The Effect of Alternating High-Sucrose and Sucrose Free-Diets, and Intermittent One-Day Fasting on the Estrous Cycle and Sex Hormones in Female Rats
Abstract
1. Introduction
2. Materials and Methods
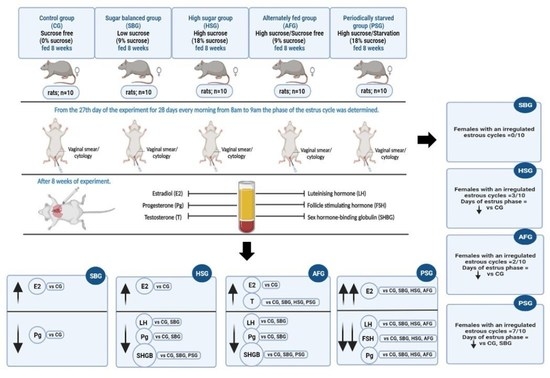
2.1. Animals and Study Design
- (i)
- The CG (control group) was fed BF;
- (ii)
- The SBG (sugar-balanced group) received MF1 containing 9.1% of the energy value from sucrose over the whole experimental period;
- (iii)
- The HSG (high-sugar group) received MF2 containing 18% of the energy value from sucrose;
- (iv)
- The AFG (alternately fed group) received BF and MF2 alternately every second week (in even weeks, BF, and in odd weeks, MF2);
- (v)
- The PSG (periodically starved group) received MF2 and were starved one day in every week (6 days on MF2 + 1 day starvation).
2.2. Sample Collection
2.3. Biochemical Analysis
2.4. Statistical Analysis
3. Results
3.1. The Estrous Cycle
3.2. Hormone Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Villegas-Romero, M.; Castrejón-Téllez, V.; Pérez-Torres, I.; Rubio-Ruiz, M.E.; Carreón-Torres, E.; Díaz-Díaz, E.; Del Valle-Mondragón, L.; Guarner-Lans, V. Short-Term Exposure to High Sucrose Levels near Weaning Has a Similar Long-Lasting Effect on Hypertension as a Long-Term Exposure in Rats. Nutrients 2018, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, X.; Cao, H.; Lv, Q.; Tong, N. Modified high-sucrose diet-induced abdominally obese and normal-weight rats developed high plasma free fatty acid and insulin resistance. Oxid. Med. Cell. Longev. 2012, 2012, 374346. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hanzawa, F.; Umeki, M.; Ikeda, S.; Mochizuki, S.; Oda, H. Time-restricted feeding suppresses excess sucrose-induced plasma and liver lipid accumulation in rats. PLoS ONE 2018, 13, e0201261. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, J.; Bruszkowska, M. Assessing the effect of sugar type and form of its intake on selected parameters of carbohydrate-lipid metabolism and plasma atherogenic indices in rats. Rocz. Panstw. Zakl. Hig. 2019, 70, 59–67. [Google Scholar] [CrossRef]
- Melo, B.F.; Sacramento, J.F.; Ribeiro, M.J.; Prego, C.S.; Correia, M.C.; Coelho, J.C.; Cunha-Guimaraes, J.P.; Rodrigues, T.; Martins, I.B.; Guarino, M.P.; et al. Evaluating the Impact of Different Hypercaloric Diets on Weight Gain, Insulin Resistance, Glucose Intolerance, and its Comorbidities in Rats. Nutrients 2019, 11, 1197. [Google Scholar] [CrossRef]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: What is the best choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef]
- Della Torre, S.; Benedusi, V.; Fontana, R.; Maggi, A. Energy metabolism and fertility: A balance preserved for female health. Nat. Rev. Endocrinol. 2014, 10, 13–23. [Google Scholar] [CrossRef]
- Nikanfar, S.; Oghbaei, H.; Rastgar Rezaei, Y.; Zarezadeh, R.; Jafari-Gharabaghlou, D.; Nejabati, H.R.; Bahrami, Z.; Bleisinger, N.; Samadi, N.; Fattahi, A.; et al. Role of adipokines in the ovarian function: Oogenesis and steroidogenesis. J. Steroid. Biochem. Mol. Biol. 2021, 209, 105852. [Google Scholar] [CrossRef]
