Do Sleep Disorders and Western Diet Influence Psoriasis? A Scoping Review
Abstract
1. Introduction
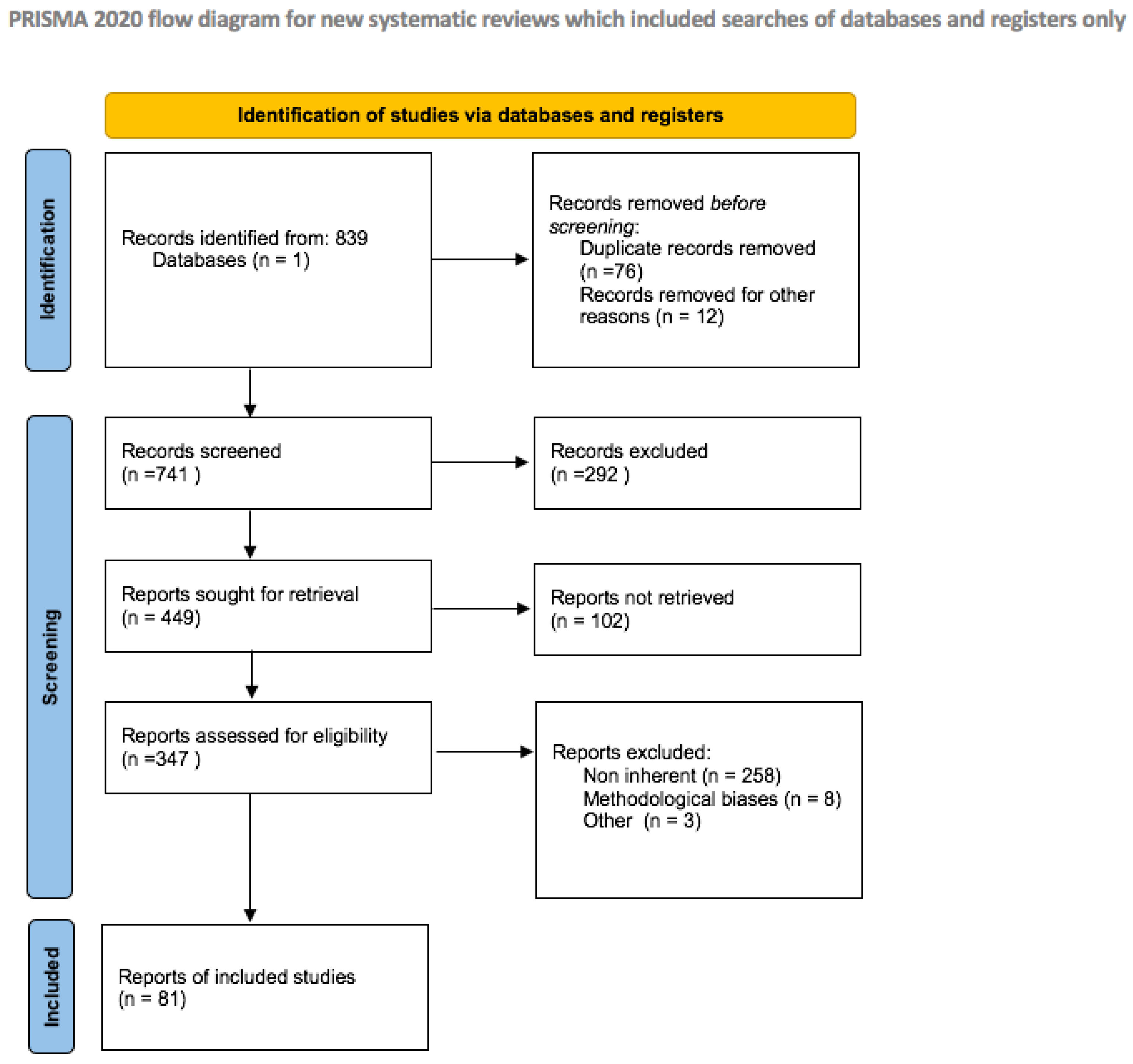
2. Evidence Acquisition
3. Sleep and Psoriasis
3.1. Pro-Inflammatory Cytokines Shared between Sleep Disorders and Psoriasis
3.2. Effects of Conventional and Biological Psoriasis Therapy on Sleep Disorders
4. Western Diet and Psoriasis
4.1. Effects of Western Diet and Psoriasis on Microbiota
4.2. How Lipids Influence Immune System Responses
4.2.1. Inflammasomes
4.2.2. Adipokines, Cytokines and Chemokines
4.2.3. n-6 PUFA-Derived Prostanoids and Leukotrienes
4.2.4. EPAs and DHAs (n-3 PUFAs)
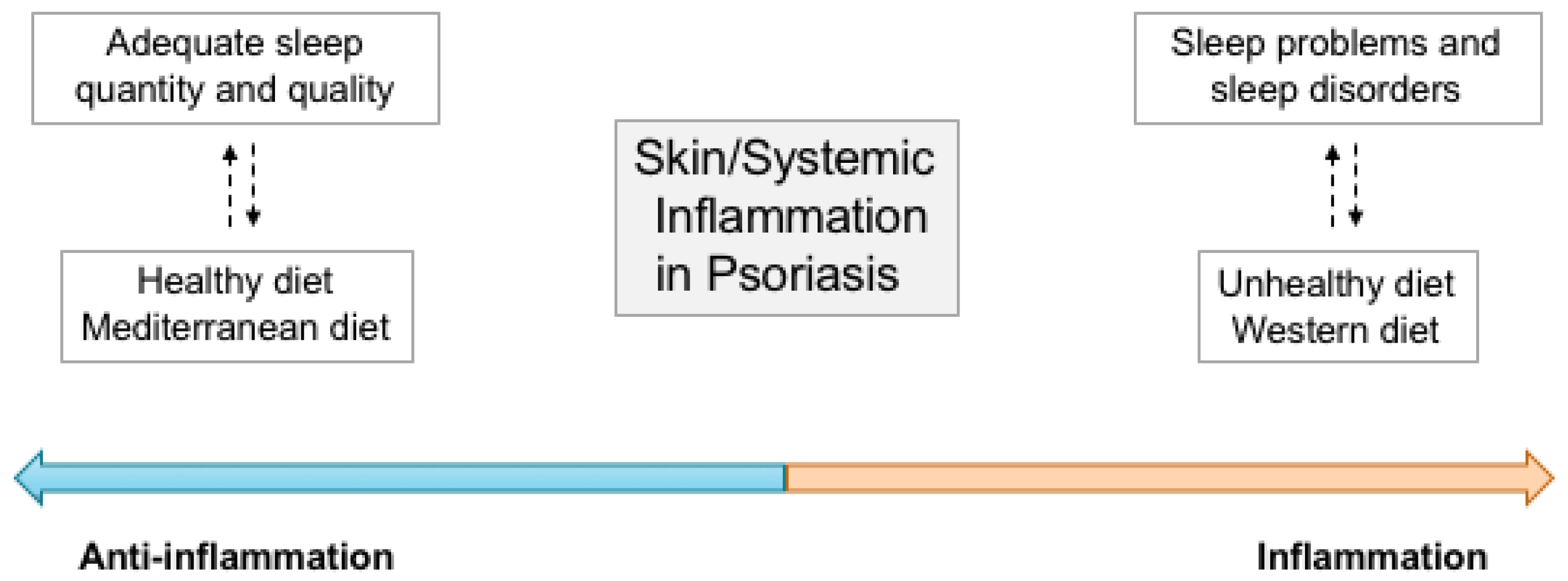
5. Sleep and Dietary Patterns: An Integrated View
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Damiani, G.; Bragazzi, N.L.; Aksut, C.K.; Wu, D.; Alicandro, G.; McGonagle, D.; Guo, C.; Dellavalle, R.; Grada, A.; Wong, P.; et al. The Global, Regional, and National Burden of Psoriasis: Results and Insights From the Global Burden of Disease 2019 Study. Front. Med. 2021, 8, 743180. [Google Scholar] [CrossRef]
- Wohlrab, J.; Fiedler, G.; Gerdes, S.; Nast, A.; Philipp, S.; Radtke, M.A.; Thaci, D.; Koenig, W.; Pfeiffer, A.F.H.; Härter, M.; et al. Recommendations for detection of individual risk for comorbidities in patients with psoriasis. Arch. Dermatol. Res. 2013, 305, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Brembilla, N.C. Pathogenesis-oriented therapy of psoriasis using biologics. Expert Opin. Biol. Ther. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Conic, R.R.; Damiani, G.; Schrom, K.P.; Ramser, A.E.; Zheng, C.; Xu, R.; McCormick, T.S.; Cooper, K.D. Psoriasis and Psoriatic Arthritis Cardiovascular Disease Endotypes Identified by Red Blood Cell Distribution Width and Mean Platelet Volume. J. Clin. Med. 2020, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Seth, D.; Ehlert, A.N.; Golden, J.B.; Damiani, G.; McCormick, T.S.; Cameron, M.J.; Cooper, K.D. Interaction of Resistin and Systolic Blood Pressure in Psoriasis Severity. J. Investig. Dermatol. 2020, 140, 1279–1282.e1. [Google Scholar] [CrossRef]
- Malerba, M.; Damiani, G.; Radaeli, A.; Ragnoli, B.; Olivini, A.; Calzavara-Pinton, P.G. Narrowband ultraviolet B phototherapy in psoriasis reduces proinflammatory cytokine levels and improves vitiligo and neutrophilic asthma. Br. J. Dermatol. 2015, 173, 1544–1545. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Pacifico, A.; Rizzi, M.; Santus, P.; Bridgewood, C.; Bragazzi, N.L.; Adawi, M.; Watad, A. Patients with psoriatic arthritis have higher levels of FeNO than those with only psoriasis, which may reflect a higher prevalence of a subclinical respiratory involvement. Clin. Rheumatol. 2020, 39, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Tacastacas, J.D.; Wuerz, T.; Miller, L.; Fastenau, P.; Bailey, C.; Chawa, M.S.; Argenas, A.; Fiore, M.; Cooper, K.D.; et al. Cognition/Psychological Burden and Resilience in Cutaneous T-Cell Lymphoma and Psoriasis Patients: Real-Life Data and Implications for the Treatment. BioMed. Res. Int. 2022, 2022, 8802469. [Google Scholar] [CrossRef] [PubMed]
- Ożóg, M.K.; Grabarek, B.O.; Wierzbik-Strońska, M.; Świder, M. Neurological Complications of Biological Treatment of Psoriasis. Life 2022, 12, 118. [Google Scholar] [CrossRef]
- Tang, X. The risk of organ-based comorbidities in psoriasis: A systematic review and meta-analysis. An. Bras. Dermatol. 2022, 97, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Nowowiejska, J.; Baran, A.; Flisiak, I. Mutual Relationship Between Sleep Disorders, Quality of Life and Psychosocial Aspects in Patients With Psoriasis. Front. Psychiatry 2021, 12, 674460. [Google Scholar] [CrossRef] [PubMed]
- Madhulika, A.; Gupta, F.; Simpson, C.; Aditya, K. Gupta Psoriasis and sleep disorders: A systematic review. Sleep Med. Rev. 2016, 29, 63–75. [Google Scholar]
- Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W. Sleep disorders in patients with psoriatic arthritis and pso-riasis. Reumatologia 2018, 56, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Kocaaga, A.; Kocaaga, M. Psoriasis: An Immunogenetic Perspective. Glob. Med. Genet. 2022, 9, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Roszkiewicz, M.; Dopytalska, K.; Szymańska, E.; Jakimiuk, A.; Walecka, I. Environmental risk factors and epigenetic alternations in psoriasis. Ann. Agric. Environ. Med. 2020, 27, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wu, X.; Rocha, C.S.; Rolston, M.; Garcia-Melchor, E.; Huynh, M.; Nguyen, M.; Law, T.; Haas, K.N.; Yamada, D.; et al. Short-Term Western Diet Intake Promotes IL-23–Mediated Skin and Joint Inflammation Accompanied by Changes to the Gut Microbiota in Mice. J. Investig. Dermatol. 2021, 141, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wu, X.; Yu, S.; Huynh, M.; Jena, P.K.; Nguyen, M.; Wan, Y.-J.Y.; Hwang, S.T. Short-Term Exposure to a Western Diet Induces Psoriasiform Dermatitis by Promoting Accumulation of IL-17A–Producing γδ T Cells. J. Investig. Dermatol. 2020, 140, 1815–1823. [Google Scholar] [CrossRef]
