Abstract
The early diagnosis of and intervention in vitamin B12 deficiency in exclusively breastfed infants by mothers with low vitamin B12 is crucial in preventing possible irreversible neurologic damage, megaloblastic anemia, and failure to thrive. We assess the usefulness of the early detection of asymptomatic B12 deficiency related to acquired conditions and highlight the importance of monitoring serum vitamin B12 levels during pregnancy. We describe demographic, clinical, dietary, and biochemical data, including the evolution of a vitamin B12 deficiency’s functional biomarkers. We enrolled 12 newborns (5 males) with an age range of 1–2 months old that were exclusively breastfed and asymptomatic. These cases were referred to our metabolic unit due to alterations in expanded newborn screening: high levels of methylmalonic acid and/or total homocysteine (tHcy). All mothers were under a vegetarian diet except three who had abnormal B12 absorption, and all presented low or borderline serum B12 level and high plasma levels of tHcy. Supplementation with oral vitB12 re-established the metabolic homeostasis of the mothers. In infants, therapy with an intramuscular injection of 1.0 mg hydroxocobalamin led to the rapid normalization of the metabolic pattern, and a healthy outcome was observed. Acquired B12 deficiency should be ruled out before proceeding in a differential diagnosis of cobalamin metabolism deficits, methylmalonic acidemia, and homocystinuria.
1. Introduction
Vitamin B12 (cobalamin, Cbl), as a regulator of fetal growth, plays an important role in cellular metabolism, affecting cell growth and differentiation by influencing DNA synthesis and epigenetic regulation [1,2].
Vitamin B12 deficiency is globally an important public health problem, albeit with still limited population data [3,4]. Some stages of life present a higher risk of deficiency, such as pregnancy and infancy [3,5,6]. Vitamin B12 deficiency has a high global incidence in pregnant women, ranging between 10% and 50% for different populations and ethnicities [7]. In a normal pregnancy, vitamin B12 levels fall by around 30% by the third trimester [5]. Newborn cobalamin levels at birth depend on maternal cobalamin stored during pregnancy, gestational age, and birth weight [6]. Maternal cobalamin deficiency, prematurity, and low birth weight are all associated with lower fetal cobalamin reserves [6]. The risk is higher in exclusively breastfed infants due to low cobalamin intake through the breast milk of mothers with vitamin B12 deficiency [6].
Vitamin B12 insufficiency is mainly associated with a high risk of failure to thrive, hematological problems, and short- and long-term effects on neurological and cognitive functions. The extent and degree of disability are assigned to the severity and duration of deficiency status. Moreover, sustained vitamin B12 deficiency may lead to irreversible neurological damage [1,2]. Early diagnosis and intervention in exclusively breastfed infants of mothers with a deficiency in or lack of vitamin B12 supplementation are crucial in preventing possible irreversible neurologic disorders [2].
Vitamin B12 is converted in the mitochondria into adenosylcobalamin (AdoCbl) and in the cytosol into methylcobalamin (MeCbl), which act as cofactors for methylmalonyl-CoA mutase (MCM) and for methionine synthase (MS), respectively. MCM metabolizes the conversion of methylmalonic acid (MMA) into succinyl-CoA, which plays a crucial role in energy metabolism by replenishing the mitochondrial succinyl-CoA pool for the TCA cycle. MS intervenes in the biosynthesis of methionine (Met) through the remethylation of homocysteine (Hcy) using the methyl group donated by the N5-methyl-tetrahydrofolate (Figure 1). Therefore, a decrease in circulating vitamin B12 leads to the disruption of the aforementioned catabolic pathways, resulting in the accumulation of MMA and Hcy. MMA and total homocysteine (tHcy) are used as second-tier test biomarkers in expanded newborn screening (NBS) for the identification of methylmalonic acidurias, defects of intracellular cobalamin metabolism, or elevated Hcy-related disorders. Thus, the alteration in these markers can also be an indication of vitamin B12 deficiency in the neonatal period. The further evaluation of vitamin B12 status via the measurement of serum cobalamin or in some cases holo-transcobalmin, the active form of cobalamin, allows for differentiating the genetic causes of intracellular cobalamin metabolism defects from acquired ones.