- Christiansen, J.J.; Djurhuus, C.B.; Gravholt, C.H.; Iversen, P.; Christiansen, J.S.; Schmitz, O.; Weeke, J.; Jørgensen, J.O.; Møller, N. Effects of cortisol on carbohydrate, lipid, and protein metabolism: Studies of acute cortisol withdrawal in adrenocortical failure. J. Clin. Endocrinol. Metab. 2007, 92, 3553–3559. [Google Scholar] [CrossRef]
- Laugero, K.D.; Falcon, L.M.; Tucker, K.L. Relationship between perceived stress and dietary and activity patterns in older adults participating in the Boston Puerto Rican Health Study. Appetite 2011, 56, 194–204. [Google Scholar] [CrossRef]
- Panth, N.; Gavarkovs, A.; Tamez, M.; Mattei, J. The influence of diet on fertility and the implications for public health nutrition in the United States. Front. Public Health 2018, 6, 211. [Google Scholar] [CrossRef]
- Eunice, O.; Homahinuchu, J.H.H.; Ariyo, J.D. Sugar intake disrupts some reproductive functions in female wistar rats. J. Infertil. Reprod. Biol. 2021, 9, 1–6. [Google Scholar]
- Volk, K.; Pogrebna, V.V.; Roberts, J.A.; Zachry, J.E.; Blythe, S.N.; Toporikova, N. High-fat, high-sugar diet disrupts the preovulatory hormone surge and induces cystic ovaries in cycling female rats. J. Endocr. Soc. 2017, 1, 1488–1505. [Google Scholar] [CrossRef]
- De Melo, G.B.; Soares, J.F.; Costa, T.; Benevides, R.; Vale, C.C.; Paes, A.; Gaspar, R.S. Early Exposure to High-Sucrose Diet Leads to Deteriorated Ovarian Health. Front. Endocrinol. 2021, 12, 656831. [Google Scholar] [CrossRef]
- Di Monaco, R.; Miele, N.A.; Cabisidan, E.K.; Cavella, S. Strategies to reduce sugars in food. Curr. Opin. Food Sci. 2018, 19, 92–97. [Google Scholar] [CrossRef]
- Larson, N.I.; Neumark-Sztainer, D.; Story, M. Weight control behaviors and dietary intake among adolescents and young adults: Longitudinal findings from Project EAT. J. Am. Diet. Assoc. 2009, 109, 1869–1877. [Google Scholar] [CrossRef]
- Niño, O.M.S.; da Costa, C.S.; Torres, K.M.; Zanol, J.F.; Freitas-Lima, L.C.; Miranda-Alves, L.; Graceli, J.B. High-refined carbohydrate diet leads to polycystic ovary syndrome-like features and reduced ovarian reserve in female rats. Toxicol. Lett. 2020, 332, 42–55. [Google Scholar] [CrossRef]
- Nwogueze, B.C.; Ojieh, A.E.; Wilson, J.I.; Ovuakporaye, S.I.; Ohwin, P.E.; Aisuodionoe, E.M.; Daubry, T.; Agbonifo-Chijiokwu, E.; Eke, C.N.; Omeru, O.; et al. Down regulatory response of reproductive potentials in stress-induced rats supplemented with clomifene citrate: The fate of infertility. Biomed. Pharmacother. 2021, 143, 112208. [Google Scholar] [CrossRef]
- Sadowska, J.; Dudzinska, W.; Skotnicka, E.; Sielatycka, K.; Daniel, I. The impact of a diet containing sucrose and systematically repeated starvation on the oxidative status of the uterus and ovary of rats. Nutrients 2019, 11, 1544. [Google Scholar] [CrossRef]
- Sadowska, J.; Dudzinska, W.; Dziaduch, I. Effects of different models of sucrose intake on the oxidative status of the uterus and ovary of rats. PLoS ONE 2021, 16, e02511789. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Lai, Z.; Tian, Y.; Fang, L.; Wu, M.; Xiong, J.; Qin, X.; Luo, A.; Wang, S. Long-Term Moderate Oxidative Stress Decreased Ovarian Reproductive Function by Reducing Follicle Quality and Progesterone Production. PLoS ONE 2016, 11, e0162194. [Google Scholar] [CrossRef]
- Shull, J.D.; Dennison, K.L.; Check, A.C.; Trentham-Dietz, A. Rat models of 17β-estradiol-induced mammary cancer reveal novel insights into breast cancer etiology and prevention. Physiol. Genom. 2018, 50, 215–234. [Google Scholar] [CrossRef]