- Nowowiejska, J.; Baran, A.; Lewoc, M.; Grabowska, P.; Kaminski, T.W.; Flisiak, I. The Assessment of Risk and Predictors of Sleep Disorders in Patients with Psoriasis—A Questionnaire-Based Cross-Sectional Analysis. J. Clin. Med. 2021, 10, 664. [Google Scholar] [CrossRef]
- Yeroushalmi, S.; Hakimi, M.; Chung, M.; Bartholomew, E.; Bhutani, T.; Liao, W. Psoriasis and Exercise: A Review. Psoriasis Targets Ther. 2022, 12, 189–197. [Google Scholar] [CrossRef]
- Damiani, G.; Bragazzi, N.; McCormick, T.S.; Pigatto, P.D.M.; Leone, S.; Pacifico, A.; Tiodorovic, D.; Di Franco, S.; Alfieri, A.; Fiore, M. Gut microbiota and nutrient interactions with skin in psoriasis: A comprehensive review of animal and human studies. World J. Clin. Cases 2020, 8, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Ciccarese, G.; Indemini, E.; Savarino, V.; Parodi, A. Psoriasis and small intestine bacterial overgrowth. Int. J. Dermatol. 2018, 57, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Hawro, T.; Hawro, M.; Zalewska-Janowska, A.; Weller, K.; Metz, M.; Maurer, M. Pruritus and sleep disturbances in patients with psoriasis. Arch. Dermatol. Res. 2020, 312, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Mostaghimi, L.; Hetzel, S. Insomnia and other sleep complaints in inflammatory versus noninflammatory skin disorders: An observational case-control study. Int. J. Dermatol. 2019, 58, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Kabeloglu Ilbay, V.; Tas, B.; Altuntas, M.; Atakli, H.D.; Soysal, A. Risk of Obstructive Sleep Apnea Syndrome in Psoriasis Patients. Arch. Iran Med. 2019, 22, 137–143. [Google Scholar]
- Saçmacı, H.; Gürel, G. Sleep disorders in patients with psoriasis: A cross-sectional study using non-polysomnographical methods. Sleep Breath. 2019, 23, 893–898. [Google Scholar] [CrossRef]
- Jensen, P.; Zachariae, C.; Skov, L.; Zachariae, R. Sleep disturbance in psoriasis: A case-controlled study. Br. J. Dermatol. 2018, 179, 1376–1384. [Google Scholar] [CrossRef]
- Gowda, S.; Goldblum, O.M.; McCall, W.V.; Feldman, S.R. Factors affecting sleep quality in patients with psoriasis. J. Am. Acad. Dermatol. 2010, 63, 114–123. [Google Scholar] [CrossRef]
- Hirotsu, C.; Rydlewski, M.; Araújo, M.S.; Tufik, S.; Andersen, M.L. Sleep Loss and Cytokines Levels in an Experimental Model of Psoriasis. PLoS ONE 2012, 7, e51183. [Google Scholar] [CrossRef]
- Henry, A.L.; Chisholm, A.; Carter, L.A.; Bundy, C.; Griffiths, C.E.; Kyle, S.D. The relationship between sleep disturbance, symptoms and daytime functioning in psoriasis: A prospective study integrating actigraphy and experience sampling methodology. Sleep Med. 2020, 72, 144–149. [Google Scholar] [CrossRef]
- Thaçi, D.; Galimberti, R.; Amaya-Guerra, M.; Rosenbach, T.; Robertson, D.; Pedersen, R.; Yang, S.; Kuligowski, M.; Boggs, R. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: Results from the PRISTINE trial. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Strober, B.E.; Sobell, J.M.; Duffin, K.C.; Bao, Y.; Guérin, A.; Yang, H.; Goldblum, O.; Okun, M.M.; Mulani, P.M. Sleep quality and other patient-reported outcomes improve after patients with psoriasis with suboptimal response to other systemic therapies are switched to adalimumab: Results from PROGRESS, an open-label Phase IIIB trial. Br. J. Dermatol. 2012, 167, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Khalid, U.; Gislason, G.; Mallbris, L.; Skov, L.; Hansen, P.R. Psoriasis and Sleep Apnea: A Dan.ish Nationwide Cohort Study. J. Clin. Sleep Med. 2016, 12, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-W.; Kang, J.-H.; Lin, H.-C. Increased risk of psoriasis following obstructive sleep apnea: A longitudinal population-based study. Sleep Med. 2012, 13, 285–289. [Google Scholar] [CrossRef]
- Cohen, J.M.; Jackson, C.; Li, T.Y.; Wu, S.; Qureshi, A.A. Sleep disordered breathing and the risk of psoriasis among US women. Arch. Dermatol. Res. 2015, 307, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Zoumakis, E.; Lin, H.-M.; Bixler, E.O.; Trakada, G.; Chrousos, G.P. Marked Decrease in Sleepiness in Patients with Sleep Apnea by Etanercept, a Tumor Necrosis Factor-? Antagonist. J. Clin. Endocrinol. Metab. 2004, 89, 4409–4413. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-Y.; Hsieh, C.-F.; Chiang, Y.-T.; Tsai, Y.-W.; Huang, W.-F.; Li, C.-Y.; Wang, T.-S.; Tsai, T.-F. Concomitant Sleep Disorders Significantly Increase the Risk of Cardiovascular Disease in Patients with Psoriasis. PLoS ONE 2016, 11, e0146462. [Google Scholar] [CrossRef]
- Karaca, S.; Fidan, F.; Erkan, F.; Nural, S.; Pinarcı, T.; Gunay, E.; Unlu, M. Might psoriasis be a risk factor for obstructive sleep apnea syndrome? Sleep Breath 2013, 17, 275–280. [Google Scholar] [CrossRef]
- Shalom, G.; Dreiher, J.; Cohen, A. Psoriasis and obstructive sleep apnea. Int. J. Dermatol. 2016, 55, e579–e584. [Google Scholar] [CrossRef]