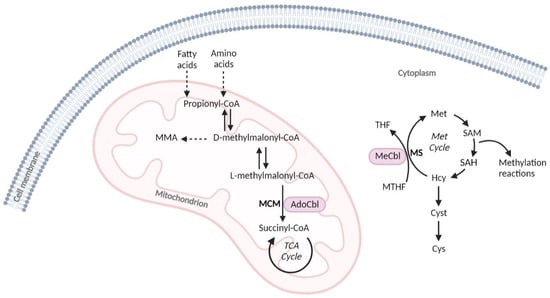
Figure 1.
Simplified scheme of the two metabolic pathways that use cobalamin cofactors. AdoCbl, adenosylcobalamin; Cys, cystine; Cyst, cystathionine; Hcy, homocysteine; MeCbl, methylcobalamin; Met cycle, methionine cycle; MCM, methylmalonyl-CoA mutase; MMA, methylmalonic acid; MS, methionine synthase; MTHF, N5-methyltetrahydrofolate; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; TCA cycle, tricarboxylic acid cycle; THF, tetrahydrofolate.
We conducted a retrospective study with the aim to assess the usefulness of early therapeutic intervention in asymptomatic infants detected by expanded NBS whose diagnosis of acquired vitamin B12 deficiency was caused by maternal vitamin B12 deficiency. Although our cohort of patients included only 12 cases, it represents a rate of vitamin B12 deficiency of 1 in 29.609 NB, which is like the one described by others [8,9]. We present here the evolution of functional biomarkers from NBS to the first hospital visit and the mothers’ pregnancy outcomes, emphasizing the importance of early diagnosis to prevent the damage of maternal vitamin B12 deficiency.
2. Materials and Methods
This study was based on a retrospective observational cohort of newborns referred to the metabolic unit at Hereditary Metabolic Disease Reference Center, Lisbon North University Hospital Center, Lisbon due to alterations in NBS, covering the period from 2017 to 2021. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Demographic data, clinical findings, diet quality and style, and pregnancy follow-up data were collected through a comprehensive review of the individual files. These data were complemented by information obtained during hospital visits. The following biochemical data were compiled: hematological indices, serum vitamin B12, plasma total homocysteine (tHcy), urine methylmalonic acid (MMA), and dried blood spot (DBS) methionine (Met) and propionylcarnitine (C3) levels from first-tier NBS, and tHcy and MMA from second-tier NBS. Functional biomarkers of vitamin B12 deficiency were reassessed in the infants at the first hospital visit with a mean age of 39.2 days (range: 13–66). The biochemical assessment of mothers was also performed at the same visit. The monitoring of functional markers and vitamin B12 serum levels after two weeks of supplementation was subsequently pursued.
The screening procedure was based on an algorithm that starts with the evaluation of first-tier tests: detection of C3 above the cutoff value (>5.25 µM) or a C3/C2 (acetylcarnitine) > 0.20. Subsequently, in second-tier tests performed on the first DBS (collected between 3rd and 6th days of life), we evaluated MMA, 3-hydroxypropanoic acid, and propionyl glycine. An MMA level above the cutoff value leads to the evaluation of tHcy.
On the basis of cutoff values usually described in the literature [6], vitamin B12 maternal status was defined as deficient and insufficient for values of vitamin B12 < 200 pg/mL, and between 200 and 300 pg/mL, respectively. Concerning the biomarkers’ normal cutoff values, the ones established by the participant laboratories (personal information) were adopted: dried blood spot (DBS), 99.5% and 1% percentile for high and low values, respectively, C3 (propionylcarnitine) < 5.25 µM; C3/C2 < 0.20; C3/Met < 0.30; MMA < 4.0; tHcy < 4.40 µM and urine MMA < 13.0 µmol/mmol creatinine in infants; plasma tHcy 4.0–6.0 µM, in infants, and ≤14.0 µM for adults [10]. Moreover, megaloblastic anemia was defined for the newborn (NB) when mean corpuscular blood volume (MCV) was >115 fL and hemoglobin < 11.0 g/dL; for adults when MCV was >98 fL and hemoglobin < 11.9 g/dL. Due to the interplay of folates in vitamin B12 deficiency, plasma folate level was evaluated in all mothers and NBs at first hospital visit.
