Recent Studies on Protective Effects of Walnuts against Neuroinflammation
Abstract
1. Introduction
2. Studies of the Inhibitory Effects of Walnuts on Neuroinflammatory Cascades Using In Vivo and In Vitro Models
3. Anti-Inflammatory Components of Walnuts
3.1. Polyunsaturated Fatty Acids
3.2. Phenolic Compounds
3.3. Walnut Protein-Derived Peptides
4. Possible Mechanisms
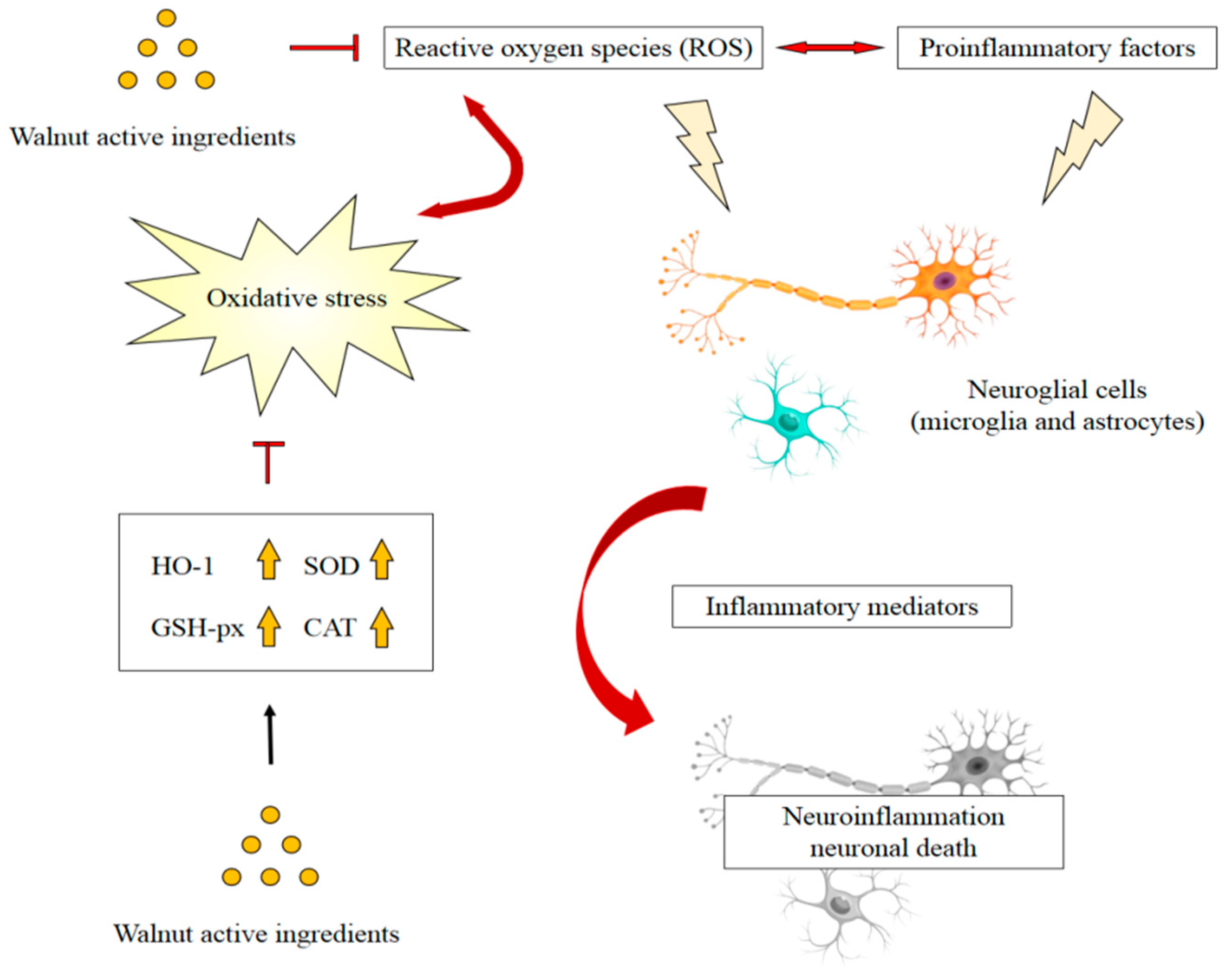
4.1. Antioxidant and Anti-Inflammatory Activity
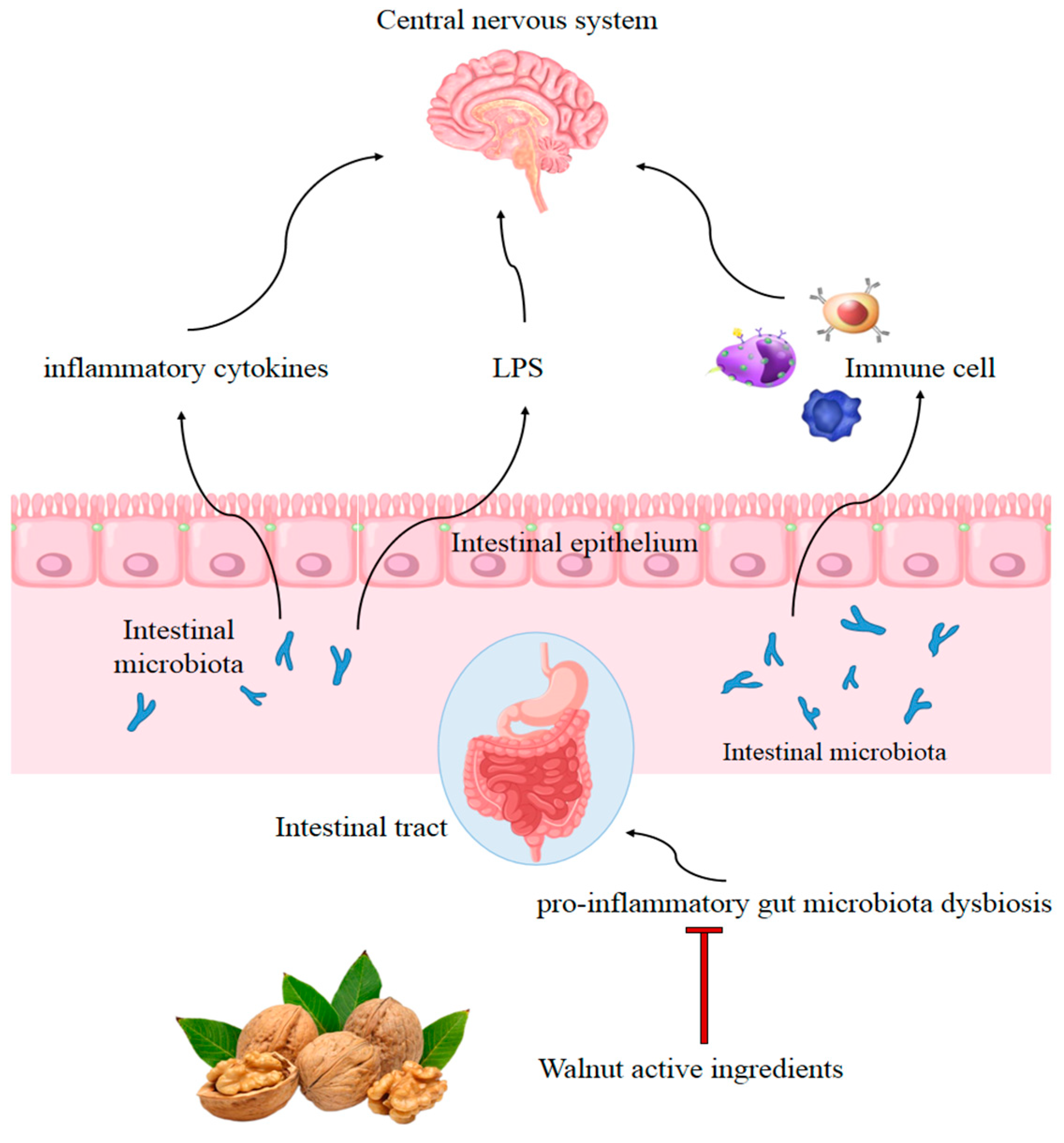
4.2. Gut Modulation Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mcquade, A.; Blurton-Jones, M. Microglia in Alzheimer’s Disease: Exploring How Genetics and Phenotype Influence Risk. J. Mol. Biol. 2019, 431, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Bayer, T.A.; Wirths, O. Intracellular Accumulation of Amyloid-Beta—A Predictor for Synaptic Dysfunction and Neuron Loss in Alzheimer’s Disease. Front. Aging Neurosci. 2010, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Sha, L.; Jia, Z.; Bu, S.; Jian, Z. Andrographolide attenuates microglia-mediated Aβ neurotoxicity partially through inhibiting NF-κB and JNK-MAPK signaling pathway. Immunopharmacol. Immunotoxicol. 2017, 39, 276–284. [Google Scholar]
- Ali, T.; Yoon, G.H.; Shah, S.A.; Lee, H.Y.; Kim, M.O. Osmotin attenuates amyloid beta-induced memory impairment, tau phosphorylation and neurodegeneration in the mouse hippocampus. Sci. Rep. 2015, 5, 11708. [Google Scholar] [CrossRef]
- Wong-Riley, M. Cytochrome oxidase: An endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989, 12, 94–101. [Google Scholar] [CrossRef]
- Meda, L.; Cassatella, M.A.; Szendrei, G.I.; Otvos, L.; Baron, P.; Villalba, M.; Ferrari, D.; Rossi, F. Activation of microglial cells by β-amyloid protein and interferon-γ. Nature 1995, 374, 647–650. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of proinflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar]
- Howcroft, T.K.; Campisi, J.; Louis, G.B.; Smith, M.T.; Sierra, F. The role of inflammation in age-related disease. Aging 2013, 5, 84–93. [Google Scholar] [CrossRef]
- Griciuc, A.; Tanzi, R.E. The role of innate immune genes in Alzheimer’s disease. Curr. Opin. Neurol. 2021, 34, 228–236. [Google Scholar] [CrossRef]
- Ros, E.; Izquierdo-Pulido, M.; Sala-Vila, A. Beneficial effects of walnut consumption on human health: Role of micronutrients. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 498–504. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Fleming, J.; Kris-Etherton, P.; Ros, E. Impact of α-Linolenic Acid, the Vegetable ω-3 Fatty Acid, on Cardiovascular Disease and Cognition. Adv. Nutr. 2022, 13, 1584–1602. [Google Scholar] [CrossRef]
- Muthaiyah, B.; Essa, M.M.; Lee, M.; Chauhan, V.; Chauhan, A. Dietary Supplementation of Walnuts Improves Memory Deficits and Learning Skills in Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 42, 1397–1405. [Google Scholar] [CrossRef]
- Willis, L.M.; Shukitt-Hale, B.; Cheng, V.; Joseph, J.A. Dose-dependent effects of walnuts on motor and cognitive function in aged rats. Br. J. Nutr. 2009, 101, 1140–1144. [Google Scholar] [CrossRef]
- Martinez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvado, J.; San Julian, B.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M.A. Mediterranean diet improves cognition: The predimed-navarra randomised trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; Ros, E. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. Jama Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef]
- O’Brien, J.; Okereke, O.; Devore, E.; Rosner, B.; Grodstein, F. Long-term intake of nuts in relation to cognitive function in older women. J. Nutr. Health Aging 2014, 18, 496–502. [Google Scholar] [CrossRef]
- Cahoon, D.; Shertukde, S.P.; Avendano, E.E.; Tanprasertsuk, J.; Scott, T.M.; Johnson, E.J.; Chung, M.; Nirmala, N. Walnut intake, cognitive outcomes and risk factors: A systematic review and meta-analysis. Ann. Med. 2021, 53, 972–998. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Valls-Pedret, C.; Rajaram, S.; Coll-Padrós, N.; Cofán, M.; Serra-Mir, M.; Pérez-Heras, A.M.; Roth, I.; Freitas-Simoes, T.M.; Doménech, M.; et al. Effect of a 2-year diet intervention with walnuts on cognitive decline. the walnuts and healthy aging (waha) study: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 111, 590–600. [Google Scholar] [CrossRef]