- Andrews, W.W.; Ojeda, S.R. A detailed analysis of the serum luteinizing hormone secretory profile in conscious, free-moving female rats during the time of puberty. Endocrinology 1981, 109, 2032–2039. [Google Scholar]
- Auta, T.; Hassan, A.T. Alteration in oestrus cycle and implantation in Mus musculus administered aqueous wood ash extract of Azadirachta indica (neem). Asian Pacific J. Reprod. 2016, 5, 188–192. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jeddi, S.; Kashfi, K. The laboratory rat: Age and body weight matter. EXCLI J. 2021, 20, 1431–1445. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76 rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Z.; Gregg, E.W.; Flanders, W.D.; Merritt, R.; Hu, F.B. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern. Med. 2014, 174, 516–524. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- AOAC, Association of Official Analytical and Chemists. Official Methods of Analysis, 20th ed.; AOAC, Association of Official Analytical and Chemists: Gaithersburg, MD, USA, 2016. [Google Scholar]
- FAO. Chapter 2: Methods of Food Analysis. In Food Energy—Methods of Analysis and Conversion Factors; Food Nutrition Paper; FAO: Rome, Italy, 2003; Volume 77, pp. 12–14. [Google Scholar]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef]
- McQuillan, H.J.; Han, S.Y.; Cheong, I.; Herbison, A.E. GnRH pulse generator activity across the estrous cycle of female mice. Endocrinology 2019, 160, 1480–1491. [Google Scholar] [CrossRef]
- Smith, J.T.; Popa, S.M.; Clifton, D.K.; Hoffman, G.E.; Steiner, R.A. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J. Neurosci. 2006, 26, 6687–6694. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.D.; Srouji, S.S.; Histed, S.N.; Hall, J.E. Differential effects of aging on estrogen negative and positive feedback. Am. J. Physiol. Endocrinol. Metab. 2011, 301, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Hall, J. Chapter 7—Neuroendocrine Control of the Menstrual Cycle. In Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management; Strauss, J., III, Barbieri, R., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2004; pp. 195–211. [Google Scholar]
- Kauffman, A.S.; Clifton, D.K.; Steiner, R.A. Emerging ideas about kisspeptin–GPR54 signaling in the neuroendocrine regulation of reproduction. Trends. Neurosci. 2007, 30, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Roa, J.; Aguilar, E.; Diéguez, C.; Pinilla, L. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front. Neuroendocrinol. 2008, 29, 48–69. [Google Scholar] [CrossRef]
- Minabe, S.; Uenoyama, Y.; Tsukamura, H.; Maeda, K. Analysis of pulsatile and surge-like luteinizing hormone secretion with frequent blood sampling in female mice. J. Reprod. Develop. 2011, 57, 660–664. [Google Scholar] [CrossRef]
- Quennell, J.H.; Howell, C.S.; Roa, J.; Augustine, R.A.; Grattan, D.R.; Anderson, G.M. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology 2011, 152, 1541–1550. [Google Scholar] [CrossRef]
- Sliwowska, J.H.; Fergani, C.; Gawełek, M.; Skowronska, B.; Fichna, P.; Lehman, M. Insulin: Its role in the central control of reproduction. Physiol. Behav. 2014, 22, 197–206. [Google Scholar] [CrossRef]
