | 0.979 (0.970–0.988) | <0.001 |
| T1 (Reference) | 1.000 | 1.000 | ||
| T2 | 0.741 (0.531–1.034) | 0.069 | 0.721 (0.576–0.902) | 0.004 |
| T3 | 0.497 (0.335–0.738) | 0.006 | 0.510 (0.389–0.668) | <0.001 |
| AHEI | ||||
| Continuous scales | 0.974 (0.963–0.986) | 0.002 | 0.974 (0.966–0.982) | <0.001 |
| T1 (Reference) | 1.000 | 1.000 | ||
| T2 | 0.722 (0.541–0.963) | 0.034 | 0.698 (0.581–0.838) | <0.001 |
| T3 | 0.535 (0.379–0.754) | 0.005 | 0.519 (0.403–0.669) | <0.001 |
| DASH | ||||
| Continuous scales | 0.918 (0.892–0.945) | <0.001 | 0.920 (0.898–0.943) | <0.001 |
| T1 (Reference) | 1.000 | 1.000 | ||
| T2 | 0.743 (0.571–0.967) | 0.034 | 0.765 (0.608–0.962) | 0.022 |
| T3 | 0.527 (0.397–0.699) | 0.002 | 0.548 (0.437–0.688) | <0.001 |
| MED | ||||
| Continuous scales | 0.832 (0.719–0.962) | 0.021 | 0.847 (0.756–0.949) | 0.004 |
| T1 (Reference) | 1.000 | 1.000 | ||
| T2 | 0.737 (0.573–0.947) | 0.026 | 0.759 (0.624–0.922) | 0.005 |
| T3 | 0.637 (0.433–0.939) | 0.031 | 0.679 (0.519–0.887) | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, T.; Zhang, J.; Xie, W.; Ni, Y.; Fang, X.; Liu, M.; Peng, X.; Wang, J.; Dai, Y.; Zhou, Y. Dietary Quality and Relationships with Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) among United States Adults, Results from NHANES 2017–2018. Nutrients 2022, 14, 4505. https://doi.org/10.3390/nu14214505
Tian T, Zhang J, Xie W, Ni Y, Fang X, Liu M, Peng X, Wang J, Dai Y, Zhou Y. Dietary Quality and Relationships with Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) among United States Adults, Results from NHANES 2017–2018. Nutrients. 2022; 14(21):4505. https://doi.org/10.3390/nu14214505
Chicago/Turabian StyleTian, Ting, Jingxian Zhang, Wei Xie, Yunlong Ni, Xinyu Fang, Mao Liu, Xianzhen Peng, Jie Wang, Yue Dai, and Yonglin Zhou. 2022. "Dietary Quality and Relationships with Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) among United States Adults, Results from NHANES 2017–2018" Nutrients 14, no. 21: 4505. https://doi.org/10.3390/nu14214505
APA StyleTian, T., Zhang, J., Xie, W., Ni, Y., Fang, X., Liu, M., Peng, X., Wang, J., Dai, Y., & Zhou, Y. (2022). Dietary Quality and Relationships with Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) among United States Adults, Results from NHANES 2017–2018. Nutrients, 14(21), 4505. https://doi.org/10.3390/nu14214505





