Postprandial Glucose and Gastrointestinal Hormone Responses of Healthy Subjects to Wheat Biscuits Enriched with L-Arginine or Branched-Chain Amino Acids of Plant Origin
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Test Foods
2.3. Study Design and Experimental Protocol
2.4. Blood Analyses
2.4.1. Biochemical Parameters and Gastrointestinal Hormones
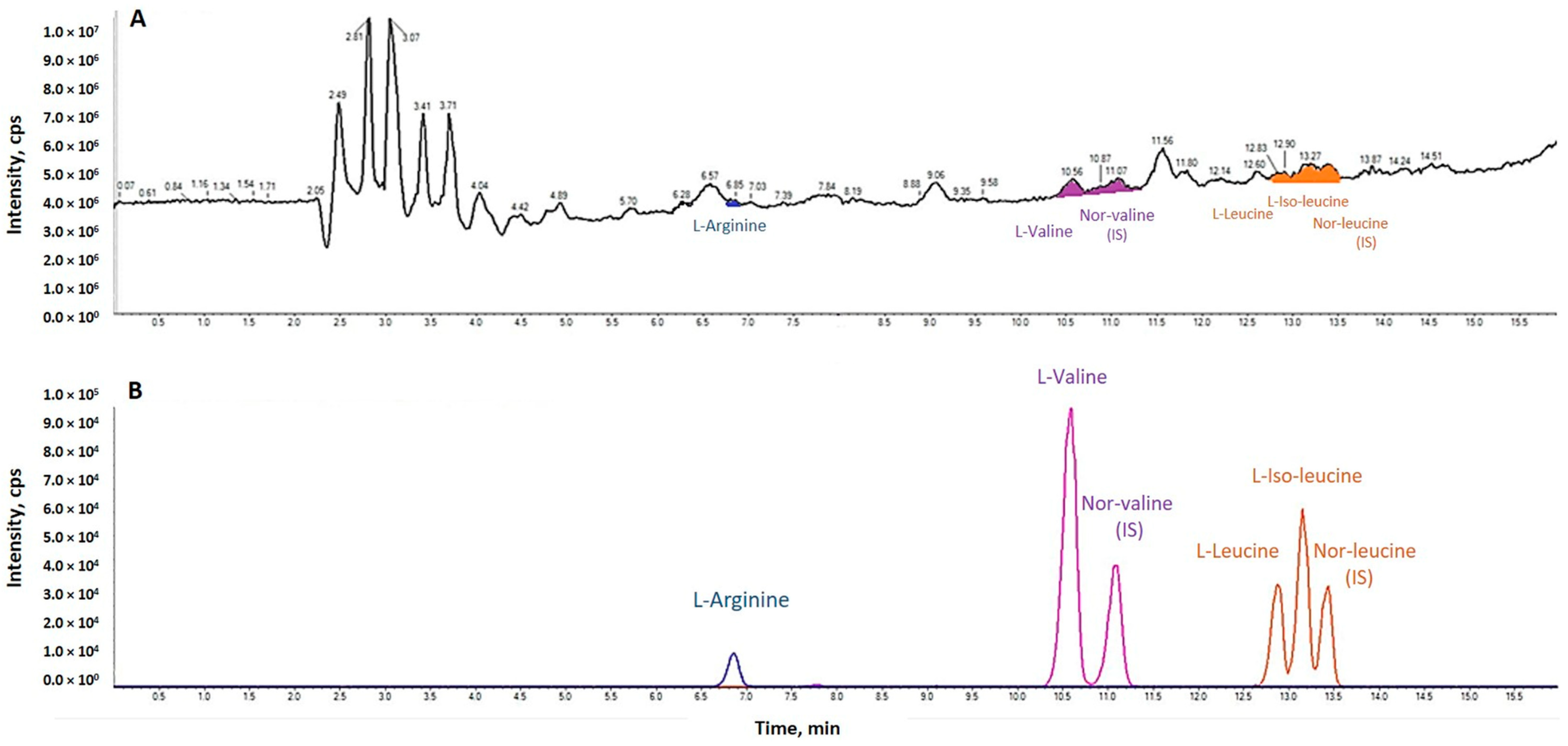
2.4.2. Plasma Amino Acids
Sample Preparation
Separation and Detection
Identification and Quantitation
Method Validation
2.5. Subjective Satiety Measures
2.6. Sensory Evaluation
2.7. Calculation and Statistical Analysis
3. Results
3.1. Subjects
3.2. Test Food Analysis
3.3. Blood Analyses
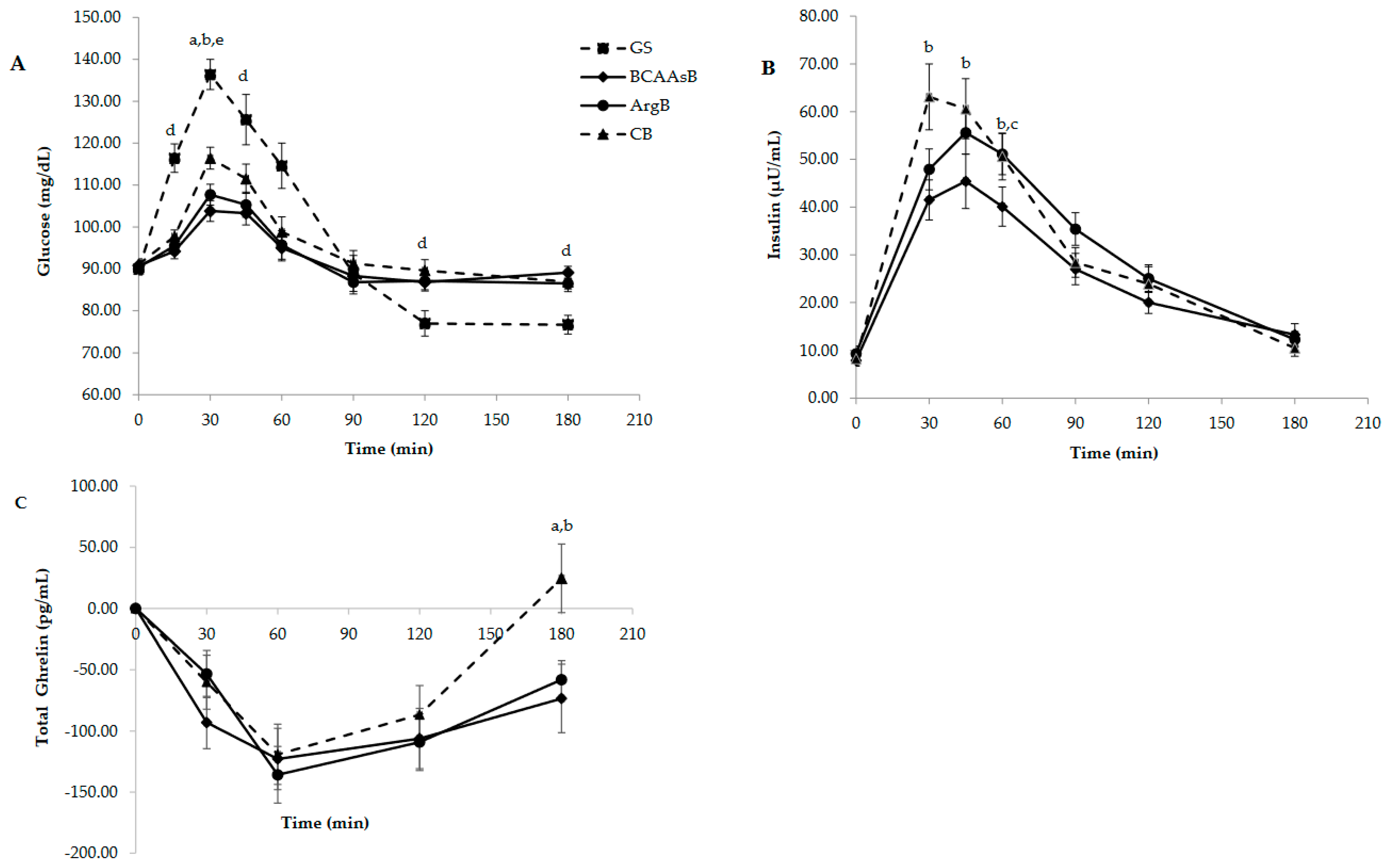
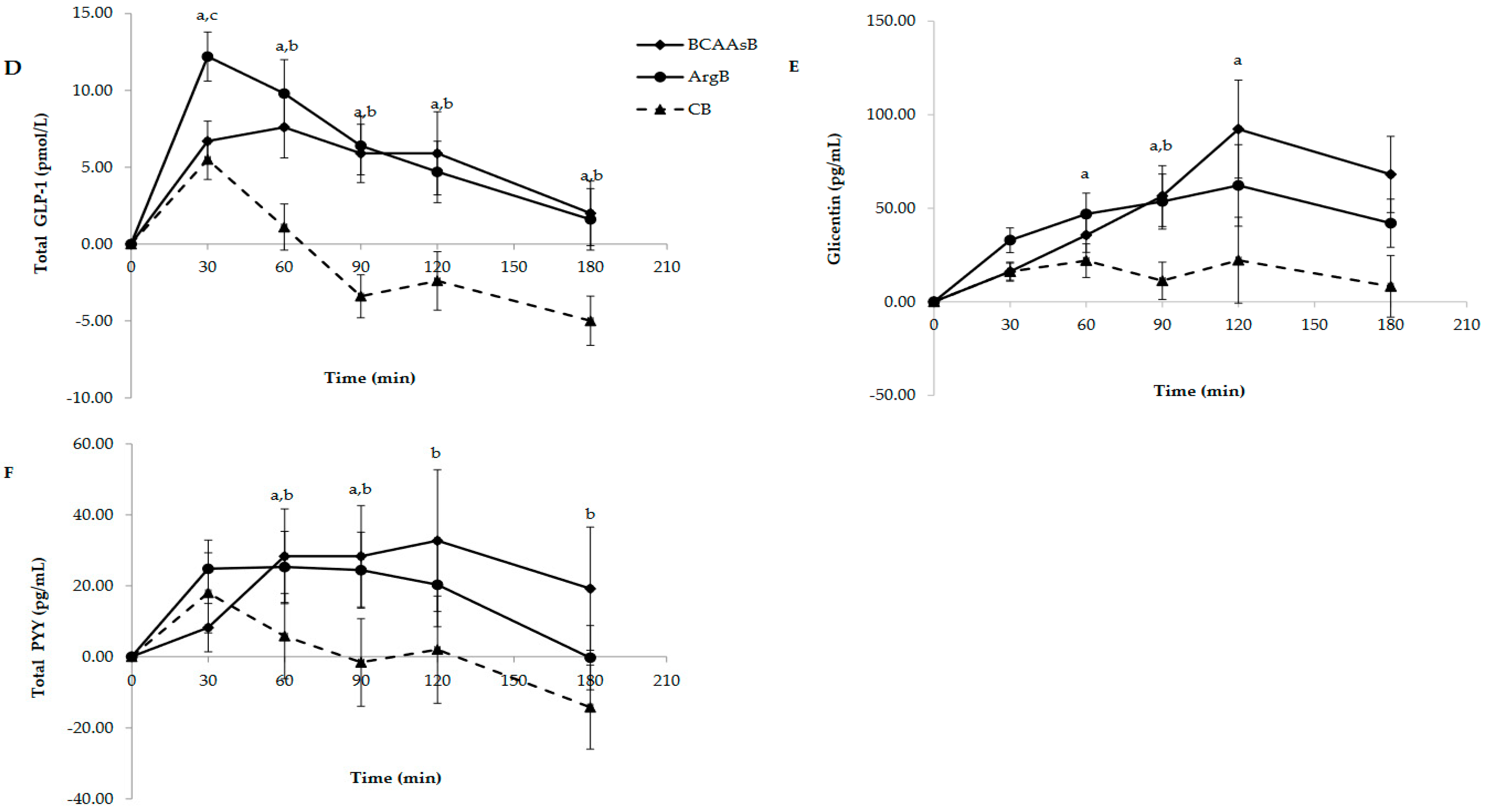
3.3.1. Glucose and Hormone Responses
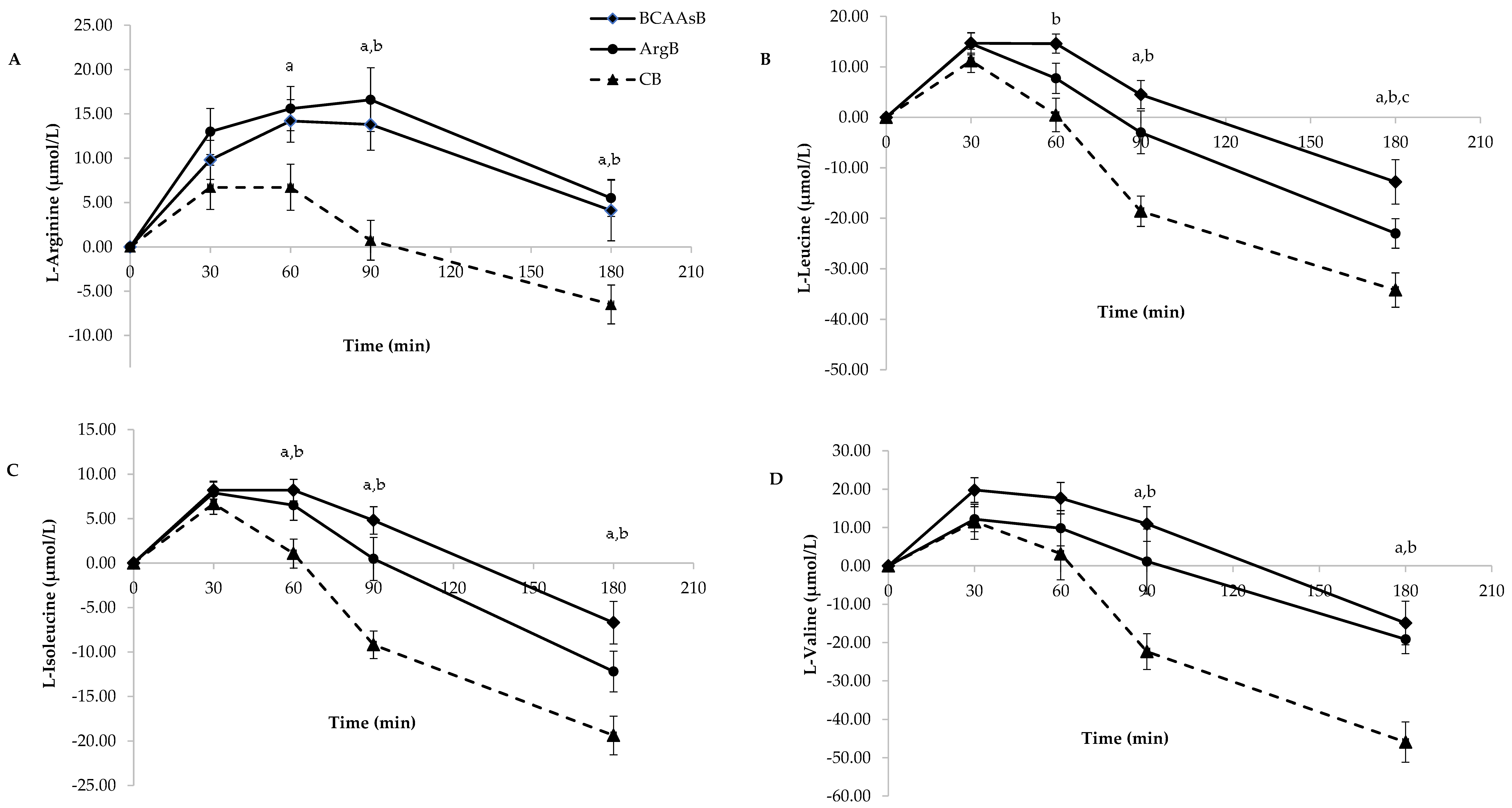
3.3.2. Amino Acid Responses
3.4. Subjective Satiety Measures
3.5. Sensory Evaluation
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bornet, F.R.J.; Jardy-Gennetier, A.E.; Jacquet, N.; Stowell, J. Glycaemic response to foods: Impact on satiety and long-term weight regulation. Appetite 2007, 49, 535–553. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.B. High-glycemic index foods, hunger and obesity: Is there a connection? Nutr. Rev. 2000, 58, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Schultes, B.; Panknin, A.K.; Hallschmid, M.; Jauch-Chara, K.; Wilms, B.; de Courbière, F.; Lehnert, H.; Schmid, S.M. Glycemic increase induced by intravenous glucose infusion fails to affect hunger, appetite, or satiety following breakfast in healthy men. Appetite 2016, 105, 562–566. [Google Scholar] [CrossRef]
- Zhu, R.; Fogelholm, M.; Larsen, T.M.; Poppitt, S.D.; Silvestre, M.P.; Vestentoft, P.S.; Jalo, E.; Navas-Carretero, S.; Huttunen-Lenz, M.; Taylor, M.A.; et al. A High-Protein, Low Glycemic Index Diet Suppresses Hunger but Not Weight Regain After Weight Loss: Results From a Large, 3-Years Randomized Trial (PREVIEW). Front. Nutr. 2021, 8, 685648. [Google Scholar] [CrossRef] [PubMed]
- Esteves de Oliveira, F.C.; Pinheiro Volp, A.C.; Alfenas, R.C. Impact of different protein sources in the glycemic and insulinemic responses. Nutr. Hospital. 2011, 26, 669–676. [Google Scholar]
- Psichas, A.; Reimann, F.; Gribble, F.M. Gut chemosensing mechanisms. J. Clin. Investig. 2016, 125, 908–917. [Google Scholar] [CrossRef]
- Lynch, H.; Johnston, C.; Wharton, C. Plant-based diets: Considerations for environmental impact, protein quality, and exercise performance. Nutrients 2018, 10, 1841. [Google Scholar] [CrossRef]