The diagnosis of maternal vitamin B12 deficiency was established when a mother’s vitamin B12 level was below the defined cutoff value and/or functional biomarkers were elevated. In the cases where maternal vitamin B12 deficiency was not explained by dietary reasons, mothers were referred to internal medicine for further work-up and treatment. All mothers were also referred to a nutritional clinic.
The diagnosis of acquired vitamin B12 deficiency was established in all NBs on the basis of second-tier NBS test results performed on the first DBS and on the data tracked at the first hospital visit: vitamin B12 serum level below cutoff value (<280 pg/mL) concomitant with altered functional biomarker values, the confirmation of maternal vitamin B12 deficiency, and the normalization of NB values after hydroxocobalamin supplementation.
3. Results
In the period of 2017–2021, 12 NBs with altered NBS with suspicion of acquired vitamin B12 insufficiency were studied, and results are presented in Table 1. C3 values varied from high (n = 8) to borderline (n = 2) and normal (n = 2) levels; for Met values, the majority (n = 10) were in the normal range, and only two (n = 2) displayed Met values slightly below the low range. However, the ratios C3/Met (mean = 0.45; range: 0.23–1.76) and C3/C2 (mean = 0.22; range: 0.13–0.32) for both or one of the two were high. These results led to a second-tier test in the first DBS, and in all cases, MMA (mean = 16.6 µM; range: 6.0–67.8) was above the cutoff level, but for tHcy (mean = 6.9 µM; range: 1.4–16.5), only 7 of the 12 studied cases had a tHcy level above the cutoff value.

Table 1.
Infant birth outcomes and DBS biomarkers at first and second NBS tiers.
The first visit to the hospital occurred at the mean age of 39 days of life (range: 13–66), and the clinical outcome, hematological indices, and functional biomarkers were evaluated (Table 2). All infants were exclusively breastfed and asymptomatic with normal psychomotor development and good weight evolution, except two of them who did not have good weight gain. Four patients included in this study presented transitory anemia with slightly low hemoglobin (9.3 to 10.6 g/dL), and megaloblastic anemia was not detected in any of the infants.

Table 2.
Infant outcomes and evaluated biomarkers at first hospital visit.
All NBs revealed very low serum vitamin B12 (<100 pg/mL) and very high levels of tHcy (mean = 71.2 µM; range: 28.0–163.4) (Figure 2), which suggests that the Hcy remethylation pathway under a low vitamin B12 steady state is compromised in a cumulative mode along time. The MMA metabolism seemed to be grossly affected since its urinary excretion was very high on first hospital visit. Met levels were not as informative as could be expected, but the diet also contributes to the cellular Met pool. Plasma Met levels at the first hospital visit were within the respective reference values in all cases (Figure 2).
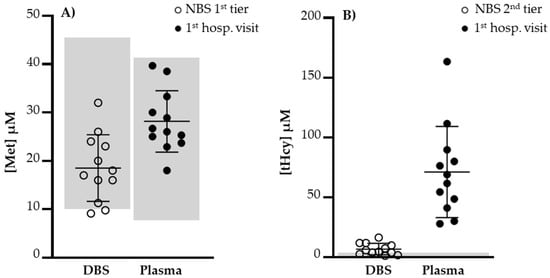
Figure 2.
(A) Levels of DBS Met at first NBS tier and plasma Met at first hospital visit. Met values did not reflect a compromise of remethylation reaction due to vitamin B12 deficiency. (B) Levels of DBS tHcy at second NBS tier and plasma tHcy at second hospital visit. A significant increase in tHcy was observed at first hospital visit, 32.9 days of age (average, range 13–65). Met, methionine; NBS, newborn screening; tHcy, total homocysteine. Normal value ranges limited by gray areas.
Therapy with intramuscular vitamin B12 led to the rapid normalization of metabolic abnormalities. At the last visit, all patients with a mean age of one year were asymptomatic with normal levels of the functional biomarkers.