- Cofán, M.; Rajaram, S.; Sala-Vila, A.; Valls-Pedret, C.; Serra-Mir, M.; Roth, I.; Freitas-Simoes, T.M.; Bitok, E.; Sabaté, J.; Ros, E. Effects of 2-year walnut-supplemented diet on inflammatory biomarkers. J. Am. Coll. Cardiol. 2020, 76, 2282–2284. [Google Scholar] [CrossRef]
- Poulose, S.M.; Bielinski, D.F.; Shukitt-Hale, B. Walnut diet reduces accumulation of polyubiquitinated proteins and inflammation in the brain of aged rats. J. Nutr. Biochem. 2013, 24, 912–919. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Zhao, T.; Zhang, Q.; Liu, Y.; Sun, B.; Su, G.; Zhao, M. Inhibitory Effects of Walnut (Juglans regia) Peptides on Neuroinflammation and Oxidative Stress in Lipopolysaccharide-Induced Cognitive Impairment Mice. J. Agric. Food Chem. 2020, 68, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhen, Y.F.; Song, L.G.; Kong, W.N.; Shao, T.M.; Li, X.; Chai, X.Q. Salidroside attenuates beta amyloid-induced cognitive deficits via modulating oxidative stress and inflammatory mediators in rat hippocampus. Behav. Brain Res. 2013, 244, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative stress and b-amyloid protein in Alzheimer’s disease. Neuro. Mol. Med. 2011, 13, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Cai, P.S.; Xiong, C.M.; Ruan, J.L. Neuroprotective effect of peptides extracted from walnut (Juglans sigilata Dode) proteins on Aβ25-35-induced memory impairment in mice. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Lee, U.; Kang, J.Y.; Park, S.K.; Shin, E.J.; Kim, H.-J.; Kim, C.-W.; Kim, M.-J.; Heo, H.J. Anti-Amnesic Effect of Walnut via the Regulation of BBB Function and Neuro-Inflammation in Aβ 1-42-Induced Mice. Antioxidants 2020, 9, 976–1000. [Google Scholar]
- Li, G.; Yu, J.; Zhang, L.; Wang, Y.; Wang, C.; Chen, Q. Onjisaponin B prevents cognitive impairment in a rat model of D -galactose-induced aging. Biomed. Pharmacother. 2018, 99, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, X.; Peng, F.; Liao, J.; Nai, Y.; Lei, H.; Li, M.; Xu, H. Walnut Protein Hydrolysates Play a Protective Role on Neurotoxicity Induced by D -Galactose and Aluminum Chloride in Mice. Molecules 2018, 23, 2308. [Google Scholar] [CrossRef] [PubMed]
- Eggen, B.; Raj, D.; Hanisch, U.K.; Boddeke, H. Microglial Phenotype and Adaptation. J. Neuroimmune Pharmacol. 2013, 8, 807–823. [Google Scholar] [CrossRef]
- André, C.; Guzman-Quevedo, O.; Rey, C.; Rémus-Borel, J.; Clark, S.; Castellanos-Jankiewicz, A.; Ladeveze, E.; Leste-Lasserre, T.; Nadjar, A.; Abrous, D.N. Inhibiting microglia expansion prevents diet-induced hypothalamic and peripheral inflammation. Diabetes 2017, 66, 908–919. [Google Scholar] [CrossRef]
- Kim, E.-A.; Hwang, K.; Kim, J.-E.; Ahn, J.-Y.; Choi, S.Y.; Yang, S.-J.; Cho, S.-W. Anti-inflammatory effects of N-cyclooctyl-5-methylthiazol-2-amine hydrobromide on lipopolysaccharide-induced inflammatory response through attenuation of NLRP3 activation in microglial cells. BMB Rep. 2021, 54, 557–562. [Google Scholar] [CrossRef]
- Thangthaeng, N.; Poulose, S.M.; Fisher, D.R.; Shukitt-Hale, B. Walnut extract modulates activation of microglia through alteration in intracellular calcium concentration. Nutr. Res. 2018, 49, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.M.; Bielinski, D.F.; Fisher, D.R.; Matthan, N.R.; Joseph, J.A. Walnut extract inhibits LPS-induced activation of BV-2 microglia via internalization of TLR4: Possible involvement of phospholipase D2. Inflammation 2010, 33, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.R.; Poulose, S.M.; Bielinski, D.F.; Shukitt-Hale, B. Serum metabolites from walnut-fed aged rats attenuate stress-induced neurotoxicity in BV-2 microglial cells. Nutr. Neurosci. 2016, 20, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Shen, B.; Gao, Q.; Zhu, J.; Dong, J.; Zhang, L.; Zhang, Y. Caspase-1 inhibition attenuates activation of BV2 microglia induced by LPS-treated RAW264.7 macrophages. J. Biomed. Res. 2016, 30, 225–233. [Google Scholar]