- Li, P.; Lu, B.; Gong, J.; Li, L.; Chen, G.; Zhang, J.; Chen, Y.; Tian, X.; Han, B.; Guo, Y.; et al. Chickpea extract ameliorates metabolic syndrome symptoms via restoring intestinal ecology and metabolic profile in type 2 diabetic rats. Mol. Nutr. Food Res. 2021, 65, e2100007. [Google Scholar] [CrossRef]
- Binou, P.; Yanni, A.E.; Karahtanos, V.T. Physical properties, sensory acceptance, postprandial glycemic response, and satiety of cereal-based foods enriched with legume flours: A review. Crit. Rev. Food Sci. Nutr. 2020, 11, 2722–2740. [Google Scholar] [CrossRef]
- Ropelle, E.R.; Pauli, J.R.; Fernandes, M.F.A.; Rocco, S.A.; Marin, R.M.; Morari, J.; Souza, K.K.; Dias, M.M.; Gomes-Marcondes, M.C.; Gontijo, J.A.; et al. A Central Role for Neuronal AMP-Activated Protein Kinase (AMPK) and Mammalian Target of Rapamycin (mTOR) in High-Protein Diet–Induced Weight Loss. Diabetes 2008, 57, 594–605. [Google Scholar] [CrossRef]
- Laeger, T.; Reed, S.D.; Henagan, T.M.; Fernandez, D.H.; Taghavi, M.; Addington, A.; Münzberg, H.; Martin, R.J.; Hutson, S.M.; Morrison, C.D. Leucine acts in the brain to suppress food intake but does not function as a physiological signal of low dietary protein. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R310–R320. [Google Scholar] [CrossRef] [PubMed]
