Physicochemical Properties of the Soluble Dietary Fiber from Laminaria japonica and Its Role in the Regulation of Type 2 Diabetes Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. SDF Preparation
2.3. Functional Properties
2.3.1. Water-Holding Capacity (WHC)
2.3.2. Oil-Holding Capacity (OHC)
2.3.3. Water-Swelling Capacity (WSC)
2.3.4. Glucose Absorption Capacity (GAC)
2.3.5. Cholesterol Adsorption Capacity (CAC)
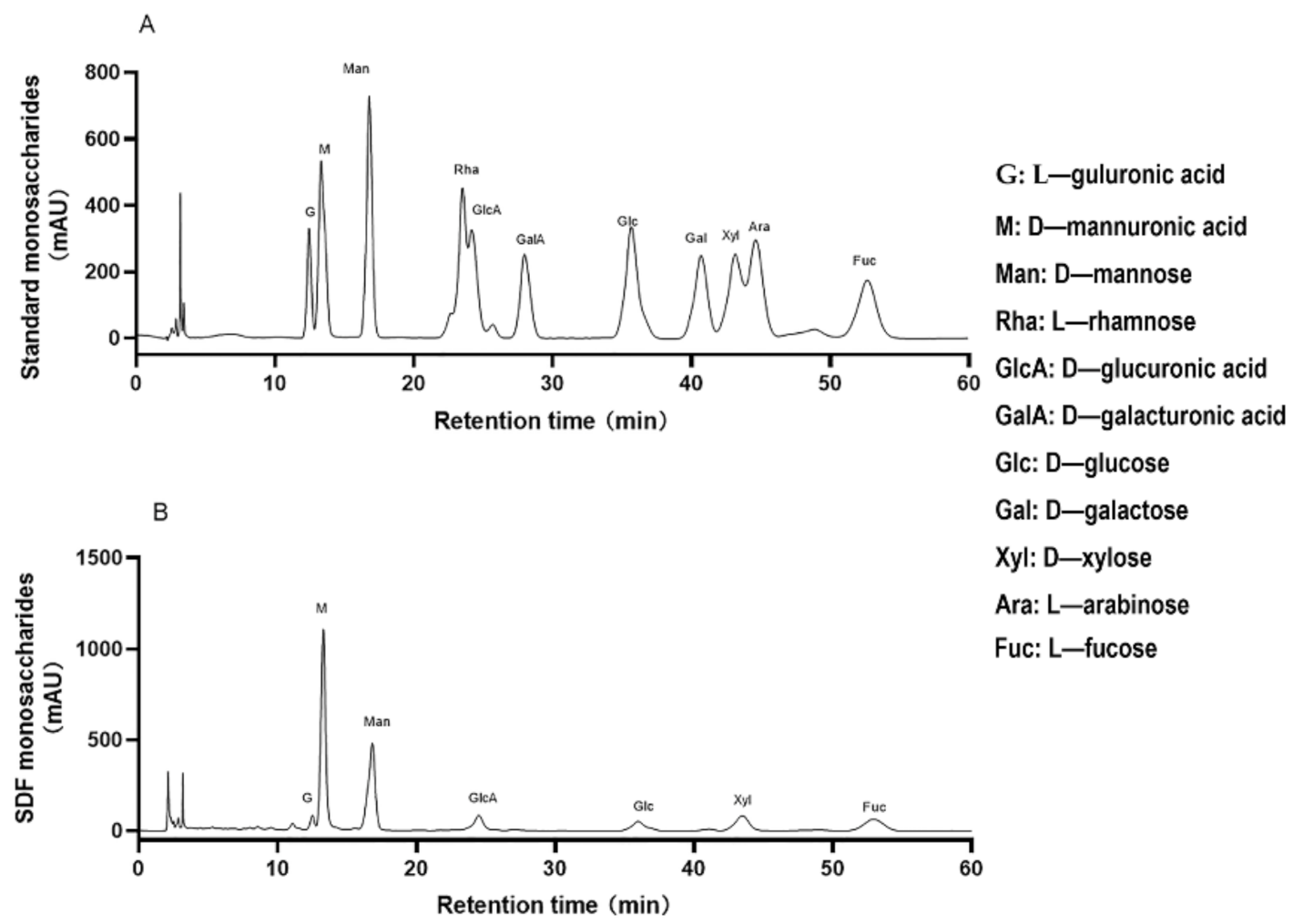
2.4. Monosaccharide Composition and Molecular Weight of SDF
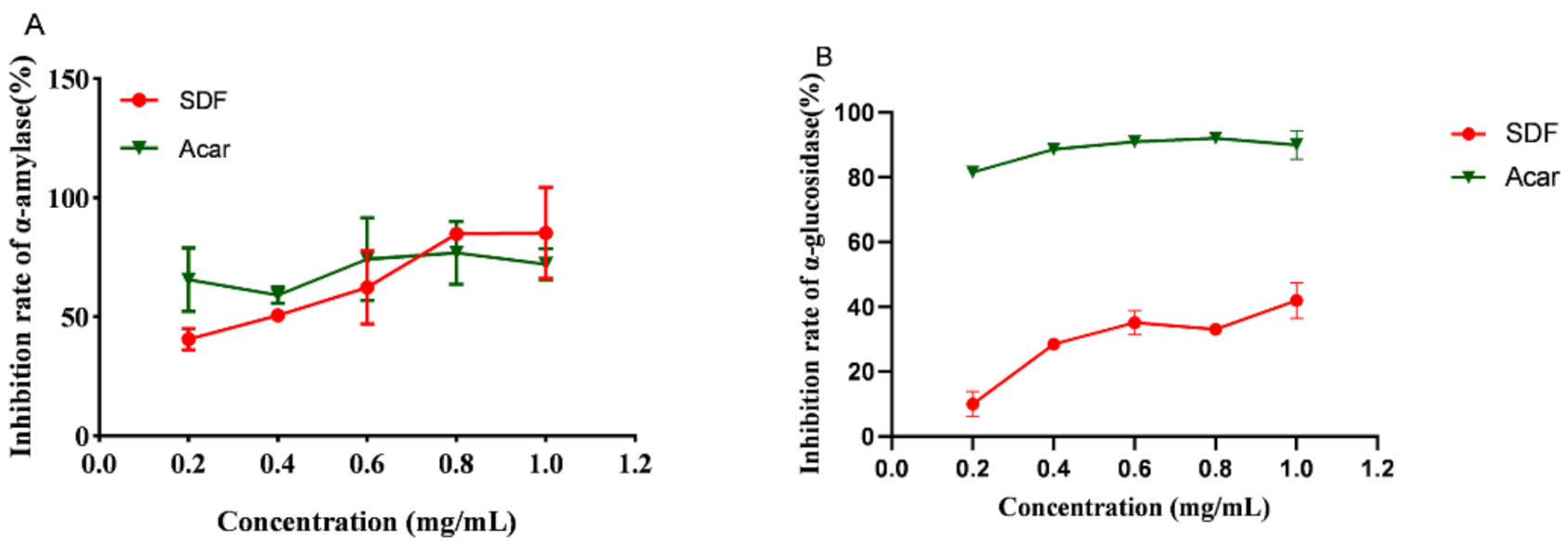
2.5. Inhibition of α-Amylase Activity by SDF
2.6. Inhibition of α-Glucosidase Activity by SDF
2.7. Animal Treatments and Sample Collection