Demographic data, dietary regimen, and biochemical data from the NBs’ mothers are summarized in Table 3. Maternal demographic characteristics show that the affected mothers in our study were from a variety of ethnic backgrounds: Portugal (n = 4), India (n = 4), Brazil (n = 3), and Angola (n = 1). Their dietary history and nonvitamin supplementation during pregnancy were potential causes of vitamin B12 deficiency, which was later validated with confirmatory tests. Vitamin B12 deficiency was due to different conditions: decreased intake—nine women adhered to a strict vegetarian diet before and/or during pregnancy; excessive consumption—one was a multipara woman (6th pregnancy) who had multiple vitamin deficiencies; decreased absorption—one was identified with malabsorption syndrome after bariatric surgery; and autoimmune condition—one had a pernicious anemia that had not been previously identified. All mothers showed low levels of vitamin B12 (mean = 137.5 pg/mL; range: <100–267), matching the classification of vitamin B12 deficiency or insufficiency associated with high levels of homocysteine (mean = 18.3 µM; range: 13.4–77.6), and only two displayed slightly high MCV indices, but without anemia (Table 3). The coexistence of folate deficiency was detected only in one case.

Table 3.
Maternal demographic and clinical findings, dietary regimen, and biochemical data.
Maternal vitamin B12 concentration status was not associated with any perinatal outcomes (birthweight and gestational age at birth, Table 2). Mothers were also supplemented with oral vitamin B12 and folic acid if necessary.
4. Discussion
A correctly planned vegetarian diet is nutritionally adequate and healthy [11]. However, nonsupplemented populations who circumvent food of animal origin are at high risk of acquiring a vitamin B12 deficiency or insufficiency status, which relies exclusively on intake from dietary sources [6,10,11,12,13]. Women of childbearing age and pregnant women globally have a high incidence of vitamin B12 deficiency ranging between 10% and 50% for different populations and ethnicities [11,12,13,14].
Vitamin B12 deficiency becomes a relevant health issue specially during pregnancy since vitamin B12 is a relevant regulator of placentation and fetal growth. Vitamin B12 deficiency is frequently underdiagnosed in pregnant women, not only due to a restricted diet, but also due to other causes, as our study shows (excessive consumption, malabsorption, and autoimmune disorders). The most common cause of vitamin B12 deficiency in adults is pernicious anemia [13,15]. However, in our study, only one mother was diagnosed with this condition, showing that a vegetarian diet is likely becoming more common. Several studies have shown a high adherence to vegetarian diets. The global number of vegetarians can represent up to 10% of the total population [11].
Women are frequently clinically asymptomatic in the presence of atrophic gastritis [13]. Pilot project Newborn Screening 2020 (NBS 2020) carried out in Heidelberg, Germany [13,16] performed a diagnostic work-up for the mothers of newborns with vitamin B12 deficiency that led to the diagnosis of previously unrecognized gastrointestinal malabsorption due to autoimmune gastritis, ulcerative colitis, gastric bypass, hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome, severe pancytopenia, and carbamazepine treatment. However, no gastrointestinal cause for vitamin B12 deficiency was established in most cases and 89% of mothers reported a balanced diet. A vegetarian diet was reported only in a few cases, but some of them had hyperemesis or aversion to meat during pregnancy, which may explain some cases of vitamin deficiency [12,13,16]. Two of the mothers included in our study also developed an aversion to meat or dairy products during pregnancy. It is also important to analyze the fact that 8 of the 12 mothers included in our study were immigrants, showing the possible effect, referred by Reischl-Hajiabadi A.T. et al., of a lack of information, language barriers, or greater hesitancy to attend preventive prenatal care in this group [16].
Vitamin B12 deficiency in newborns is mainly maternal in origin [13,16,17,18]. The best means of preventing neonatal deficiency is to ensure that the mother is vitamin B12-replete during pregnancy and breastfeeding. Women following vegetarian diets should have nutritional counseling, preconception, and monitoring throughout the pregnancy, and start early micronutrient supplementation, including vitamin B12 [1,2,5,11,19]. However, maternity guidelines usually do not include the routine evaluation of vitamin B12 status and vitamin B12 supplementation, so none of the affected mothers in our study had taken vitamin supplementation before or throughout pregnancy. Caregivers of pregnant women should increase awareness of vitamin B12 deficiency. The well-established recommendation and accepted global guidance to start folic acid supplementation preconceptionally may explain the fact that only one of the mothers included in our study showed folate deficiency.
An additional benefit for NBS may be the detection of undiagnosed vitamin B12-de-ficient NBs from vitamin B12-deficient mothers. The prevalence of vitamin B12 deficiency detected by NBS was described in several studies in the last few years [2,15,16,19,20,21]. The percentage of detected cases varies between countries depending on the strategies and cutoffs applied in each NBS [16].