- Haugeberg, G.; Hoff, M.; Kavanaugh, A.; Michelsen, B. Psoriatic arthritis: Exploring the occurrence of sleep disturbances, fatigue, and depression and their correlates. Arthritis Res. Ther. 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Luca, M.; Luca, A.; Musumeci, M.L.; Fiorentini, F.; Micali, G.; Calandra, C. Psychopathological Variables and Sleep Quality in Psoriatic Patients. Int. J. Mol. Sci. 2016, 17, 1184. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.L.; Kyle, S.D.; Chisholm, A.; Griffiths, C.E.M.; Bundy, C. A cross-sectional survey of the nature and correlates of sleep dis-turbance in people with psoriasis. Br. J. Dermatol. 2017, 177, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Kaaz, K.; Szepietowski, J.C.; Matusiak, Ł. Influence of Itch and Pain on Sleep Quality in Atopic Dermatitis and Psoriasis. Acta Derm. Venereol. 2019, 99, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.A.; Simpson, F.C.; Vujcic, B.; Gupta, A.K. Obstructive sleep apnea and dermatologic disorders. Clin. Dermatol. 2017, 35, 319–327. [Google Scholar] [CrossRef]
- Walia, H.K.; Mehra, R. Overview of Common Sleep Disorders and Intersection with Dermatologic Conditions. Int. J. Mol. Sci. 2016, 17, 654. [Google Scholar] [CrossRef]
- Papadavid, E.; Dalamaga, M.; Vlami, K.; Koumaki, D.; Gyftopoulos, S.; Christodoulatos, G.S.; Papiris, S.; Rigopoulos, D. Psoriasis is associated with risk of obstructive sleep apnea independently from metabolic parameters and other comorbidities: A large hospital-based case-control study. Sleep Breath. 2017, 21, 949–958. [Google Scholar] [CrossRef]
- Wong, I.T.Y.; Chandran, V.; Li, S.; Gladman, D.D. Sleep Disturbance in Psoriatic Disease: Prevalence and Associated Factors. J. Rheumatol. 2017, 44, 1369–1374. [Google Scholar] [CrossRef]
- Melikoglu, M. Sleep Quality and its Association with Disease Severity in Psoriasis. Eurasian J. Med. 2017, 49, 124–127. [Google Scholar] [CrossRef]
- Gezer, O.; Batmaz, I.; Sariyildiz, M.A.; Sula, B.; Ucmak, D.; Bozkurt, M.; Nas, K. Sleep quality in patients with psoriatic arthritis. Int. J. Rheum. Dis. 2017, 20, 1212–1218. [Google Scholar] [CrossRef]
- Tas, B.; Kabeloglu, V.; Soysal, A.; Atakli, D. Sleep Quality in Psoriasis Patients and Its Relations with Possible Affecting Factors. Med. Bull. Sisli Hosp. 2020, 54, 181–187. [Google Scholar] [CrossRef]
- Hirotsu, C.; Nogueira, H.; Albuquerque, R.G.; Tomimori, J.; Tufik, S.; Andersen, M.L. The bidirectional interactions between psoriasis and obstructive sleep apnea. Int. J. Dermatol. 2015, 54, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.; Bundy, C.; Kyle, S.; Griffiths, C.E.M.; Chisholm, A. Understanding the experience of sleep disturbance in psoriasis: A qualitative exploration using the Common-Sense Model of Self-Regulation. Br. J. Dermatol. 2019, 180, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Papadavid, E.; Vlami, K.; Dalamaga, M.; Giatrakou, S.; Theodoropoulos, K.; Gyftopoulos, S.; Stavrianeas, N.; Papiris, S.; Rigopoulos, D. Sleep apnea as a comorbidity in obese psoriasis patients: A cross-sectional study. Do psoriasis characteristics and metabolic parameters play a role? J. Eur. Acad. Dermatol. Venereol. 2013, 27, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Ger, T.-Y.; Fu, Y.; Chi, C.-C. Bidirectional Association Between Psoriasis and Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 5931. [Google Scholar] [CrossRef] [PubMed]
- Maari, C.; Bolduc, C.; Nigen, S.; Marchessault, P.; Bissonnette, R. Effect of adalimumab on sleep parameters in patients with pso-riasis and obstructive sleep apnea: A randomized controlled trial. J. Dermatol. Treat. 2014, 25, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C.; Montano, N.; Furlan, R.; Keen, C.L.; Gershwin, M.E. Inflammation and Oxidative Stress in Obstructive Sleep Apnea Syndrome. Exp. Biol. Med. 2007, 232, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- E Lewis, P.; Emasealu, O.V.; Rohrbeck, P.; Hu, Z. Risk of type II diabetes and hypertension associated with chronic insomnia among active component, U.S. Armed Forces, 1998–2013. MSMR 2014, 21, 6–13. [Google Scholar]
- Loke, Y.K.; Brown, J.W.; Kwok, C.S.; Niruban, A.; Myint, P.K. Association of obstructive sleep apnea with risk of serious cardio-vascular events: A systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.A.; Simpson, F.C. Obstructive Sleep Apnea and Psychiatric Disorders: A Systematic Review. J. Clin. Sleep Med. 2015, 11, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Togeiro, S.M.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive sleep apnea: A cardiometabolic risk in obesity and the metabolic syndrome. J. Am. Coll. Cardiol. 2013, 62, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Jean-Louis, G.; Zizi, F.; Clark, L.T.; Brown, C.D.; McFarlane, S.I. Obstructive Sleep Apnea and Cardiovascular Disease: Role of the Metabolic Syndrome and Its Components. J. Clin. Sleep Med. 2008, 4, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Lanteri, P.; Bragazzi, N.L.; Magnavita, N.; Scoditti, E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 2021, 4, 1304. [Google Scholar] [CrossRef] [PubMed]