2.8. Extraction of Genome DNA and Amplicon Generation
2.9. Detection of Fecal Metabolites
2.10. Statistical and Bioinformatics Analysis
3. Results and Discussion
3.1. The Functional Properties of SDF
3.2. Monosaccharide Composition and Molecular Weight of SDF
3.3. SDF Inhibition of α-Amylase and α-Glucosidase Activity
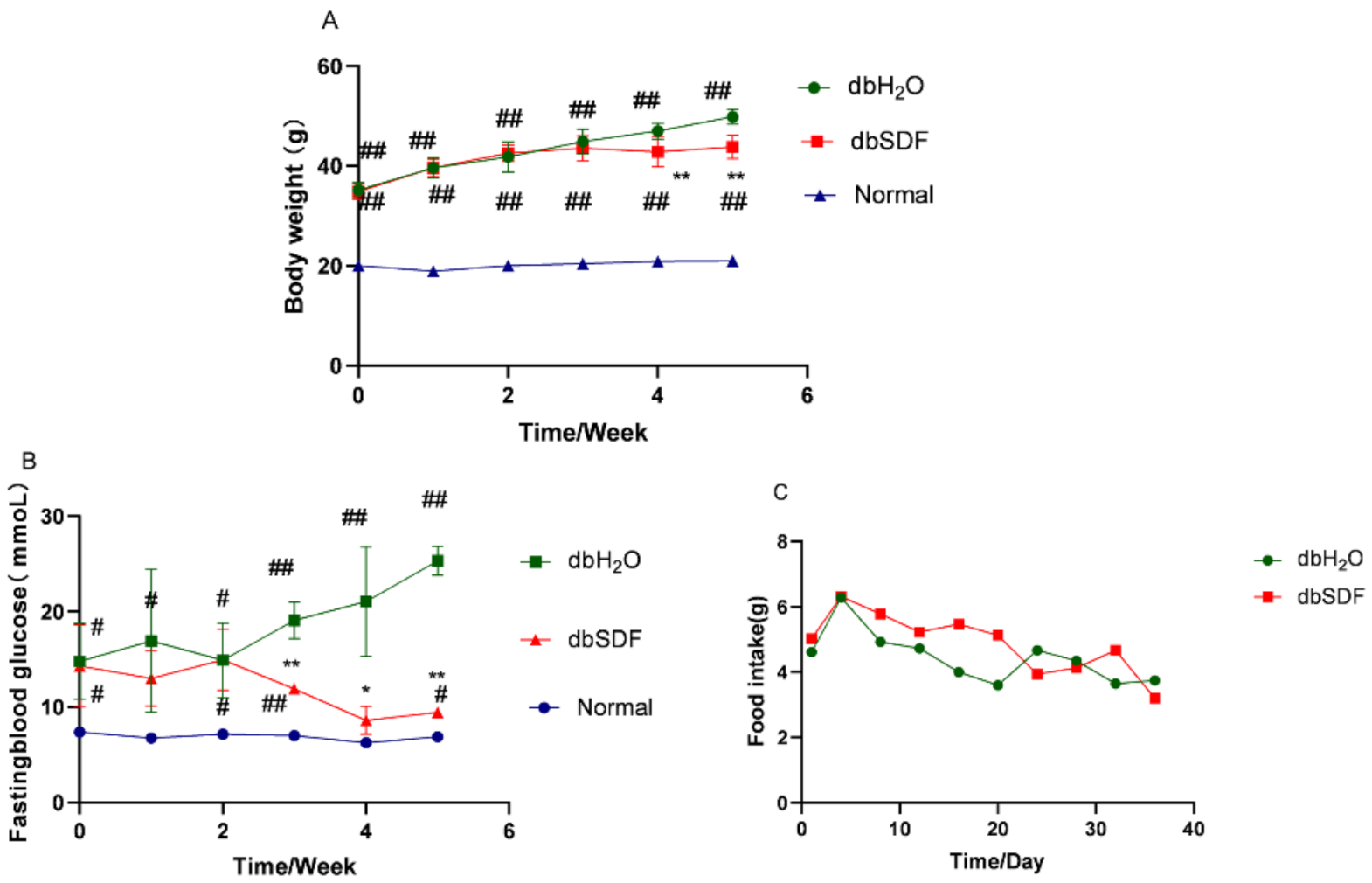
3.4. Effects of SDF on Body Weight and Fasting Blood Glucose
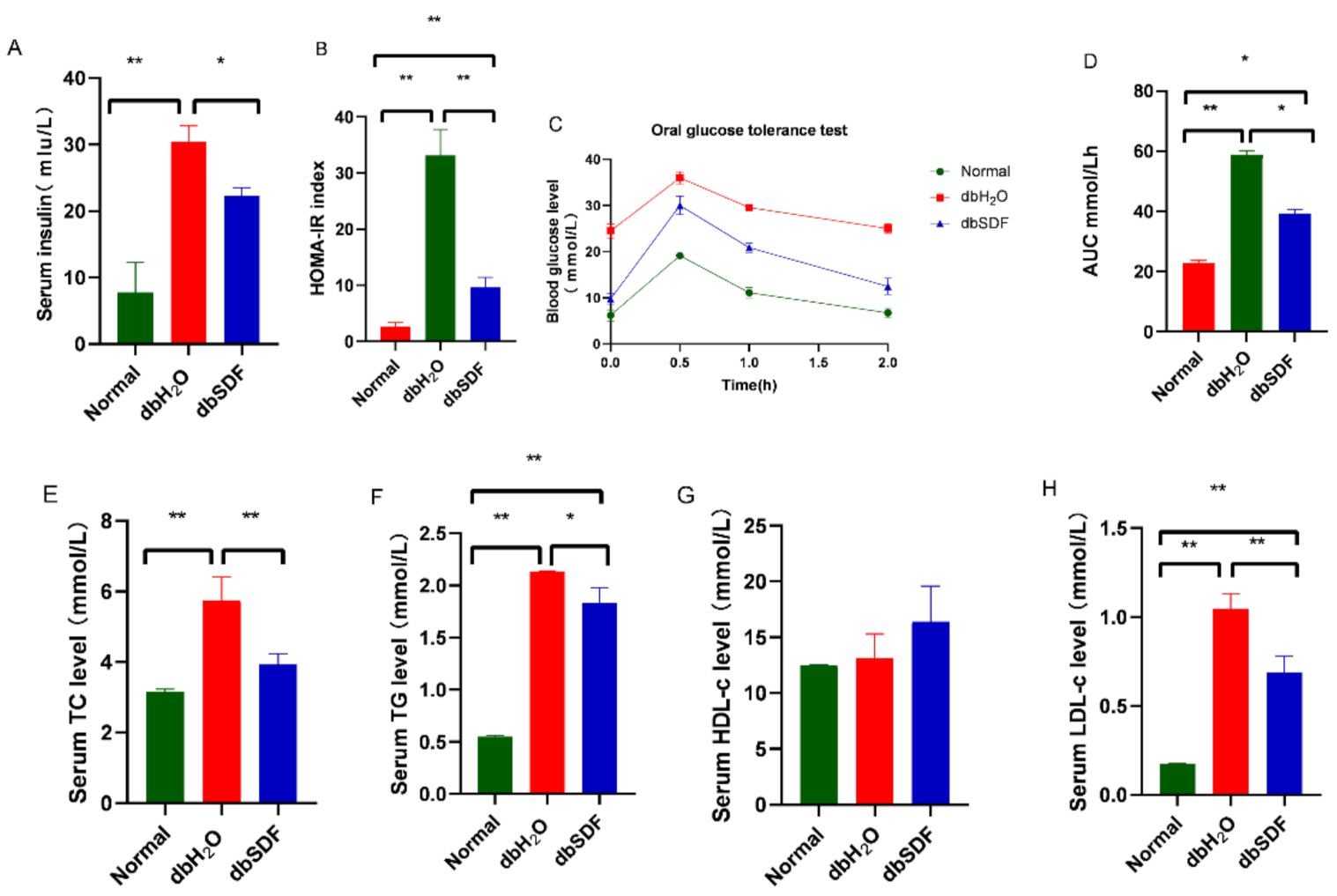
3.5. Effects of SDF on Insulin Resistance and Lipids
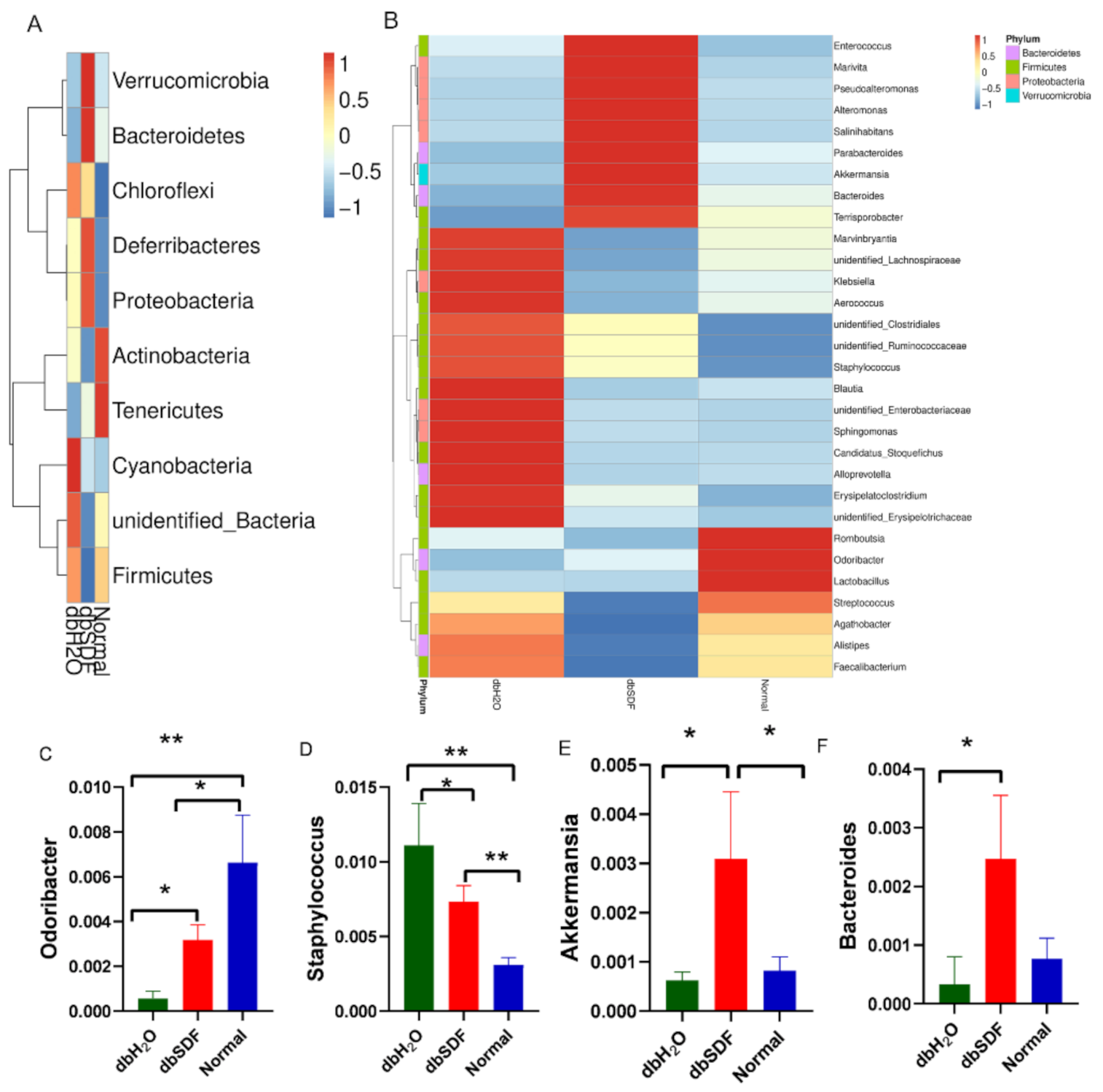
3.6. Effects of SDF on Intestinal Flora
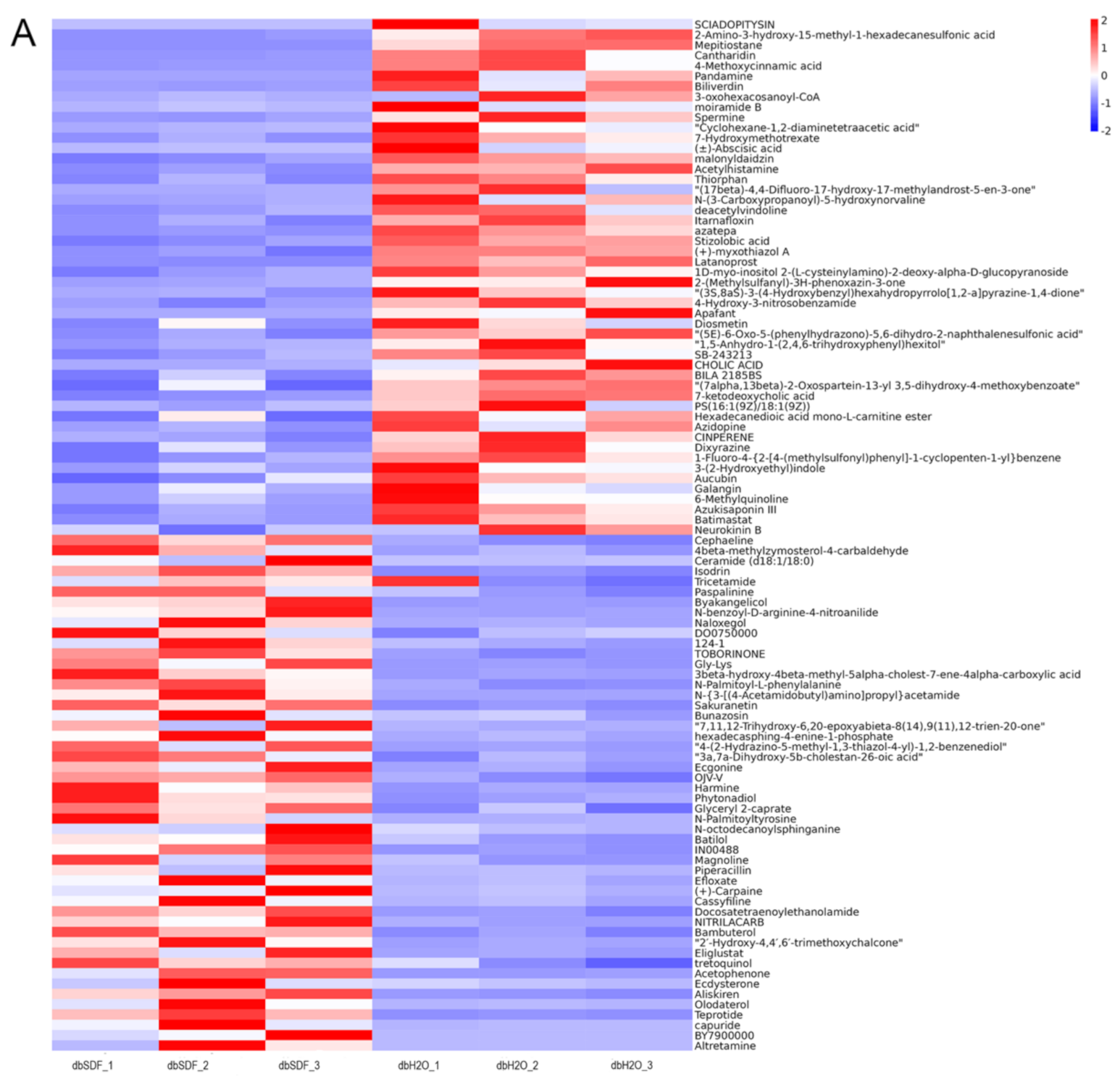
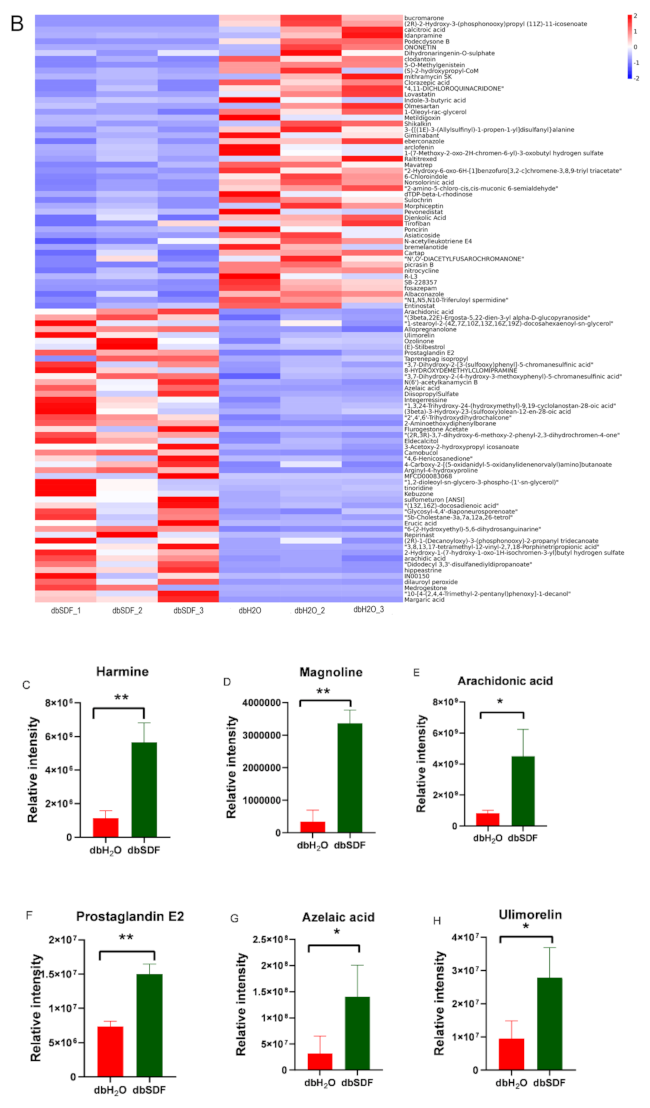