The MMA was elevated at the time of NBS in all newborns referred to our center, but the same was not observed for tHcy. This observation indicates that MMA is more sensitive than tHcy once the enzymatic reaction involved in MMA catabolism is entirely dependent on vitamin B12 availability. tHcy, as a biomarker of vitamin B12 deficiency, may be ambiguous since its metabolism is interconnected with folate. However, after a short time (1 or 2 months), plasma tHcy was significantly high. The rapid rise in biomarkers with cellular adverse effects emphasizes the impact of an early therapeutic intervention towards a healthy outcome. This point is controversial; in some studies [12], the most sensitive marker for vitamin B12 deficiency was tHcy, but it is the consensus that the combination of MMA and tHcy is needed in all cases.
There is no agreement regarding treatment in cases of vitamin B12 deficiency [13]. To all NBs included in this study, a one-time injection of 1.0 mg of hydroxocobalamin improved the metabolic status. Pilot project NBS 2020 [12,16] compared the effectiveness of oral-only vitamin B12 supplementation versus supplementation and parental therapy (including intramuscular and intravenous), showing that vitamin B12 levels and functional biomarkers were normalized with both treatments. The group treated with parenteral supplementation showed a higher response in terms of vitamin B12 levels, which may indicate excessive treatment due to the achieved supranormal vitamin B12 levels [16]. In another study, 47 children with vitamin B12 deficiency were treated with oral vitamin B12, showing its effectiveness [22]. Oral supplementation seems to be a good treatment option to avoid invasive and painful treatments in families with compliance to the treatment. Vtamin B12 deficiency in the child or mother should not be a reason to avoid breastfeeding rather than adequately monitoring and supplementing vitamin B12 [13].
Several studies [5,19,23,24,25,26] demonstrated that cobalamin deficiency in exclusively breastfed infants is essentially manifested between 3 and 6 months of age, and in older ages in strict association with the decline in a breast-milk cobalamin source. Symptoms usually start between 4 and 6 months of age if vitamin B12 levels are not corrected [13], and differ in severity, including physical, hematological, and neurological signs. All patients of our study remained without clinical symptoms at a one-year follow-up, which was expected, since early therapeutic intervention may prevent neurological and severe hematological findings.
This supports the postulated clinical benefit achieved by NBS and the consequent early treatment of still-asymptomatic children due to maternal vitamin B12 deficiency under exclusive breast-milk feeding. The percentage of cobalamin in breast milk is a major determinant of cobalamin status in exclusively breastfed infants and is strongly correlated with maternal blood cobalamin [19]. Maternal cobalamin intake is the main determinant of a breast-milk cobalamin source [19]. Therefore, most commercially prepared infant formulas are enriched with cobalamin to overcome the problem. Moreover, maternal cobalamin deficiency was related to an increased risk of preterm birth and low birth weight [6,7,12,13,23,25]; however, in our study and pilot project NBS 2020 [16], this correlation was not observed.
5. Conclusions
In our study, early detection through NBS may have prevented hematological abnormalities and irreversible neurological damage in infants with acquired vitamin B12 deficiency due to maternal vitamin B12 deficiency. Cobalamin deficiency after birth is a global public health problem, and a serious one in countries with endemic deficiency and prevalent or prolonged breastfeeding practices. Future improvements in the feedback from metabolic centers that are responsible for the ultimate diagnosis of these conditions to NBS follow-up programs result in better knowledge of the incidence of this preventable nutritional condition [2,27].
The most effective prevention strategy for both mother and child includes screening for maternal vitamin B12 deficiency throughout the pregnancy on follow-up appointments [12,16]. Healthcare providers should ask pregnant and lactating women about their diet and medical history to identify those who are at risk of an inadequate intake or malabsorption of vitamin B12. Providers should not rely solely on the measurement of serum vitamin B12 level but should cross-reference these data with vitamin B12 functional marker measurements, plasma MMA, and tHcy to confirm the status of vitamin B12 deficiency in at-risk women [2]. Caregivers of pregnant women should be aware of vitamin B12 deficiency and prevent it during pregnancy.