- Blouet, C.; Jo, Y.H.; Li, X.; Schwartz, G.J. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J. Neurosci. 2009, 29, 8302–8311. [Google Scholar] [CrossRef]
- Cota, D.; Proulx, K.; Blake Smith, K.A.; Kozma, S.C.; Thomas, G.; Woods, S.C.; Seeley, R.J. Hypothalamic mTOR signaling regulates food intake. Science 2006, 12, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Purpera, M.N.; Shen, L.; Taghavi, M.; Münzberg, H.; Martin, R.J.; Hutson, S.M.; Morrison, C.D. Impaired branched chain amino acid metabolism alters feeding behavior and increases orexigenic neuropeptide expression in the hypothalamus. J. Endocrinol. 2012, 212, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Monti, L.D.; Casiraghi, M.C.; Setola, E.; Galluccio, E.; Pagani, M.A.; Quaglia, L.; Bosi, E.; Piatti, P. L-arginine enriched biscuits improve endothelial function and glucose metabolism: A pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism 2013, 62, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen, C.; Smajilovic, S.; Smith, E.P.; Woods, S.C.; Bräuner-Osborne, H.; Seeley, R.J.; D’Alessio, D.A.; Ryan, K.K. Oral L-arginine stimulates GLP-1 secretion to improve glucose tolerance in male mice. Endocrinology 2013, 154, 3978–3983. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Neophytou, C.; Thein, S.; Martin, N.M.; Alamshah, A.; Spreckley, E.; Bloom, S.R.; Murphy, K.G. L-arginine increases postprandial circulating GLP-1 and PYY levels in humans. Obesity 2018, 26, 1721–1726. [Google Scholar] [CrossRef]