- Dong, Q.; Lazarus, R.M.; Wong, L.S.; Vellios, M.; Handelsman, D.J. Pulsatile LH secretion in streptozotocin-induced diabetes in the rat. J. Endocrinol. 1991, 131, 49–55. [Google Scholar] [CrossRef]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.M.; Le Poul, E.; Brezillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. Effect of centrally administered insulin on gonadotropin-releasing hormone neuron activity and luteinizing hormone surge in the diabetic female rat. Neuroendocrinology 2002, 76, 357–365. [Google Scholar]
- Lee, J.; Lee, H.C.; Kim, S.Y.; Cho, G.J.; Woodruff, T.K. Poorly-controlled Type 1 diabetes mellitus impairs LH-LHCGR signaling in the ovaries and decreases female fertility in mice. Yonsei Med. J. 2019, 60, 667–678. [Google Scholar] [CrossRef]
- Chen, M.J.; Yang, W.S.; Hsiao, C.K.; Yang, Y.S.; Ho, H.N. Low sex hormone-binding globulin is associated with low high-density lipoprotein cholesterol and metabolic syndrome in women with PCOS. Hum. Reprod. 2006, 21, 2266–2271. [Google Scholar] [CrossRef]
- Chosich, J.; Bradford, A.P.; Allshouse, A.A.; Reusch, E.B.; Santoro, N.; Schauer, I.E. Acute recapitulation of the hyperinsulinemia and hyperlipidemia characteristic of metabolic syndrome suppresses gonadotropins. Obesity 2017, 25, 553–560. [Google Scholar] [CrossRef]
- Qiu, X.; Dao, H.; Wang, M.; Heston, A.; Garcia, L.D.; Sangal, A.; Dowling, A.R.; Faulkner, L.D.; Molitor, S.C.; Elias, C.F.; et al. Insulin and leptin signaling interact in the mouse kiss1 neuron during the peripubertal period. PLoS ONE 2015, 10, e0121974. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, G. Intermittent fasting dietary restriction regimen negatively influences reproduction in young rats: A study of hypothalamo-hypophysial-gonadal axis. PLoS ONE 2013, 8, e52416. [Google Scholar] [CrossRef]
- Castellano, J.M.; Navarro, V.M.; Fernández-Fernández, R.; Nogueiras, R.; Tovar, S.; Roa, J.; Vazquez, M.J.; Vigo, E.; Casanueva, F.F.; Aguilar, E.; et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 2005, 146, 3917–3925. [Google Scholar] [CrossRef]
- Saben, J.L.; Asquar, Z.; Rhee, J.S.; Drury, A.; Scheaffer, S.; Moley, H. Excess maternal fructose consumption increases fetal loss and impairs endometrial decidualization in mice. Endocrinology 2016, 157, 956–968. [Google Scholar] [CrossRef]
- Tobiansky, D.J.; Kachkovski, G.V.; Enos, R.T.; Schmidt, K.L.; Murphy, E.A.; Soma, K.K. Sucrose consumption alters steroid and dopaminę signalling in the female rat brain. J. Endocrinol. 2020, 245, 231–246. [Google Scholar] [CrossRef]
- Niswender, G.D.; Juengel, J.L.; Silva, P.J.; Rollyson, M.K.; McIntush, E.W. Mechanisms controlling the function and life span of the corpus luteum. Physiol. Rev. 2000, 80, 1–29. [Google Scholar] [CrossRef]
- Sagae, S.C.; Menezes, E.F.; Bonfleur, M.L.; Vanzela, E.C.; Zacharias, P.; Lubaczeuski, C.; Franci, C.R.; Sanvitto, G.L. Early onset of obesity induces reproductive deficits in female rats. Physiol. Behav. 2012, 105, 1104–1111. [Google Scholar] [CrossRef]
- Bowen-Shauver, J.M.; Gibori, G. The Corpus Luteum of Pregnancy; Elsevier Inc.: San Diego, CA, USA, 2004; pp. 201–230. [Google Scholar]