- Imagawa, S.; Yamaguchi, Y.; Higuchi, M.; Neichi, T.; Hasegawa, Y.; Mukai, H.Y.; Suzuki, N.; Yamamoto, M.; Nagasawa, T. Levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea–hypopnea syndrome. Blood 2001, 98, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, C.; Solovan, C.; Rosenberger, A.D.; Jinping, L.; Treudler, R.; Frei, U.; Eckardt, K.-U.; Brown, L.F. Upregulation of Hypoxia-Inducible Factors in Normal and Psoriatic Skin. J. Investig. Dermatol. 2007, 127, 2445–2452. [Google Scholar] [CrossRef]
- Baran, A.; Flisiak, I.; My´sliwiec, H.; Chodynicka, B. Role of adiponectin in psoriasis. Dermatol. Rev. 2010, 97, 413–416. [Google Scholar]
- Yasuda, K.-I.; Ishiuji, Y.; Endo, T.; Tanito, K.; Kamide, R.; Nobeyama, Y.; Asahina, A. Cyclosporine Improves Sleep Quality in Patients with Atopic Dermatitis. Dermatol. Ther. 2020, 10, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Ransom, J.; Morgan, P.J.; McCaffery, P.J.; Stoney, P.N. The rhythm of retinoids in the brain. J. Neurochem. 2014, 129, 366–376. [Google Scholar] [CrossRef]
- Smolensky, M.H.; Portaluppi, F.; Manfredini, R.; Hermida, R.C.; Tiseo, R.; Sackett-Lundeen, L.L.; Haus, E.L. Diurnal and twenty-four hour patterning of human diseases: Acute and chronic common and uncommon medical conditions. Sleep Med. Rev. 2015, 21, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Buratta, S.; Mercuri, S.R.; Pellegrino, R.M.; Urbanelli, L.; Emiliani, C.; Bertuccini, L.; Iosi, F.; Huber, V.; Brianti, P.; et al. Lipidic Profile Changes in Exosomes and Microvesicles Derived From Plasma of Monoclonal Antibody-Treated Psoriatic Patients. Front Cell Dev Biol. 2022, 10, 923769. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Franchi, C.; Pigatto, P.; Altomare, A.; Pacifico, A.; Petrou, S.; Leone, S.; Pace, M.C.; Fiore, M. Outcomes assessment of hepatitis C virus-positive psoriatic patients treated using pegylated interferon in combination with ribavirin compared to new Direct-Acting Antiviral agents. World J. Hepatol. 2018, 10, 329–336. [Google Scholar] [CrossRef]
- Gisondi, P.; Cazzaniga, S.; Chimenti, S.; Giannetti, A.; Maccarone, M.; Picardo, M.; Girolomoni, G.; Naldi, L.; Psocare Study Group. Metabolic abnormalities associated with initiation of systemic treatment for psoriasis: Evidence from the Italian Psocare Registry. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e30–e41. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Cazzaniga, S.; Conic, R.R.; Naldi, L. Psocare Registry Network. Pruritus Characteristics in a Large Italian Cohort of Psoriatic Patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Herbert, D.; Franz, S.; Popkova, Y.; Anderegg, U.; Schiller, J.; Schwede, K.; Lorz, A.; Simon, J.C.; Saalbach, A. High-Fat Diet Exacerbates Early Psoriatic Skin Inflammation Independent of Obesity: Saturated Fatty Acids as Key Players. J. Investig. Dermatol. 2018, 138, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Kabashima, K. Current understanding of the role of dietary lipids in the pathophysiology of psoriasis. J. Dermatol. Sci. 2019, 94, 314–320. [Google Scholar] [CrossRef]
- Carrera-Bastos, P.; Fontes-Villalba, M.; O’Keefe, J.H.; Lindeberg, S.; Cordain, L. The western diet and lifestyle and diseases of civilization. Res. Rep. Clin. Cardiol. 2011, 2, 15–35. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Barrea, L.; Macchia, P.E.; Tarantino, G.; Di Somma, C.; Pane, E.; Balato, N.; Napolitano, M.; Colao, A.; Savastano, S. Nutrition: A key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J. Transl. Med. 2015, 13, 303. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Balato, N.; Di Somma, C.; Macchia, P.E.; Napolitano, M.; Savanelli, M.C.; Esposito, K.; Colao, A.; Savastano, S. Nutrition and psoriasis: Is there any association between the severity of the disease and adherence to the Mediterranean diet? J. Transl. Med. 2015, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Kocic, H.; Damiani, G.; Stamenkovic, B.; Tirant, M.; Jovic, A.; Tiodorovic, D.; Peris, K. Dietary compounds as potential modulators of microRNA expression in psoriasis. Ther. Adv. Chronic Dis. 2019, 10, 2040622319864805. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, H.E.; Carbone, S.; Lavie, C.J. Dietary Fats and Chronic Noncommunicable Diseases. Nutrients 2018, 10, 1385. [Google Scholar] [CrossRef]
- Yazdanpanah, M.J.; Vahabi-Amlashi, S.; Nematy, M.; Shaelaei, N.; Mohajeri, S.A.R.; Tafazzoli, Z. Association of serum lipid profiles and dietary intakes of vitamin E and fiber with psoriasis severity. Caspian J. Intern. Med. 2021, 12, 606–612. [Google Scholar]
- Myśliwiec, H.; Baran, A.; Harasim-Symbor, E.; Myśliwiec, P.; Milewska, A.J.; Chabowski, A.; Flisiak, I. Serum fatty acid profile in psoriasis and its comorbidity. Arch. Dermatol. Res. 2017, 309, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Zákostelská, Z.; Málková, J.; Klimešová, K.; Rossmann, P.; Hornová, M.; Novosádová, I.; Stehlíková, Z.; Kostovčík, M.; Hudcovic, T.; Štepánková, R.; et al. Intestinal microbiota promotes psoriasis-like skin inflammation by enhancing Th17 response. PLoS ONE 2016, 11, e0159539. [Google Scholar] [CrossRef]