- Wolfe, H.; Minogue, A.M.; Rooney, S.; Lynch, M.A. Infiltrating macrophages contribute to age-related neuroinflammation in C57/BL6 mice. Mech. Ageing Dev. 2018, 173, 84–91. [Google Scholar] [CrossRef]
- Wang, Q.; Zhi, T.; Han, P.; Li, S.; Xia, J.; Chen, Z.; Wang, C.; Wu, Y.; Jia, Y.; Ma, A. Potential anti-inflammatory activity of walnut protein derived peptide leucine-proline-phenylalanine in lipopolysaccharides-irritated RAW264.7 cells. Food Agric. Immunol. 2021, 32, 663–678. [Google Scholar] [CrossRef]
- Sharma, Y.; Srivastava, N.; Bala, K. Neuroprotective ability of TMV coat protein on rat PC-12 cells and it’s in silico study with LRRK2 receptor. Neurol. Res. 2018, 40, 1028–1039. [Google Scholar] [CrossRef]
- Yua, Z.; Jina, W.; Dong, X.; Ao, M.; Liu, H.; Yu, L. Safety evaluation and protective effects of ethanolic extract from maca (Lepidium meyenii Walp.) against corticosterone and H2O2 induced neurotoxicity—ScienceDirect. Regul. Toxicol. Pharmacol. 2020, 111, 104570. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Zhao, F.; Qin, H.; Lu, H.; Fang, L.; Wang, J.; Min, W. Potential mechanisms mediating the protective effects of a peptide from walnut (Juglans mandshurica Maxim.) against hydrogen peroxide induced neurotoxicity in PC12 cells. Food Funct. 2019, 10, 3491–3501. [Google Scholar] [CrossRef]
- Pei, Q.; Liu, Y.; Peng, S. Fatty Acid Profiling in Kernels Coupled with Chemometric Analyses as a Feasible Strategy for the Discrimination of Different Walnuts. Foods 2022, 11, 500. [Google Scholar] [CrossRef]
- Tu, T.H.; Kim, H.; Yang, S.; Kim, J.K.; Kim, J.G. Linoleic acid rescues microglia inflammation triggered by saturated fatty acid. Biochem. Biophys. Res. Commun. 2019, 513, 201–206. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, S.H.; Han, J.H.; Hong, Y.K.; Hwang, S.; Lee, S.; Kim, D.; Han, S.Y.; Kim, E.S.; Cho, K.S. The effects of hempseed meal intake and linoleic acid on Drosophila models of neurodegenerative diseases and hypercholesterolemia. Mol. Cells 2011, 31, 337–342. [Google Scholar] [CrossRef]
- Lee, S.; Youn, K.; Jun, M. Major compounds of red ginseng oil attenuate Aβ25–35-induced neuronal apoptosis and inflammation by modulating MAPK/NF-κB pathway. Food Funct. 2018, 9, 4122–4134. [Google Scholar] [CrossRef]
- Saiki, P.; Kawano, Y.; Griensven, L.J.L.D.V.; Miyazaki, K. The anti-inflammatory effect of Agaricus brasiliensis is partly due to its linoleic acid content. Food Funct. 2017, 8, 4150–4158. [Google Scholar] [CrossRef]
- Lee, K.-J.; Ko, Y.-J.; Kang, S.-K.; Kim, W.-S.; Cho, C.-S.; Choi, Y.-J. Additive anti-inflammation by a combination of conjugated linoleic acid and α-lipoic acid through molecular interaction between both compounds. Food Sci. Biotechnol. 2020, 29, 419–429. [Google Scholar] [CrossRef]
- Escartin, C.; Bonvento, G. Targeted activation of astrocytes: A potential neuroprotective strategy. Mol. Neurobiol. 2008, 38, 231–241. [Google Scholar] [CrossRef]
- Saba, F.; Sirigu, A.; Pillai, R.; Caria, P.; Cordeddu, L.; Carta, G.; Murru, E.; Sogos, V.; Banni, S. Downregulation of inflammatory markers by conjugated linoleic acid isomers in human cultured astrocytes. Nutr. Neurosci. 2017, 22, 207–214. [Google Scholar] [CrossRef]
- Murru, E.; Carta, G.; Manca, C.; Sogos, V.; Banni, S. Conjugated Linoleic Acid and Brain Metabolism: A Possible Anti-Neuroinflammatory Role Mediated by PPARα Activation. Front. Pharmacol. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Stachowska, E.; Dolegowska, B.; Dziedziejko, V.; Rybicka, M.; Kaczmarczyk, M.; Bober, J.; Rac, M.; Machalinski, B.; Chlubek, D. Prostaglandin e-2 (PGE2) and thromboxane a(2) (txa(2)) synthesis is regulated by conjugated linoleic acids (CLA) in human macrophages. J. Physiol. Pharmacol. 2009, 60, 77–85. [Google Scholar]