- Alamshah, A.; McGavigan, A.K.; Spreckley, E.; Kinsey-Jones, J.S.; Amin, A.; Tough, I.R.; O’hara, H.C.; Moolla, A.; Banks, K.; France, R.; et al. L-arginine promotes gut hormone release and reduces food intake in rodents. Diab. Obes. Metab. 2016, 18, 508–518. [Google Scholar] [CrossRef]
- Imeneo, V.; Romeo, R.; Gattuso, A.; De Bruno, A.; Piscopo, A. Functionalized Biscuits with Bioactive Ingredients Obtained by Citrus Lemon Pomace. Foods 2021, 10, 2460. [Google Scholar] [CrossRef]
- De Abreu, J.P.; Quintino, I.; Pascoal, G.; Postingher, B.; Cadena, R.; Teodoro, A. Antioxidant capacity, phenolic compound content and sensory properties of cookies produced from organic grape peel (Vitis labrusca) flour. Int. J. Food Sci. Technol. 2019, 54, 1215–1224. [Google Scholar] [CrossRef]
- Alongi, M.; Melchior, S.; Anese, M. Reducing the glycemic index of short dough biscuits by using apple pomace as a functional ingredient. LWT 2019, 100, 300–305. [Google Scholar] [CrossRef]
- ISO 1871:2009; Food and Feed Products—General Guidelines for the Determination of Nitrogen by the Kjeldahl Method. ISO: Geneva, Switzerland, 2009.