- Stehlikova, Z.; Kostovcikova, K.; Kverka, M.; Rossmann, P.; Dvorak, J.; Novosadova, I.; Kostovcik, M.; Coufal, S.; Srutkova, D.; Prochazkova, P.; et al. Crucial Role of Microbiota in Experimental Psoriasis Revealed by a Gnotobiotic Mouse Model. Front. Microbiol. 2019, 10, 236. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2017, 19, 19–30. [Google Scholar] [CrossRef]
- Fyhrquist, N.; Muirhead, G.; Prast-Nielsen, S.; Jeanmougin, M.; Olah, P.; Skoog, T.; Jules-Clement, G.; Feld, M.; Barrientos-Somarribas, M.; Sinkko, H.; et al. Microbe-host interplay in atopic dermatitis and psoriasis. Nat. Commun. 2019, 10, 4703. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Cantabrana, C.; Gómez, J.; Delgado, S.; Requena-López, S.; Queiro-Silva, R.; Margolles, A.; Coto, E.; Sánchez, B.; Coto-Segura, P. Gut microbiota dysbiosis in a cohort of patients with psoriasis. Br. J. Dermatol. 2019, 181, 1287–1295. [Google Scholar] [CrossRef]
- Chen, Y.; Ho, H.J.; Tseng, C.; Lai, Z.; Shieh, J.-J.; Wu, C.-Y. Intestinal microbiota profiling and predicted metabolic dysregulation in psoriasis patients. Exp. Dermatol. 2018, 27, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Fang, Z.; Cui, S.; Zhao, J.; Zhang, H.; Chen, W.; Lu, W. Evaluation of probiotics for inhibiting hyperproliferation and in-flammation relevant to psoriasis in vitro. J. Funct. Foods 2021, 81, 104433. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wu, C.-S.; Chao, Y.-H.; Lin, C.-C.; Tsai, H.-Y.; Li, Y.-R.; Chen, Y.Z.; Tsai, W.-H.; Chen, Y.-K. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J. Food Drug Anal. 2017, 25, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M. Bifidobacterium infantis35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.K.; Fung, M.A.; Chen, H.-L. Role of skin and gut microbiota in the pathogenesis of psoriasis, an inflammatory skin disease. Med. Microecol. 2020, 4, 100016. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wu, L.; Xiao, S.; Ji, Y.; Tan, Y.; Jiang, C.; Zhang, G. Dysregulation of the gut-brain-skin axis and key overlapping inflammatory and immune mechanisms of psoriasis and depression. Biomed. Pharmacother. 2021, 137, 111065. [Google Scholar] [CrossRef]
- Martinez, K.B.; Leone, V.; Chang, E.B. Western diets, gut dysbiosis, and metabolic diseases: Are they linked? Gut Microbes 2017, 8, 130–142. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and Psoriasis. Int. J. Mol. Sci. 2020, 21, 5405. [Google Scholar] [CrossRef]
- Endo, Y.; Asou, H.K.; Matsugae, N.; Hirahara, K.; Shinoda, K.; Tumes, D.J.; Tokuyama, H.; Yokote, K.; Nakayama, T. Obesity Drives Th17 Cell Differentiation by Inducing the Lipid Metabolic Kinase, ACC1. Cell Rep. 2015, 12, 1042–1055. [Google Scholar] [CrossRef]
- Stelzner, K.; Herbert, D.; Popkova, Y.; Lorz, A.; Schiller, J.; Gericke, M.; Klöting, N.; Blüher, M.; Franz, S.; Simon, J.C.; et al. Free fatty acids sensitize dendritic cells to amplify TH1/TH17-immune responses. Eur. J. Immunol. 2016, 46, 2043–2053. [Google Scholar] [CrossRef]
- Shimoura, N.; Nagai, H.; Fujiwara, S.; Jimbo, H.; Nishigori, C. Exacerbation and Prolongation of Psoriasiform Inflammation in Diabetic Obese Mice: A Synergistic Role of CXCL5 and Endoplasmic Reticulum Stress. J. Investig. Dermatol. 2018, 138, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.B.; Gregersen, I.; Sandanger, Ø.; Yang, K.; Sokolova, M.; Halvorsen, B.E.; Gullestad, L.; Broch, K.; Aukrust, P.; Louwe, M.C. Targeting the Inflammasome in Cardiovascular Disease. JACC Basic Transl. Sci. 2021, 7, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Fekri, S.Z.; Sigurdardottir, G.; Eding, C.B.; Sandin, C.; Enerbäck, C. Enhanced Inflammasome Activity in Patients with Psoriasis Promotes Systemic Inflammation. J. Investig. Dermatol. 2021, 141, 586–595.e5. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Kawashima, A.; Usui-Kawanishi, F.; Watanabe, S.; Kimura, H.; Kamata, R.; Shirasuna, K.; Koyama, Y.; Sato-Tomita, A.; Matsuzaka, T.; et al. Saturated Fatty Acids Undergo Intracellular Crystallization and Activate the NLRP3 Inflammasome in Macrophages. Arter. Thromb. Vasc. Biol. 2018, 38, 744–756. [Google Scholar] [CrossRef]
- Christ, A.; Günther, P.; Lauterbach, M.A.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 2018, 172, 162–175.e14. [Google Scholar] [CrossRef]
- Setty, A.R.; Curhan, G.; Choi, H.K. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study II. Arch. Intern. Med. 2007, 167, 1670–1675. [Google Scholar] [CrossRef]
- Higashi, Y.; Yamakuchi, M.; Fukushige, T.; Ibusuki, A.; Hashiguchi, T.; Kanekura, T. High-fat diet exacerbates imiquimod-induced psoriasis-like dermatitis in mice. Exp. Dermatol. 2018, 27, 178–184. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, Y.; Liao, Y.; Shi, Y.; Zhang, L.J. Emerging Roles of Adipose Tissue in the Pathogenesis of Psoriasis and Atopic Der-matitis in Obesity. JID Innov. 2021, 2, 100064. [Google Scholar] [CrossRef]
- Kyriakou, A.; Patsatsi, A.; Sotiriadis, D.; Goulis, D.G. Serum Leptin, Resistin, and Adiponectin Concentrations in Psoriasis: A Meta-Analysis of Observational Studies. Dermatology 2017, 233, 378–389. [Google Scholar] [CrossRef]