3.7. Effects of SDF on Intestinal Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Thomas, R.L.; Halim, S.; Gurudas, S.; Sivaprasad, S.; Owens, D.R. IDF diabetes atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res. Clin. Pract. 2019, 157, 107840. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 46, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, M.; Knight, R.; Leibel, R.L. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol. Metab. 2015, 26, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Van, H.M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Feng, B.; Li, P.; Tang, Z.; Wang, L. Microflora disturbance during progression of glucose intolerance and effect of sitagliptin: An animal study. J. Diabetes Res. 2016, 2016, 2093171. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, Y.; Zhang, M.; Pang, X.; Xu, J.; Kang, C.; Li, M.; Zhang, C.; Zhang, Z.; Zhang, Y.; et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS ONE 2012, 7, e42529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascale, A.; Marchesi, N.; Govoni, S.; Coppola, A.; Gazzaruso, C. The role of gut microbiota in obesity, diabetes mellitus, and effect of metformin: New insights into old diseases. Curr. Opin. Pharmacol. 2019, 49, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.; Souto, S.B.; Sánchez-López, E.; Machado, A.L.; Severino, P.; Jose, S.; Santini, A.; Fortuna, A.; García, M.L.; Silva, A.M.; et al. Sugar-lowering drugs for type 2 diabetes mellitus and metabolic syndrome-review