- AOAC. AOAC Official Method 991.43 Total, Soluble and Insoluble Dietary Fiber in Foods. Cereal Foods 1995, 32, 7. [Google Scholar]
- European Union. Commission Regulation (EC) No 152/2009 of 27 January 2009: Laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 2009, 54, 23–37. [Google Scholar]
- Stamataki, N.S.; Nikolidaki, E.K.; Yanni, A.E.; Stoupaki, M.; Konstantopoulos, P.; Tsigkas, A.P.; Perrea, D.; Tentolouris, N.; Karathanos, V.T. Evaluation of high nutritional quality snack based on oat flakes and inulin: Effects on postprandial glucose, insulin and ghrelin responses of healthy subjects. Food Funct. 2016, 7, 3295–3303. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Bioanalytical Method Validation EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2** Committee for Medicinal Products for Human Use (CHMP); European Medicines Agency: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M.S. Glycaemic index methodology. Nutr. Res. Rev. 2005, 8, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Ricardo-Silgado, M.L.; McRae, A.; Acosta, A. Role of Enteroendocrine Hormones in Appetite and Glycemia. Obes. Med. 2021, 23, 100332. [Google Scholar] [CrossRef]
- Blühe, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Bensaïd, A.; Tomé, D.; Gietzen, D.; Even, P.; Morens, C.; Gausseres, N.; Fromentin, G. Protein is more potent than carbohydrate for reducing appetite in rats. Physiol. Behav. 2002, 75, 577–582. [Google Scholar] [CrossRef]
- Halton, T.L.; Hu, F.B. The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef]
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015, 101, 1320S–1329S. [Google Scholar] [CrossRef]
- Pesta, D.H.; Samuel, V.T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. 2014, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; Kendall, C.W.C.; Esfahani, A.; Wong, J.M.W.; Carleton, A.J.; Jiang, H.Y.; Bazinet, R.P.; Vidgen, E.; Jenkins, D.J.A. Effect of non-oil-seed pulses on glycaemic control: A systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 2009, 52, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Simpson, H.; Lousley, S.; Geekie, M.; Simpson, R.; Carter, R.; Hockaday, T.; Mann, J. A high carbohydrate leguminous fibre diet improves all aspects of diabetic control. Lancet 1981, 317, 1–5. [Google Scholar] [CrossRef]
- Hall, R.S.; Thomas, S.J.; Johnson, S.K. Australian sweet lupin flour addition reduces the glycaemic index of a white bread breakfast without affecting palatability in healthy human volunteers. Asia Pac. J. Clin. Nutr. 2005, 14, 91–97. [Google Scholar]
- Gbenga-Fabusiwa, F.J.; Oladele, E.P.; Oboh, G.; Adefegha, S.A.; Fabusiwa, O.F.; Osho, P.O.; Enikuomehin, A.; Oshodi, A.A. Glycemic Response in Diabetic Subjects to Biscuits Produced from Blends of Pigeon Pea and Wheat Flour. Mater. Veg. 2019, 74, 553–559. [Google Scholar] [CrossRef]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating Ghrelin Levels Are Decreased in Human Obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef]
- Cummings, D.E. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006, 89, 71–84. [Google Scholar] [CrossRef]
- Raffort, J.; Lareyre, F.; Massalou, D.; Fénichel, P.; Panaïa-Ferrari, P.; Chinetti, G. Insights on glicentin, a promising peptide of the proglucagon family. Biochem. Med. 2017, 27, 308–324. [Google Scholar] [CrossRef]
- Ohneda, A.; Kobayashi, T.; Nihei, J.; Takahashi, H. Effect of intraluminal administration of amino acids upon plasma glicentin. Diabetes Res. Clin. Pract. 1988, 14, 265–270. [Google Scholar] [CrossRef]