- Lee, A.Y.; Lee, M.H.; Lee, S.; Cho, E.J. Neuroprotective effect of alpha-linolenic acid against Aβ-mediated inflammatory responses in C6 glial cell. J. Agric. Food Chem. 2018, 66, 4853–4861. [Google Scholar] [CrossRef]
- Pauls, S.D.; Rodway, L.A.; Winter, T.; Taylor, C.G.; Zahradka, P.; Aukema, H.M. Anti-inflammatory effects of α-linolenic acid in M1-like macrophages are associated with enhanced production of oxylipins from α-linolenic and linoleic acid. J. Nutr. Biochem. 2018, 57, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Chung, S.H. Anti-inflammatory effect of alpha-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-kappaB and mitogen-activated protein kinase pathways. J. Agric. Food Chem. 2007, 55, 5073–5080. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lu, Q.; Cui, L.; Zong, M.; Guo, Y.; Liu, L.; Pan, D.; Wu, Z. The fatty acid profiles of mixed fermented milk and its anti-inflammation properties in an LPS-induced RAW264.7 cell model. Food Funct. 2022, 13, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Han, E.J.; Chung, S.H. In vivo and in vitro anti-inflammatory activities of alpha-linolenic acid isolated from Actinidia polygama fruits. Arch. Pharmacal Res. 2007, 30, 708–714. [Google Scholar] [CrossRef]
- Alam, S.-I.; Kim, M.-W.; Shah, F.A.; Saeed, K.; Ullah, R.; Kim, M.-O. Alpha-Linolenic Acid Impedes Cadmium-Induced Oxidative Stress, Neuroinflammation, and Neurodegeneration in Mouse Brain. Cell 2021, 10, 2274. [Google Scholar] [CrossRef]
- Regueiro, J.; Sanchez-Gonzalez, C.; Vallverdu-Queralt, A.; Simal-Gandara, J.; Lamuela-Raventos, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap-Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef]
- Vu, D.; Vo, P.; Coggeshall, M.; Lin, C.H. Identification and characterization of phenolic compounds in black walnut kernels. J. Agric. Food Chem. 2018, 66, 4503–4511. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Z.; Dai, S.; Che, X.; Liu, W. Identification and quantification of bioactive compounds in diaphragma juglandis fructus by uhplc-q-orbitrap hrms and uhplc-ms/ms. J. Agric. Food Chem. 2019, 67, 3811–3825. [Google Scholar] [CrossRef]
- Trandafir, I.; Cosmulescu, S. Total Phenolic Content, Antioxidant Capacity and Individual Phenolic Compounds of Defatted Kernel from Different Cultivars of Walnut. Erwerbs-Obstbau 2020, 62, 309–314. [Google Scholar] [CrossRef]
- Wu, S.; Shen, D.; Wang, R.; Li, Q.; Liu, Y. Phenolic profiles and antioxidant activities of free, esterified and bound phenolic compounds in walnut kernel. Food Chem. 2021, 350, 129217. [Google Scholar] [CrossRef]
- Fakiha, F.; Faraz, Z.M.; Ehraz, A.; Masood, A.; Mohammad, A. Ellagic acid attenuates arsenic induced neuro-inflammation and mitochondrial dysfunction associated apoptosis. Toxicol. Rep. 2018, 5, 411–417. [Google Scholar]
- He, X.-m.; Zhou, Y.-z.; Sheng, S.; Li, J.-j.; Wang, G.-q.; Zhang, F. Ellagic Acid Protects Dopamine Neurons via Inhibition of NLRP3 Inflammasome Activation in Microglia. Oxidative Med. Cell. Longev. 2020, 2020, 2963540. [Google Scholar] [CrossRef]
- Guo, X.; Wang, O.; Wang, Y.; Wang, K.; Zhou, F. Phenolic acids alleviate high-fat and high-fructose diet-induced metabolic disorders in rats. J. Food Biochem. 2017, 41, e12419. [Google Scholar] [CrossRef]
- Fan, Y.; Piao, C.H.; Hyeon, E.; Jung, S.Y.; Eom, J.-E.; Shin, H.S.; Song, C.H.; Cha, O.H. Gallic acid alleviates nasal inflammation via activation of Th1 and inhibition of Th2 and Th17 in a mouse model of allergic rhinitis. Int. Immunopharmacol. 2019, 70, 512–519. [Google Scholar] [CrossRef]
- Seo, C.S.; Jeong, S.J.; Yoo, S.R.; Lee, N.R.; Shin, H.K. Quantitative Analysis and In vitro Anti-inflammatory Effects of Gallic Acid, Ellagic Acid, and Quercetin from Radix Sanguisorbae. Pharmacogn. Mag. 2016, 12, 104–108. [Google Scholar]
- Abdullah, A.; Maged, M.; Hairul-Islam, M.I.; Osama, I.A.; Manal, A.; Hamza, H. Activation of aryl hydrocarbon receptor signaling by a novel agonist ameliorates autoimmune encephalomyelitis. PLoS ONE 2019, 14, e0215981. [Google Scholar] [CrossRef]