Author Contributions
Conceptualization, P.L.P. and I.T.d.A.; methodology, I.T.d.A.; software, C.F.; validation, P.J., S.M., R.L.S., H.R., I.T.d.A., A.G. and L.V.; formal analysis, C.F.; resources, P.L.P. and C.F.; data curation, P.L.P., I.T.d.A., P.J. and C.F.; writing—original draft preparation, P.L.P.; writing—review and editing, P.L.P., I.T.d.A., P.J., H.R. and C.F.; visualization, C.F.; supervision, I.T.d.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Lisbon North University Hospital Center, EPE (No. 26722) (September 2022).
Informed Consent Statement
Patient consent was waived due to the fact that participating patients were not identified in this study.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.L.; Brito, A.; Guéant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B12 deficiency. Nat. Rev. 2017, 3, 17040. [Google Scholar] [CrossRef]
- Hinton, C.F.; Ojodu, J.A.; Fernhoff, P.M.; Rasmussen, S.A.; Scanlon, K.S.; Hannon, W.H. Maternal and neonatal vitamin B12 deficiency detected through expanded newborn screening-United States, 2003-2017. J. Pediatr. 2010, 157, 162–163. [Google Scholar] [CrossRef]
- Darnton-Hill, I. Public Health Aspects in the Prevention and Control of Vitamin Deficiencies. Curr. Dev. Nutr. 2019, 3, nzz075. [Google Scholar] [CrossRef] [PubMed]
- Siddiqua, T.; Allen, L.; Raqib, R.; Ahmed, T. Vitamin B12 Deficiency in Pregnancy and Lactation: Is there a Need for Pre and Post-natal Supplementation? J. Nutr. Disorders Ther. 2014, 4, 142. [Google Scholar] [CrossRef]
- Behere, R.V.; Deshmukh, A.S.; Otiv, S.; Gupte, M.D.; Yajnik, C.S. Maternal Vitamin B12 Status During Pregnancy and Its Association with Outcomes of Pregnancy and Health of the Offspring: A Systematic Review and Implications for Policy in India. Front. Endocrinol. 2021, 12, 619176. [Google Scholar] [CrossRef]
- Hannibal, L.; Lysne, V.; Bjørke-Monsen, A.-L.; Behringer, S.; Grünert, S.C.; Spiekerkoetter, U.; Jacobsen, D.W.; Blom, H.J. Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front. Mol. Biosci. 2016, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Rogne, T.; Tielemans, M.J.; Chong, M.F.-F.; Yajnik, C.S.; Krishnaveni, G.V.; Poston, L.; Jaddoe, V.W.V.; Steegers, E.A.P.; Joshi, S.; Chong, Y.-S.; et al. Associations of maternal vitamin B12 concentration in pregnancy with the risks of preterm birth and low birthweight: A systematic review and meta-analysis of individual participant data. Am. J. Epidemiol. 2017, 185, 212–223. [Google Scholar]
- Röschinger, W.; Sonnenschein, S.; Schuhmann, E.; Nennstiel-Ratzel, U.; Roscher, A.; Olgemöller, B. Neue Zielerkrankungen im Neugeborenenscreening. Mon. Kinderheilkd. 2015, 163, 142–149. [Google Scholar] [CrossRef]
- Sarafoglou, K.; Rodgers, J.; Hietala, A.; Matern, D.; Bentler, K. Expanded Newborn Screening for Detection of Vitamin B12 Deficiency. JAMA 2011, 305, 1198–1200. [Google Scholar] [CrossRef]
- Caldeira-Araújo, H.; Ramos, R.; Florindo, C.; Rivera, I.; Castro, R.; de Almeida, I.T. Homocysteine Metabolism in Children and Adolescents: Influence of Age on Plasma Biomarkers and Correspondent Genotype Interactions. Nutrients 2019, 11, 646. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Sá-Azevedo, R.; Balinha, J.; Ferro, G. Vegetarianism during pregnancy: Risks and benefits. Trends Food Sci. Technol. 2018, 79, 28–34. [Google Scholar] [CrossRef]