- Perakakis, N.; Mantzoros, C.S. The role of glicentin and oxyntomodulin in human metabolism: New evidence and new directions. J. Clin. Endocrinol. Metab. 2020, 105, 329. [Google Scholar] [CrossRef]
- Perakakis, N.; Kokkinos, A.; Peradze, N.; Tentolouris, N.; Ghaly, W.; Pilitsi, E.; Upadhyay, J.; Alexandrou, A.; Mantzoros, C.S. Circulating levels of gastrointestinal hormones in response to the most common types of bariatric surgery and predictive value for weight loss over one year: Evidence from two independent trials. Metabolism 2019, 101, 153997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Oiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.; Adams, S.H. Branched-chain amino acids in metabolic signaling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.-P.; Brandon, A.E.; et al. Branched-chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef]
- Elovaris, R.A.; Bitarafan, V.; Agah, S.; Ullrich, S.S.; Lange, K.; Horowitz, M.; Feinle-Bisset, C. Comparative Effects of the Branched-Chain Amino Acids, Leucine, Isoleucine and Valine, on Gastric Emptying, Plasma Glucose, C-Peptide and Glucagon in Healthy Men. Nutrients 2021, 13, 1613. [Google Scholar] [CrossRef]
- Uchida, M.; Kobayashi, O.; Saito, C. Correlation Between Gastric Emptying and Gastric Adaptive Relaxation Influenced by Amino Acids. J. Neurogastroenterol. Motil. 2017, 23, 400–408. [Google Scholar] [CrossRef][Green Version]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72. [Google Scholar] [CrossRef]
- Hjørne, A.N.; Modvig, I.M.; Holst, J.J. The Sensory Mechanisms of Nutrient-Induced GLP-1 Secretion. Metabolites 2022, 12, 420. [Google Scholar] [CrossRef]
- Binou, P.; Yanni, A.E.; Kartsioti, K.; Barmpagianni, K.; Konstantopoulos, P.; Karathanos, V.T.; Kokkinos, A. Wheat biscuits enriched with plant-based protein contribute to weight loss and beneficial metabolic effects in subjects with overweight/obesity. Nutrients 2022, 14, 2516. [Google Scholar] [CrossRef]

| Characteristic | Subjects | Range |
|---|---|---|
| n | 30 | - |
| Sex (male/female) | 15/15 | - |
| Age (years) | 26.5 ± 5.3 | 18–39 |
| Weight (kg) | 77.8 ± 16.3 | 44.0–102.6 |
| BMI (kg/m2) | 25.7 ± 4.6 | 19.1–34.5 |
| WHR | 0.8 ± 0.2 | 0.68–1.03 |
| Body fat (%) | 25.5 ± 10.0 | 11.3–44.1 |
| SBP (mmHg) | 109.6 ± 22.8 | 100.3–141.0 |
| DBP (mmHg) | 67.8 ± 15.3 | 57.3–93.3 |
| Fasting plasma glucose (mg/dL) | 90.8 ± 7.2 | 72.6–100.0 |
| Fasting serum insulin (μU/mL) | 7.9 ± 5.8 | 2.1–22.0 |
| Biscuit | Energy Content (kJ) | Available Carbohydrates (g) | Fat (g) | Protein (g) | Total Dietary Fibers (g) | Soluble Dietary Fibers (g) | Insoluble Dietary Fibers (g) | L-Arg (g) | L-Leu (g) | L-Ile (g) | L-Val (g) | BCAAs (g) | IAA (g) | TAA (g) | AAA (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| per 100 g | |||||||||||||||
| CB | 1873.6 | 71.5 | 14.0 | 7.3 | 2.0 | 0.5 | 1.5 | 0.241 | 0.464 | 0.232 | 0.288 | 0.984 | 1.954 | 6.622 | 0.594 |
| BCAAsB | 1874.9 | 60.0 | 15.7 | 14.0 | 4.5 | 1.7 | 2.8 | 0.747 | 1.130 | 0.547 | 0.637 | 2.314 | 4.621 | 12.920 | 1.252 |
| ArgB | 1893.3 | 56.8 | 17.2 | 14.5 | 5.6 | 1.6 | 4.0 | 1.050 | 0.938 | 0.494 | 0.615 | 2.047 | 4.236 | 12.986 | 1.254 |
| per 50 g of available carbohydrates | |||||||||||||||
| CB | 1310.5 | 50.0 | 9.8 | 5.1 | 1.4 | 0.4 | 1.0 | 0.169 | 0.325 | 0.162 | 0.201 | 0.688 | 1.366 | 4.631 | 0.415 |
| BCAAsB | 1564.0 | 50.0 | 13.1 | 11.7 | 3.8 | 1.4 | 2.4 | 0.623 | 0.942 | 0.456 | 0.531 | 1.928 | 3.851 | 10.767 | 1.044 |
| ArgB | 1665.5 | 50.0 | 15.1 | 12.8 | 4.9 | 1.4 | 3.5 | 0.924 | 0.826 | 0.435 | 0.541 | 1.802 | 3.729 | 11.431 | 1.104 |