- Hermawati, E.; Arfian, N.; Mustofa, M.; Partadiredja, G. Chlorogenic acid ameliorates memory loss and hippocampal cell death after transient global ischemia. Eur. J. Neurosci. 2019, 51, 651–669. [Google Scholar] [CrossRef]
- Li, P.; Stetler, R.A.; Leak, R.K.; Shi, Y.; Li, Y.; Yu, W.; Bennett, M.; Chen, J. Oxidative stress and DNA damage after cerebral ischemia: Potential therapeutic targets to preserve the genome and improve stroke recovery. Neuropharmacology 2017, 134, 208–217. [Google Scholar] [CrossRef]
- Lee, T.-K.; Kang, I.-J.; Kim, B.; Sim, H.J.; Kim, D.-W.; Ahn, J.H.; Lee, J.-C.; Ryoo, S.; Shin, M.C.; Cho, J.H.; et al. Experimental Pretreatment with Chlorogenic Acid Prevents Transient Ischemia-Induced Cognitive Decline and Neuronal Damage in the Hippocampus through Anti-Oxidative and Anti-Inflammatory Effects. Molecules 2020, 25, 3578. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Zeng, J.; Wan, X.; Liu, T.; Xiong, Y.; Xiang, G.; Peng, Y.; Zhu, R.; Zhou, Y.; Liu, C. Chlorogenic acid ameliorates Klebsiella pneumoniae-induced pneumonia in immunosuppressed mice via inhibiting the activation of NLRP3 inflammasomes. Food Funct. 2021, 12, 9466–9475. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xu, J.; Miao, S.; Wei, K.; Peng, L.; Wang, Y.; Wei, X. Recent advances in the utilization of tea active ingredients to regulate sleep through neuroendocrine pathway, immune system and intestinal microbiota. Crit. Rev. Food Sci. Nutr. 2022, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Liu, W.; Zhou, H.-Y.; Gui, Y.-R.; Yang, Y.-H.; Wu, M.-J.; Xiao, Y.-F.; Shang, J.-T.; Long, G.-F.; Shu, X.-J. Epigallocatechin-3-gallate Alleviates Cognitive Deficits in APP/PS1 Mice. Curr. Med. Sci. 2020, 40, 18–27. [Google Scholar] [CrossRef]
- Kawai, K.; Tsuno, N.H.; Kitayama, J.; Okaji, Y.; Yazawa, K.; Asakage, M.; Hori, N.; Watanabe, T.; Takahashi, K.; Nagawa, H. Epigallocatechin gallate attenuates adhesion and migration of CD8+ T cells by binding to CD11b. J. Allergy Clin. Immunol. 2004, 113, 1211–1217. [Google Scholar] [CrossRef]
- Li, J. Neuroprotective effect of ()-epigallocatechin-3-gallate on autoimmune thyroiditis in a rat model by an anti-inflammation effect, anti-apoptosis and inhibition of TRAIL signaling pathway. Exp. Ther. Med. 2018, 15, 1087–1092. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Barro, L.; Tsai, S.T.; Feng, T.W.; Ming, F.H. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. Int. J. Mol. Sci. 2021, 22, 3037. [Google Scholar] [CrossRef]
- Yang, D.-J.; Liu, S.-C.; Chen, Y.-C.; Hsu, S.-H.; Chang, Y.-P.; Lin, J.-T. Three Pathways Assess Anti-Inflammatory Response of Epicatechin with Lipopolysaccharide-Mediated Macrophage RAW264.7 Cells. J. Food Biochem. 2015, 39, 334. [Google Scholar] [CrossRef]
- Carullo, G.; Cappello, A.R.; Frattaruolo, L.; Badolato, M.; Armentano, B.; Aiello, F. Quercetin and derivatives: Useful tools in inflammation and pain management. Future Med. Chem. 2017, 9, 79–93. [Google Scholar] [CrossRef]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential Implications of Quercetin in Autoimmune Diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef]
- Okoko, T.; Oruambo, I.F. Inhibitory activity of quercetin and its metabolite on lipopolysaccharide-induced activation of macrophage U937 cells. Food Chem. Toxicol. 2009, 47, 809–812. [Google Scholar] [CrossRef]
- Lv, Y.; Wei, K.; Meng, X.; Huang, Y.; Zhang, T.; Li, Z. Separation and identification of iron-chelating peptides from defatted walnut flake by nanoLC-ESI–MS/MS and de novo sequencing—ScienceDirect. Process Biochem. 2017, 59, 223–228. [Google Scholar] [CrossRef]
- Wang, S.; Su, G.; Zhang, Q.; Zhao, T.; Zhao, M. Walnut (Juglans regia) Peptides Reverse Sleep Deprivation-Induced Memory Impairment in Rat via Alleviating Oxidative Stress. J. Agric. Food Chem. 2018, 66, 10617–10627. [Google Scholar] [CrossRef]
- Gao, Y.; Qin, H.; Wu, D.; Liu, C.; Fang, L.; Wang, J.; Liu, X.; Min, W. Walnut peptide WEKPPVSH in alleviating oxidative stress and inflammation in lipopolysaccharide-activated BV-2 microglia via the Nrf2/HO-1 and NF-κB/p38 MAPK pathways. J. Biosci. Bioeng. 2021, 132, 496–504. [Google Scholar] [CrossRef]