- Dopytalska, K.; Baranowska-Bik, A.; Roszkiewicz, M.; Bik, W.; Walecka, I. The role of leptin in selected skin diseases. Lipids Health Dis. 2020, 19, 215. [Google Scholar] [CrossRef]
- Zhu, K.-J.; Zhang, C.; Li, M.; Zhu, C.-Y.; Shi, G.; Fan, Y.-M. Leptin levels in patients with psoriasis: A meta-analysis. Clin. Exp. Dermatol. 2013, 38, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Tada, Y.; Hau, C.S.; Mitsui, A.; Kamata, M.; Asano, Y.; Sugaya, M.; Kadono, T.; Masamoto, Y.; Kurokawa, M.; et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from γδ-T cells. Nat. Commun. 2015, 6, 7687. [Google Scholar] [CrossRef] [PubMed]
- Lihn, A.S.; Bruun, J.M.; He, G.; Pedersen, S.B.; Jensen, P.F.; Richelsen, B. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol. Cell. Endocrinol. 2004, 219, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef]
- Ganguly, D.; Chamilos, G.; Lande, R.; Gregorio, J.; Meller, S.; Facchinetti, V.; Homey, B.; Barrat, F.J.; Zal, T.; Gilliet, M. Self-RNA–antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009, 206, 1983–1994. [Google Scholar] [CrossRef]
- Kanemaru, K.; Matsuyuki, A.; Nakamura, Y.; Fukami, K. Obesity exacerbates imiquimod-induced psoriasis-like epidermal hy-perplasia and interleukin-17 and interleukin-22 production in mice. Exp. Dermatol. 2015, 24, 436–442. [Google Scholar] [CrossRef]
- Vasseur, P.; Serres, L.; Jégou, J.-F.; Pohin, M.; Delwail, A.; Petit-Paris, I.; Levillain, P.; Favot, L.; Samson, M.; Yssel, H.; et al. High-Fat Diet–Induced IL-17A Exacerbates Psoriasiform Dermatitis in a Mouse Model of Steatohepatitis. Am. J. Pathol. 2016, 186, 2292–2301. [Google Scholar] [CrossRef]
- Nakamizo, S.; Honda, T.; Adachi, A.; Nagatake, T.; Kunisawa, J.; Kitoh, A.; Otsuka, A.; Dainichi, T.; Nomura, T.; Ginhoux, F.; et al. High fat diet exacerbates murine psoriatic dermatitis by increasing the number of IL-17-producing γδ T cells. Sci. Rep. 2017, 7, 14076. [Google Scholar] [CrossRef]
- Honda, T.; Kabashima, K. Prostanoids and leukotrienes in the pathophysiology of atopic dermatitis and psoriasis. Int. Immunol. 2019, 31, 589–595. [Google Scholar] [CrossRef]
- Ueharaguchi, Y.; Honda, T.; Kusuba, N.; Hanakawa, S.; Adachi, A.; Sawada, Y.; Otsuka, A.; Kitoh, A.; Dainichi, T.; Egawa, G.; et al. Thromboxane A2 facilitates IL-17A pro-duction from Vγ4+ γδ T cells and promotes psoriatic dermatitis in mice. J. Allergy Clin. Immunol. 2018, 142, 680–683.e2. [Google Scholar] [CrossRef]
- Schirmer, C.; Klein, C.; von Bergen, M.; Simon, J.C.; Saalbach, A. Human fibroblasts support the expansion of IL-17–producing T cells via up-regulation of IL-23 production by dendritic cells. Blood 2010, 116, 1715–1725. [Google Scholar] [CrossRef]
- Lee, J.; Aoki, T.; Thumkeo, D.; Siriwach, R.; Yao, C. T cell-intrinsic prostaglandin E2-EP2/EP4 signaling is critical in pathogenic TH17 cell-driven inflammation. J. Allergy Clin. Immunol. 2019, 143, 631–643. [Google Scholar] [CrossRef]
- Sumida, H.; Yanagida, K.; Kita, Y.; Abe, J.; Matsushima, K.; Nakamura, M.; Ishii, S.; Sato, S.; Shimizu, T. Interplay between CXCR2 and BLT1 Facilitates Neutrophil Infiltration and Resultant Keratinocyte Activation in a Murine Model of Imiquimod-Induced Psoriasis. J. Immunol. 2014, 192, 4361–4369. [Google Scholar] [CrossRef] [PubMed]
- Black, A.K.; Camp, R.D.R.; Mallet, A.I.; Cunningham, F.M.; Hofbauer, M.; Greaves, M.W.; Derm, F.F. Pharmacologic and Clinical Effects of Lonapalene (RS 43179), a 5-Lipoxygenase Inhibitor, in Psoriasis. J. Investig. Dermatol. 1990, 95, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Mari, N.L.; Simão, A.N.C.; Dichi, I. n-3 polyunsaturated fatty acids supplementation in psoriasis: A review. Nutrire 2017, 42, 5. [Google Scholar] [CrossRef]
- Qin, S.; Wen, J.; Bai, X.C.; Chen, T.Y.; Zheng, R.C.; Zhou, G.B.; Ma, J.; Feng, J.Y.; Zhong, B.L.; Li, Y.M. Endogenous n-3 polyunsaturated fatty acids protect against imiquimod-induced psoriasis-like inflammation via the IL-17/IL-23 axis. Mol. Med. Rep. 2014, 9, 2097–2104. [Google Scholar] [CrossRef]
- Allen, M.J.; Fan, Y.Y.; Monk, J.M.; Hou, T.Y.; Barhoumi, R.; McMurray, D.N.; Chapkin, R.S. n-3 PUFAs reduce T-helper 17 cell differen-tiation by decreasing responsiveness to interleukin-6 in isolated mouse splenic CD4⁺ T cells. J. Nutr. 2014, 144, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Yen, J.H.; Ganea, D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2011, 25, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Seki, H.; Tani, Y.; Arita, M. Omega-3 PUFA derived anti-inflammatory lipid mediator resolvin E1. Prostaglandins Other Lipid Mediat. 2009, 89, 126–130. [Google Scholar] [CrossRef]
- Sawada, Y.; Honda, T.; Hanakawa, S.; Nakamizo, S.; Murata, T.; Ueharaguchi-Tanada, Y.; Ono, S.; Amano, W.; Nakajima, S.; Egawa, G.; et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 2015, 212, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Otsuka, A.; Ogawa, N.; Kobayashi, Y.; Nakamura, M.; Kabashima, K. Resolvin E1 attenuates murine psoriatic dermatitis. Sci. Rep. 2018, 8, 11873. [Google Scholar] [CrossRef]