- Lund, S.A.; Van Kirk, E.A.; Murdoch, W.J. Mitogenic and antioxidant mechanisms of estradiol action in preovulatory ovine follicles: Relevance to luteal function. Biol. Reprod. 1999, 61, 388–392. [Google Scholar] [CrossRef]
- Günzel-Apel, A.U.C.; Wolf, K.; Einspanier, A.; Oei, C.; Piechotta, M. Serum progesterone in pregnant bitches supplemented with progestin because of expected or suspected luteal insufficiency. Reprod. Domest. Anim. 2012, 47 (Suppl. 6), 55–60. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80–90. [Google Scholar] [CrossRef]
- Patterson, R.E.; Sears, D.D. Metabolic effects of intermittent fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef]
- Sun, J.; Shen, X.; Liu, H.; Peng, J.; Kuang, H. Caloric restriction in female reproduction: Is it beneficial or detrimental? Reprod. Biol. Endocrinol. 2021, 19, 1. [Google Scholar] [CrossRef]
- Chalvon-Demersay, T.; Blachier, F.; Tome, D.; Blais, A. Animal models for the study of the relationships between diet and obesity: A focus on dietary protein and estrogen deficiency. Front. Nutr. 2017, 4, 5. [Google Scholar] [CrossRef]
- Even, P.C.; Virtue, S.; Morton, N.M.; Fromentin, G.; Semple, R.K. Editorial: Are rodent models fit for investigation of human obesity and related diseases? Front. Nutr. 2017, 4, 58. [Google Scholar] [CrossRef]

| Trait | CG | SBG | HSG | AFG | PSG |
|---|---|---|---|---|---|
| Initial b.w. (g) | 205 ± 17.8 | 205 ± 16.0 | 204 ± 13.0 | 205 ± 15.8 | 205 ± 16.0 |
| Final b.w. (g) | 230 ± 19.8 | 232 ± 17.8 | 231 ± 14.1 | 233 ± 16.8 | 230 ± 16.7 |
| Feed intake (g/100 g b.w.) | 335 ± 8.5 | 328 ± 13.8 | 326 ± 8.3 | 330 ± 6.9 (169 BF + 161 MF1) | 333 ± 10.2 |
| Energy intake (kcal/100 g b.w.) | 1142 ± 28.5 a | 1154 ± 45.3 a,b | 1157 ± 27.1 a,b | 1147 ± 24.6 a | 1181 ± 33.2 b |
| Sucrose intake (g/100 g b.w.) | 0 a | 26.2 ± 1.1 b | 52.2 ± 1.3 c | 25.8 ± 1.0 b | 52.3 ± 1.6 c |
| Contribution of sucrose-derived energy to the total dietary energy content (%) | 0 | 9.1% | 18% | 9% | 18% |
| Trait | CG | SBG | HSG | AFG | PSG |
|---|---|---|---|---|---|
| Duration of the estrous phase (days) | 7.7 ± 1.25 c | 6.6 ± 0.84 b,c | 5.6 ± 0.7 a,b | 5.7 ± 1.16 a,b | 4.6 ± 1.07 a |
| Females with irregular cycles (number) | 0/10 | 0/10 | 3/10 | 2/10 | 7/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska, J.; Dudzińska, W.; Dziaduch, I. The Effect of Alternating High-Sucrose and Sucrose Free-Diets, and Intermittent One-Day Fasting on the Estrous Cycle and Sex Hormones in Female Rats. Nutrients 2022, 14, 4350. https://doi.org/10.3390/nu14204350
Sadowska J, Dudzińska W, Dziaduch I. The Effect of Alternating High-Sucrose and Sucrose Free-Diets, and Intermittent One-Day Fasting on the Estrous Cycle and Sex Hormones in Female Rats. Nutrients. 2022; 14(20):4350. https://doi.org/10.3390/nu14204350
Chicago/Turabian StyleSadowska, Joanna, Wioleta Dudzińska, and Izabela Dziaduch. 2022. "The Effect of Alternating High-Sucrose and Sucrose Free-Diets, and Intermittent One-Day Fasting on the Estrous Cycle and Sex Hormones in Female Rats" Nutrients 14, no. 20: 4350. https://doi.org/10.3390/nu14204350
APA StyleSadowska, J., Dudzińska, W., & Dziaduch, I. (2022). The Effect of Alternating High-Sucrose and Sucrose Free-Diets, and Intermittent One-Day Fasting on the Estrous Cycle and Sex Hormones in Female Rats. Nutrients, 14(20), 4350. https://doi.org/10.3390/nu14204350