- Martinon, F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010, 40, 616–619. [Google Scholar] [CrossRef]
- Zhu, X.; Su, B.; Wang, X.; Smith, M.A.; Perry, G. Causes of oxidative stress in Alzheimer disease. Cell. Mol. Life Sci. 2007, 64, 2202–2210. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Yang, C.M. Role of Redox Signaling in Neuroinflammation and Neurodegenerative Diseases. BioMed Res. Int. 2013, 2013, 484613. [Google Scholar] [CrossRef]
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef]
- Schwab, C.; Mcgeer, P.L.; Miklossy, J.; Martins, R.N. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J. Alzheimer’s Dis. 2008, 13, 359–369. [Google Scholar] [CrossRef]
- Erdinest, N.; Shmueli, O.; Grossman, Y.; Ovadia, H.; Solomon, A. Anti-Inflammatory Effects of Alpha Linolenic Acid on Human Corneal Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2012, 53, 4396–4406. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Potential Beneficial Effects of a Diet with Walnuts in Aging and Alzheimer’s Disease. Brain Aging Ther. Interv. 2012, 239–252. [Google Scholar] [CrossRef]
- Pandareesh, M.D.; Chauhan, V.; Chauhan, A. Walnut Supplementation in the Diet Reduces Oxidative Damage and Improves Antioxidant Status in Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 1295–1305. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Beneficial Effects of Walnuts on Cognition and Brain Health. Nutrients 2020, 12, 550. [Google Scholar] [CrossRef]
- Radan, M.; Dianat, M.; Badavi, M.; Mard, S.A.; Bayati, V.; Goudarzi, G. In vivo and in vitro evidence for the involvement of Nrf2-antioxidant response element signaling pathway in the inflammation and oxidative stress induced by particulate matter (PM10): The effective role of gallic acid. Free. Radic. Res. 2018, 53, 210–225. [Google Scholar] [CrossRef]
- Rahimifard, M.; Baeeri, M.; Bahadar, H.; Moini-Nodeh, S.; Abdollahi, M. Therapeutic Effects of Gallic Acid in Regulating Senescence and Diabetes; an In Vitro Study. Molecules 2020, 25, 5875. [Google Scholar] [CrossRef] [PubMed]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and Mental Health: A Review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Villarán, R.F.; Espinosa-Oliva, A.M.; Sarmiento, M.; De Pablos, R.M.; Argüelles, S.; Delgado-Cortés, M.J.; Sobrino, V.; Van Rooijen, N.; Venero, J.L.; Herrera, A.J.; et al. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: Potential risk factor in Parkinson’s disease. J. Neurochem. 2010, 114, 1687–1700. [Google Scholar] [CrossRef] [PubMed]
- Giau, V.V.; Wu, S.Y.; Jamerlan, A.; An, S.S.A.; Kim, S.; Hulme, J. Gut Microbiota and Their Neuroinflammatory Implications in Alzheimer’s Disease. Nutrients 2018, 10, 1765. [Google Scholar] [CrossRef]
- Holscher, H.D.; Guetterman, H.M.; Swanson, K.S.; Ruopeng, A.; Matthan, N.R.; Lichtenstein, A.H.; Novotny, J.A.; Baer, D.J. Walnut Consumption Alters the Gastrointestinal Microbiota, Microbially Derived Secondary Bile Acids, and Health Markers in Healthy Adults: A Randomized Controlled Trial. J. Nutr. 2018, 148, 861–867. [Google Scholar] [CrossRef]
- Byerley, L.O.; Samuelson, D.; Blanchard, E.; Luo, M.; Lorenzen, B.N.; Banks, S.; Ponder, M.A.; Welsh, D.A.; Taylor, C.M. Changes in the gut microbial communities following addition of walnuts to the diet. J. Nutr. Biochem. 2017, 48, 94–102. [Google Scholar] [CrossRef]
- Miao, F.; Shan, C.; Shah, S.A.H.; Akhtar, R.W.; Wang, X.; Ning, D. Effect of walnut (Juglans sigillata) oil on intestinal antioxidant, anti-inflammatory, immunity, and gut microbiota modulation in mice. J. Food Biochem. 2020, 45, 13567. [Google Scholar] [CrossRef]
- Ren, S.M.; Zhang, Q.Z.; Chen, M.L.; Jiang, M.; Liu, X.Q. Anti-NAFLD effect of defatted walnut powder extract in high fat diet-induced C57BL/6 mice by modulating the gut microbiota. J. Ethnopharmacol. 2021, 270, 113814. [Google Scholar] [CrossRef]