- Xu, J.; Duan, X.; Hu, F.; Poorun, D.; Liu, X.; Wang, X.; Zhang, S.; Gan, L.; He, M.; Zhu, K.; et al. Resolvin D1 attenuates imiquimod-induced mice psoriasiform dermatitis through MAPKs and NF-κB pathways. J. Dermatol. Sci. 2018, 89, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zuraikat, F.M.; Wood, R.A.; Barragán, R.; St-Onge, M.-P. Sleep and Diet: Mounting Evidence of a Cyclical Relationship. Annu. Rev. Nutr. 2021, 41, 309–332. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep dura¬tion is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 2004, 1, e62. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.V.; Borba, M.E.; Lopes, R.V.; Fisberg, R.M.; Paim, S.L.; Teodoro, V.V.; Zimberg, I.Z.; Araújo, L.B.; Shivappa, N.; Hébert, J.R.; et al. Association between inflammatory potential of the diet and sleep parameters in sleep apnea patients. Nutrition 2019, 66, 5–10. [Google Scholar] [CrossRef]
- Campanini, M.Z.; Guallar-Castillón, P.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Mediterranean Diet and Changes in Sleep Du-ration and Indicators of Sleep Quality in Older Adults. Sleep 2017, 40, zsw083. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.S.; Simas, T.A.M.; Pagoto, S.L.; Person, S.D.; Rosal, M.C.; Waring, M.E. Sleep Duration and Diet Quality Among Women Within 5 Years of Childbirth in the United States: A Cross-Sectional Study. Matern. Child Health J. 2016, 20, 1869–1877. [Google Scholar] [CrossRef]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite 2013, 64, 71–80. [Google Scholar] [CrossRef]
- Dashti, H.S.; Scheer, F.A.; Jacques, P.F.; Lamon-Fava, S.; Ordovás, J.M. Short sleep duration and dietary intake: Epidemiologic evi-dence, mechanisms, and health implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Kripke, D.F.; Naidoo, N.; Langer, R.D. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010, 11, 180–184. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Alén, M.; Cheng, S.M.; Mikkola, T.M.; Tenhunen, J.; Lyytikäinen, A.; Wiklund, P.; Cong, F.; Saarinen, A.; Tarkka, I.; et al. Associations of disordered sleep with body fat distribu-tion, physical activity and diet among overweight middle-aged men. J. Sleep Res. 2015, 24, 414–424. [Google Scholar] [CrossRef]
- Stelmach-Mardas, M.; Mardas, M.; Iqbal, K.; Tower, R.; Boeing, H.; Piorunek, T. Quality of life, depression and dietary intake in Obstructive Sleep Apnea patients. Health Qual. Life Outcomes 2016, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.M.; Goodwin, J.L.; Drescher, A.A.; Smith, T.W.; Quan, S.F. Associations of Dietary Intake and Physical Activity with Sleep Disordered Breathing in the Apnea Positive Pressure Long-term Efficacy Study (APPLES). J. Clin. Sleep Med. 2008, 4, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.W.; Li, Y.; Winkelman, J.W.; Hu, F.B.; Rimm, E.B.; Gao, X. Probable insomnia is associated with future total energy intake and diet quality in men. Am. J. Clin. Nutr. 2016, 104, 462–469. [Google Scholar] [CrossRef]
- Katagiri, R.; Asakura, K.; Kobayashi, S.; Suga, H.; Sasaki, S. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middle-aged female Japanese workers. J. Occup. Health 2014, 56, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Sleep symptoms associated with intake of specific dietary nutrients. J. Sleep Res. 2014, 23, 22–34. [Google Scholar] [CrossRef]
- Godos, J.; Grosso, G.; Castellano, S.; Galvano, F.; Caraci, F.; Ferri, R. Association between diet and sleep quality: A systematic review. Sleep Med. Rev. 2021, 57, 101430. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Sellami, M.; Salem, I.; Conic, R.Z.; Kimak, M.; Pigatto, P.D.M.; Damiani, G. Fasting and Its Impact on Skin Anatomy, Physiology, and Physiopathology: A Comprehensive Review of the Literature. Nutrients 2019, 11, 249. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Trabelsi, K.; Garbarino, S.; Ammar, A.; Chtourou, H.; Pacifico, A.; Malagoli, P.; Kocic, H.; Conic, R.R.Z.; Young Dermatologists Italian Network; et al. Can intermittent, time-restricted circadian fasting modulate cutaneous severity of dermatological disorders? Insights from a multicenter, observational, prospective study. Dermatol Ther. 2021, 34, e14912. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Mahroum, N.; Pigatto, P.D.M.; Pacifico, A.; Malagoli, P.; Tiodorovic, D.; Conic, R.R.; Amital, H.; Bragazzi, N.L.; Watad, A.; et al. The Safety and Impact of a Model of Intermittent, Time-Restricted Circadian Fasting (“Ramadan Fasting”) on Hidradenitis Suppurativa: Insights from a Multicenter, Observational, Cross-Over, Pilot, Exploratory Study. Nutrients 2019, 11, 1781. [Google Scholar] [CrossRef]
- Adawi, M.; Damiani, G.; Bragazzi, N.L.; Bridgewood, C.; Pacifico, A.; Conic, R.R.Z.; Morrone, A.; Malagoli, P.; Pigatto, P.D.M.; Amital, H.; et al. The Impact of Intermittent Fasting (Ramadan Fasting) on Psoriatic Arthritis Disease Activity, Enthesitis, and Dactylitis: A Multicentre Study. Nutrients 2019, 11, 601. [Google Scholar] [CrossRef]