- Gramer, G.; Hoffman, J.; Feyh, P. Newborn Screening for Vitamin B12 Deficiency in Germany-Strategies, Results and Public Health Implications. J. Pediatr. 2020, 216, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Gramer, G.; Hoffmann, G. Vitamin B12 Deficiency in Newborns and their Mothers—Novel Approaches to Early Detection, Treatment and Prevention of a Global Health Issue. Curr. Med. Sci. 2020, 40, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P.; Allen, R.H. Vitamin b12 deficiency as a worldwide problem. Annu. Rev. Nutr. 2004, 24, 299–326. [Google Scholar] [CrossRef]
- Scolamiero, E.; Villani, G.R.D.; Ingenito, L.; Pecce, R.; Albano, L.; Caterino, M.; di Girolamo, M.G.; Di Stefano, C.; Franzese, I.; Gallo, G.; et al. Maternal vitamin B12 deficiency detected in expanded newborn screening. Clin. Biochem. 2014, 47, 312–317. [Google Scholar] [CrossRef]
- Reischl-Hajiabadi, A.T.; Garbade, S.F.; Feyh, P.; Weiss, K.H.; Mütze, U.; Kölker, S.; Hoffmann, G.F.; Gramer, G. Maternal Vitamin B12 Deficiency Detected by Newborn Screening—Evaluation of Causes and Characteristics. Nutrients 2022, 14, 3767. [Google Scholar] [CrossRef]
- Roumeliotis, N.; Dix, D.; Lipson, A. Vitamin B12 deficiency in infants secondary to maternal causes. Can. Med. Assoc. J. 2012, 184, 1593–1598. [Google Scholar] [CrossRef][Green Version]
- Weiss, R.; Fogelman, Y.; Bennett, M. Severe Vitamin B12 Deficiency in an Infant Associated with a Maternal Deficiency and a Strict Vegetarian Diet. J. Pediatr. Hematol. 2004, 26, 270–271. [Google Scholar] [CrossRef]
- Obeid, R.; Murphy, M.; Solé-Navais, P.; Yajnik, C. Cobalamin Status from Pregnancy to Early Childhood: Lessons from Global Experience. Adv. Nutr. Int. Rev. J. 2017, 8, 971–979. [Google Scholar] [CrossRef]
- Marble, M.; Copeland, S.; Khanfar, N.; Rosenblatt, D.S. Neonatal Vitamin B12 Deficiency Secondary to Maternal Subclinical Pernicious Anemia: Identification by Expanded Newborn Screening. J. Pediatr. 2008, 152, 731–733. [Google Scholar] [CrossRef]
- Pajares, S.; Arranz, J.A.; Ormazabal, A.; Del Toro, M.; García-Cazorla, Á.; Navarro-Sastre, A.; López, R.M.; Meavilla, S.M.; Santos, M.M.d.L.; García-Volpe, C.; et al. Implementation of second-tier tests in newborn screening for the detection of vitamin B12 related acquired and genetic disorders: Results on 258,637 newborns. Orphanet J. Rare Dis. 2021, 16, 195. [Google Scholar] [CrossRef]
- Bahadir, A.; Reis, P.G.; Erduran, E. Oral vitamin B12 treatment is effective for children with nutritional vitamin B12 deficiency. J. Paediatr. Child Health 2014, 50, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Golding, J.; Gregory, S.; Clark, R.; Iles-Caven, Y.; Ellis, G.; Taylor, C.M.; Hibbeln, J. Maternal prenatal vitamin B12 intake is associated with speech development and mathematical abilities in childhood. Nutr. Res. 2021, 86, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.S.; Na’im Mohamad Ayob, M.; Cai, S.; Quah, P.L.; Gluckman, P.D.; Shek, L.P.; Yap, F.; Tan, K.H.; Chong, Y.S.; Godfrey, K.M.; et al. Maternal plasma vitamin B12 concentration during pregnancy and infant cognitive outcomes at 2 years of age. Br. J. Nutr. 2019, 121, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.L.; Fothergill, A.; Krisher, J.T.; Thomas, T.; Kurpad, A.V.; Dwarkanath, P. Maternal vitamin B12 deficiency and perinatal outcomes in southern India. PLoS ONE 2021, 16, e0248145. [Google Scholar] [CrossRef]
- Dubaj, C.; Czyż, K.; Furmaga-Jabłońska, W. Vitamin B12 deficiency as a cause of severe neurological symptoms in breast fed infant—A case report. Ital. J. Pediatr. 2020, 46, 40. [Google Scholar] [CrossRef]
- Hawthorne, B.S.; Levy, H.L. Can newborn screening for vitamin B12 deficiency be incorporated into all newborn screening programs? J. Pediatr. 2020, 216, 9–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).