- Zhi, T.; Hong, D.; Zhang, Z.; Li, S.; Xia, J.; Wang, C.; Wu, Y.; Jia, Y.; Ma, A. Anti-inflammatory and gut microbiota regulatory effects of walnut protein derived peptide LPF in vivo—ScienceDirect. Food Res. Int. 2022, 152, 110875. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Zhang, F.; Lin, Y.; Ma, Y.; Zhao, S.; Chen, C.; Wang, X.; Liu, J. Preventive effect of pressed degreased walnut meal extracts on T2DM rats by regulating glucolipid metabolism and modulating gut bacteria flora. J. Funct. Foods 2020, 64, 103694. [Google Scholar] [CrossRef]
- Wang, G.; Zhong, D.; Liu, H.; Yang, T.; Zhang, Y. Water soluble dietary fiber from walnut meal as a prebiotic in preventing metabolic syndrome. J. Funct. Foods 2021, 78, 104358. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, L.; Liu, Y.; Wu, Z.; Weng, P. The intestinal microbiota links tea polyphenols with the regulation of mood and sleep to improve immunity. Food Rev. Int. 2021, 1–14. [Google Scholar] [CrossRef]
- Sarkar, S.; Siddiqui, A.A.; Mazumder, S.; De, R.; Saha, S.J.; Banerjee, C.; Iqbal, M.S.; Adhikari, S.; Alam, A.; Roy, S.; et al. Ellagic acid, a dietary polyphenol, inhibits tautomerase activity of human macrophage migration inhibitory factor and its proinflammatory responses in human peripheral blood mononuclear cells. J. Agric. Food Chem. 2015, 63, 4988–4998. [Google Scholar] [CrossRef]
- Sang-Hyun, K.; Chang-Duk, J.; Kyongho, S.; Byung-Ju, C.; Hyunjeung, L.; Seunja, P.; Ho, L.S.; Hye-Young, S.; Dae-Keun, K.; Tae-Yong, S. Gallic Acid Inhibits Histamine Release and Proinflammatory Cytokine Production in Mast Cells. Toxicol. Sci. 2006, 91, 123–131. [Google Scholar]
- Zhang, F.; Li, N.; Jiang, L.; Chen, L.; Huang, M. Neuroprotective effects of (−)-Epigallocatechin-3-gallate against focal cerebral ischemia/reperfusion injury in rats through attenuation of inflammation. Neurochem. Res. 2015, 40, 1691–1698. [Google Scholar] [CrossRef]
| Types of Active Ingredient | Model | Dose | Results | Ref. | |
|---|---|---|---|---|---|
| Fatty acids | Linoleic acid | Aβ25−35-treated PC12 cells | 10, 50 or 100 μM | Decreased the Aβ25-35-elevated TNF-α and IL-1β levels by 50%; inhibited increased NO production by reducing iNOS; inhibited PGE2 by decreasing COX-2; decreased the level of p-p65 and p-IκB. | [43] |
| Linoleic acid trans-10, cis-12 CLA cis-9, trans-11 CLA | Human macrophages | 20 or 40 μM | Reduced PGE2 concentration by 23%; reduced COX-2 activity. Reduced PGE2 concentration by 39%; reduced the quantity of the active p65 NF-κB subunit by 55%. Reduced PGE2 concentration by 32%; reduced the quantity of the active p65 NF-κB subunit by 58%. | [49] | |
| Alpha-linolenic acid | LPS-stimulated RAW 264.7 cells | 5, 10, 20 or 40 μg/mL | Inhibited translocation of the NF-κB subunit; downregulated inflammatory iNOS, COX-2, and TNF-α gene expression in a dose-dependent manner. | [52] | |
| LPS-stimulated RAW 264.7 cells | 50 μM | Decreased expression levels of TNF-α and IL-6; increased the secretion of the anti-inflammatory cytokines IL-10. | [53] | ||
| Carrageenan-induced hind paw edema in SD rats LPS-stimulated RAW 264.7 cells | 5 or 10 mg/kg | Reduced rat paw edema; inhibited the accumulation of nitrite and PGE2. Inhibited the protein and mRNA expression levels of iNOS and COX-2 enzymes in a dose-dependent manner. | [54] | ||
| Phenolic acids | Ellagic acid | Arsenic-treated rats | 10–20 mg/kg by mouth, in drinking water for 8–11 days | Decreased levels of mRNA and proteins TNF-α, IL-1β, and INF-γ in the hippocampus. | [61] |
| LPS-elicited DA neuronal loss in SD rats LPS-stimulated BV-2 cells | 50 mg/kg (oral) 1 μM | Suppressed LPS-induced activation of NLRP3 inflammasome signaling and IL-1β, TNF-α, and IL-18 protein expressions in the rat brain. Inhibited LPS-induced activation of microglial NLRP3 inflammasome signaling; eliminated production of TNF-α, IL-1β, and IL-18 in the culture medium. | [62] | ||
| Macrophage migration inhibitory factor (MIF)-treated human peripheral blood mononuclear cells | 50 μM | Inhibited MIF-mediated nuclear translocation of NF-κB. | [107] | ||