- Damiani, G.; Watad, A.; Bridgewood, C.; Pigatto, P.D.M.; Pacifico, A.; Malagoli, P.; Bragazzi, N.L.; Adawi, M. The Impact of Ramadan Fasting on the Reduction of PASI Score, in Moderate-To-Severe Psoriatic Patients: A Real-Life Multicenter Study. Nutrients 2019, 11, 277. [Google Scholar] [CrossRef]
- Scoditti, E.; Tumolo, M.R.; Garbarino, S. Mediterranean Diet on Sleep: A Health Alliance. Nutrients 2022, 14, 2998. [Google Scholar] [CrossRef]
- Castro-Diehl, C.; Wood, A.C.; Redline, S.; Reid, M.; Johnson, D.A.; Maras, J.E.; Jacobs, D.R., Jr.; Shea, S.; Crawford, A.; St-Onge, M.P. Medi-terranean diet pattern and sleep duration and insomnia symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep 2018, 41, zsy158. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Roberts, A.; Shechter, A.; Choudhury, A.R. Fiber and Saturated Fat Are Associated with Sleep Arousals and Slow Wave Sleep. J. Clin. Sleep Med. 2016, 12, 19–24. [Google Scholar] [CrossRef]
- Mercuri, S.R.; Gregorio, G.; Brianti, P. Quality of life of psoriasis patients measured by the PSOdisk: A new visual method for assessing the impact of the disease. G. Ital. Dermatol. Venereol. 2017, 152, 424–431. [Google Scholar] [CrossRef]
- Naldi, L.; Mercuri, S.R. Epidemiology of comorbidities in psoriasis. Dermatol Ther. 2010, 23, 114–118. [Google Scholar] [CrossRef]
- Paolino, G.; Di Nicola, M.R.; Currado, M.; Brianti, P.; Mercuri, S.R. Serum tryptase levels in patients with psoriasis: A pilot study for a possible useful biomarker. Clin. Exp. Dermatol. 2022, 47, 178–179. [Google Scholar] [CrossRef]
- Paolino, G.; Di Nicola, M.R.; Brianti, P.; Bianchi, V.G.; Mercuri, S.R. New onset atopic dermatitis and psoriasis in the same patients under biologic treatments: The role of systemic treatments as a possible trigger. Dermatol. Ther. 2022, e15814. [Google Scholar] [CrossRef]
- Damiani, G.; Allocco, F.; Young Dermatologists Italian Network; Malagoli, P. COVID-19 vaccination and patients with psoriasis under biologics: Real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers. Clin. Exp. Dermatol. 2021, 46, 1106–1108. [Google Scholar] [CrossRef]
- Zhou, Q.; Vadakekolathu, J.; Watad, A.; Sharif, K.; Russell, T.; Rowe, H.; Khan, A.; Millner, P.A.; Loughenbury, P.; Rao, A.; et al. SARS-CoV-2 Infection Induces Psoriatic Arthritis Flares and Enthesis Resident Plasmacytoid Dendritic Cell Type-1 Interferon Inhibition by JAK Antagonism Offer Novel Spondyloarthritis Pathogenesis Insights. Front Immunol. 2021, 12, 635018. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Cazzaniga, S.; Chimenti, S.; Maccarone, M.; Picardo, M.; Girolomoni, G.; Naldi, L.; Psocare Study Group. Latent tuberculosis infection in patients with chronic plaque psoriasis: Evidence from the Italian Psocare Registry. Br. J. Dermatol. 2015, 172, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, S.; Lo Scocco, G.; Schincaglia, E.; Mercuri, S.R.; Chimenti, S.; Saraceno, R.; Naldi, L. Randomized, within-patient, clinical trial comparing fluorine-synthetic fiber socks with standard cotton socks in improving plantar pustulosis. Dermatology 2014, 228, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, S.; Lo Scocco, G.; Schincaglia, E.; Mercuri, S.R.; Chimenti, S.; Saraceno, R.; Naldi, L. Double-blind, within-patient, randomized, clinical trial comparing fluorine-synthetic fiber socks with standard cotton socks in improving plantar psoriasis. J. Dermatol. Treat. 2014, 25, 26–29. [Google Scholar] [CrossRef]
- Naldi, L.; Mercuri, S.R. Smoking and psoriasis: From epidemiology to pathomechanisms. J. Investig. Dermatol. 2009, 129, 2741–2743. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Riccò, M.; Pacifico, A.; Malagoli, P.; Kridin, K.; Pigatto, P.; Damiani, G. COVID-19 knowledge prevents biologics discontinuation: Data from an Italian multicenter survey during RED-ZONE declaration. Dermatol. Ther. 2020, 33, e13508. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Controne, I.; Scoditti, E.; Buja, A.; Pacifico, A.; Kridin, K.; Fabbro, M.D.; Garbarino, S.; Damiani, G. Do Sleep Disorders and Western Diet Influence Psoriasis? A Scoping Review. Nutrients 2022, 14, 4324. https://doi.org/10.3390/nu14204324
Controne I, Scoditti E, Buja A, Pacifico A, Kridin K, Fabbro MD, Garbarino S, Damiani G. Do Sleep Disorders and Western Diet Influence Psoriasis? A Scoping Review. Nutrients. 2022; 14(20):4324. https://doi.org/10.3390/nu14204324
Chicago/Turabian StyleControne, Ilaria, Egeria Scoditti, Alessandra Buja, Alessia Pacifico, Khalaf Kridin, Massimo Del Fabbro, Sergio Garbarino, and Giovanni Damiani. 2022. "Do Sleep Disorders and Western Diet Influence Psoriasis? A Scoping Review" Nutrients 14, no. 20: 4324. https://doi.org/10.3390/nu14204324
APA StyleControne, I., Scoditti, E., Buja, A., Pacifico, A., Kridin, K., Fabbro, M. D., Garbarino, S., & Damiani, G. (2022). Do Sleep Disorders and Western Diet Influence Psoriasis? A Scoping Review. Nutrients, 14(20), 4324. https://doi.org/10.3390/nu14204324