| iAUC0-180 min | GS | CB | BCAAsB | ArgB |
|---|---|---|---|---|
| Glucose (mg*min*dL−1) | 2508.3 ± 276.6 | 1307.7 ± 131.7 d | 739.5 ± 126.8 b,d | 861.7 ± 127.9 a,d |
| Insulin (μIU*min*mL−1) | - | 4333.5 ± 336.5 | 3329.8 ± 322.6 b,c | 4153.3 ± 341.3 |
| Ghrelin (pg*min*mL−1) | - | −15,135.9 ± 2031.0 | −18,409.8 ± 3320.5 | −17,509.8 ± 2863.7 |
| GLP-1 (pmol*min*L−1) | - | 510.3 ± 112.5 | 1193.8 ± 245.7 | 1358.8 ± 205.3 a |
| PYY (pg*min*mL−1) | - | 2904.0 ± 655.6 | 5076.0 ± 1104.7 | 4141.5 ± 755.1 |
| Glicentin (pg*min*mL−1) | - | 4729.0 ± 1499.8 | 10,387.5 ± 2247.2 | 8328.8 ± 1943.3 a |
| Hunger (cm*min) | −125.6 ± 28.3 | −355.8 ± 44.0 d | −499.8 ± 65.4 d | −552.0 ± 60.4 a,d |
| Fullness (cm*min) | 149.9 ± 32.3 | 390.5 ± 60.6 d | 517.4 ± 65.9 b,d | 647.3 ± 65.1 a,d |
| Desire to eat (cm*min) | −119.2 ± 26.3 | −321.4 ± 53.8 d | −506.3 ± 62.1 b,d | −549.5 ± 48.8 a,d |
| iAUC0-180 min | CB | BCAAsB | ArgB |
|---|---|---|---|
| L-arginine (μmol*min*L−1) | 829.7 ± 221.1 | 2036.5 ± 247.0 b | 2388.2 ± 234.3 a |
| L- leucine (μmol*min*L−1) | 634.9 ± 226.3 | 1435.3 ± 191.3 b | 995.1 ± 157.4 |
| L- isoleucine (μmol*min*L−1) | 352.6 ± 84.4 | 891.8 ± 107.4 b | 681.0 ± 100.7 a |
| L-valine (μmol*min*L−1) | 1088.2 ± 423.8 | 2164.6 ± 340.3 | 1608.8 ± 237.3 |
| Organoleptic Characteristic | BCAAsB | ArgB | CB | |
|---|---|---|---|---|
| Appearance | Surface homogeneity | 3.79 ± 2.20 | 3.93 ± 1.96 | 3.89 ± 2.47 |
| Appearance defects | 4.07 ± 2.31 | 3.79 ± 2.06 | 3.21 ± 2.10 | |
| Color intensity | 6.32 ± 1.70 a | 5.50 ± 1.45 a | 3.50 ± 1.67 | |
| Texture | Fracturability | 5.00 ± 2.50 | 5.36 ± 2.58 | 5.04 ± 2.70 |
| Crunchiness | 3.67 ± 2.25 | 3.89 ± 2.15 | 3.37 ± 2.02 | |
| Hardness | 3.22 ± 1.76 a | 2.93 ± 1.27 a | 5.15 ± 2.03 | |
| Gumminess | 5.22 ± 2.21 a | 4.33 ± 1.98 a | 3.67 ± 1.88 | |
| Odor | 3.81 ± 2.42 | 3.59 ± 2.39 | 2.89 ± 1.89 | |
| Taste | 3.19 ± 1.50 a | 4.08 ± 1.61 a | 5.89 ± 1.55 | |
| Aftertaste | 4.04 ± 2.06 | 4.54 ± 2.16 | 4.35 ± 2.06 | |
| Overall acceptance | 5.06 ± 2.00 a | 5.40 ± 1.77 a | 3.80 ± 2.39 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanni, A.E.; Kokkinos, A.; Binou, P.; Papaioannou, V.; Halabalaki, M.; Konstantopoulos, P.; Simati, S.; Karathanos, V.T. Postprandial Glucose and Gastrointestinal Hormone Responses of Healthy Subjects to Wheat Biscuits Enriched with L-Arginine or Branched-Chain Amino Acids of Plant Origin. Nutrients 2022, 14, 4381. https://doi.org/10.3390/nu14204381
Yanni AE, Kokkinos A, Binou P, Papaioannou V, Halabalaki M, Konstantopoulos P, Simati S, Karathanos VT. Postprandial Glucose and Gastrointestinal Hormone Responses of Healthy Subjects to Wheat Biscuits Enriched with L-Arginine or Branched-Chain Amino Acids of Plant Origin. Nutrients. 2022; 14(20):4381. https://doi.org/10.3390/nu14204381
Chicago/Turabian StyleYanni, Amalia E., Alexander Kokkinos, Panagiota Binou, Varvara Papaioannou, Maria Halabalaki, Panagiotis Konstantopoulos, Stamatia Simati, and Vaios T. Karathanos. 2022. "Postprandial Glucose and Gastrointestinal Hormone Responses of Healthy Subjects to Wheat Biscuits Enriched with L-Arginine or Branched-Chain Amino Acids of Plant Origin" Nutrients 14, no. 20: 4381. https://doi.org/10.3390/nu14204381
APA StyleYanni, A. E., Kokkinos, A., Binou, P., Papaioannou, V., Halabalaki, M., Konstantopoulos, P., Simati, S., & Karathanos, V. T. (2022). Postprandial Glucose and Gastrointestinal Hormone Responses of Healthy Subjects to Wheat Biscuits Enriched with L-Arginine or Branched-Chain Amino Acids of Plant Origin. Nutrients, 14(20), 4381. https://doi.org/10.3390/nu14204381