| LPS-stimulated RAW 264.7 cells | 6.25 μM 25 μM | Inhibited LPS-stimulated TNF-α. Inhibited LPS-stimulated IL-6 and PGE2 production. | [65] | ||
| Gallic acid | LPS-stimulated RAW 264.7 cells | 6.25 μM | Inhibited LPS-stimulated PGE2 production. | [65] | |
| MOG 35-55-immunized C57BL/6 mice | 2 mg/day for 10 days, injected intraperitoneally | Reduced infiltration of CD4+CD45+T cells and monocytes into the central nervous system. | [66] | ||
| Phorbol 12-myristate 13-acetate (PMA) + calcium ionophore A23187-stimulated human mast cells (HMC-1) | 1–10 µM for 2–4 h | Inhibited TNF-α and IL-6 gene expression, degradation of IκBα, and nuclear translocation of p65 NF-κB induced by PMA with A23187. | [108] | ||
| Chlorogenic acid | LPS-stimulated RAW 264.7 cells | 2–20 µM for 24 h | Attenuated NO, IL-1β, TNF-α, IL-6, cyclooxygenase-2, and NF-κB expression. | [70] | |
| Mongolian gerbil model of transient forebrain ischemia | 30 mg/kg | Attenuated IL-2 and IL-4 protein expressions in pyramidal neurons. | [69] | ||
| Flavonoids | EGCG | Isolated peripheral blood mononuclear cells and CD8+T cells | 25–100 µM | Inhibited infiltration of CD8+T cells into the sites of inflammation. | [75] |
| Autoimmune thyroiditis rat model | 0.5 mg/kg, three times at a 1 h interval for 3 h, injected intraperitoneally | Reduced IL-1β, INF-γ, and TNF-α levels in thyroid tissue through suppression of the NF-κB pathway. | [76] | ||
| Rat model of cerebral ischemia/reperfusion injury | 50 mg/kg, intraperitoneal injection | Inhibited cerebral ischemia/reperfusion injury by ameliorating inflammation-related molecules TNF-α, IL-1β, IL-6, NF-κB/p65, COX-2, and iNOS in the cerebellum. | [109] | ||
| Quercetin | Human mast cells HMC-1 | 10 μM | Inhibited mast cell tryptase and IL-6 release. | [80] | |
| LPS-stimulated U937 macrophages | 30 μM | Reduced the levels of TNF-α, IL-6, and IL-1. | [81] | ||
| LPS-stimulated RAW 264.7 cells | 12.5 μM | Inhibited LPS-stimulated IL-6 and PGE2 production. | [65] | ||
| Peptides | Hydrolysate (<3 kDa) Viscozyme L + pancreatin | LPS-treated mice | 666 mg/kg for 21 days | Reduced NO content, normalized the overproduction of IL-6, IL-1β, and TNF-α in the brain. | [21] |
| Hydrolysate | Aβ25−35-injected mice | 400 or 800 mg/kg for 5 weeks | Decreased the levels of NO, iNOS, NF-κB p65, TNF-α, IL-1β, and IL-6 in the hippocampus. | [24] | |
| Hydrolysate (<1 kDa) pepsin + pancreatin | D-gal + AlCl3-treated mice | 1 g/kg for 90 days | Suppressed the expression of TNF-α and IL-1β in the hippocampus. | [27] | |
| LPF | LPS-stimulated RAW264.7 cells | 250, 500, or 1000 μg/mL for 24 h or 48 h | Suppressed the mRNA expression of iNOS, COX-2, and TNF-α. | [36] | |
| LPF, GVYY, APTLW | LPS-stimulated BV-2 cells | 0.10 mM | Inhibited the overproduction of proinflammatory mediators (NO and PGE2); reduced the expression level of TNF-α,IL-1β, and IL-6. | [21] | |
| WEKPPVSH | LPS-stimulated BV-2 cells | 25 or 50 mM | Mitigated the secretion of TNF-α, IL-1β, and IL-6; downregulated the expression of iNOS, COX-2, and p-IkB/IkB. | [84] | |
| EVSGPGLSPN | H2O2-treated PC12 cells | 100 μM | Suppressed the expression of IKKβ and p65 to inhibit NF-κB pathway activation; attenuated the neurotoxic cascade by overexpression of IL-1β and TNF-α. | [39] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, B.; Wang, Y.; Zhang, X.; Sun, X. Recent Studies on Protective Effects of Walnuts against Neuroinflammation. Nutrients 2022, 14, 4360. https://doi.org/10.3390/nu14204360
Tan B, Wang Y, Zhang X, Sun X. Recent Studies on Protective Effects of Walnuts against Neuroinflammation. Nutrients. 2022; 14(20):4360. https://doi.org/10.3390/nu14204360
Chicago/Turabian StyleTan, Bing, Yuxi Wang, Xudong Zhang, and Xiangjun Sun. 2022. "Recent Studies on Protective Effects of Walnuts against Neuroinflammation" Nutrients 14, no. 20: 4360. https://doi.org/10.3390/nu14204360
APA StyleTan, B., Wang, Y., Zhang, X., & Sun, X. (2022). Recent Studies on Protective Effects of Walnuts against Neuroinflammation. Nutrients, 14(20), 4360. https://doi.org/10.3390/nu14204360