of classical and new compounds: Part-I. Pharmaceuticals 2019, 12, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenglu, W.; Shuchang, L. A summary of kelp breeding and processing in Weihai. Mod. Fish. Inf. 1997, 11, 1–5. [Google Scholar]
- Yang, G.P.; Li, X.J.; Yuan, S.Y.; Zhang, Q.S.; Weeks, C.Y.; Li, T. Progress and achievements in the study of brown algae of kelp. J. Ocean. Univ. China Nat. Sci. Ed. 2005, 35, 564–570. [Google Scholar]
- Miao, J.L.; Li, G.Y.; Li, J. The Invention Discloses a Soluble Kelp Cellulose Water and a Preparation Method Thereof. CN102805407B, 21 August 2013. [Google Scholar]
- Li, F.C.; Tang, Z.H.; Cui, B.W.; Xi, Z.L.; Wang, H.R. Hypoglycemic effects of three kinds of kelp polysaccharides. China Mar. Med. 2000, 19, 12–15. [Google Scholar]
- Zhan, Y. Green Fine Chemical; Scientific and Technical Documentation Press: Beijing, China, 2009. [Google Scholar]
- Çakır, N.; Bilgiçli, N.; Yaver, E. Impact of xylanase-treated wheat milling by-products on the physical and chemical properties of cakes. J. Sci. Food Agric. 2021, 101, 6331–6337. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Yuan, F.; Fan, R.; Gao, Y. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem. 2015, 185, 90–98. [Google Scholar] [CrossRef]
- Femenia, A.; Lefebvre, A.C.; Thebaudin, J.Y. Physical and sensory properties of model foods supplemented with cauliflower fiber. J. Food Sci. 1997, 62, 635–639. [Google Scholar] [CrossRef]
- Yang, M. Study on Extraction of Soluble Dietary Fiber from Mung Bean Peel and Its Lowering Blood Lipid. Ph.D. Thesis, Jilin Agricultural University, Changchun, China, 2018. [Google Scholar]
- Ou, S.Y.; Zheng, Y.; Liu, Z.L.; Bao, H.Y.; Zhang, N. Adsorption of fat and cholesterol by different wheat bran materials. Mod. Food Technol. 2004, 20, 24–26. [Google Scholar]
- Qin, L.; He, M.; Yang, Y.; Fu, Z.; Tang, C.; Shao, Z.; Zhang, J.; Mao, W. Anticoagulant-active sulfated arabinogalactan from Chaetomorpha linum: Structural characterization and action on coagulation factors. Carbohydr. Polym. 2020, 242, 116394. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Huang, J.; Yuan, X.; Wang, Q.; Ma, L. Dietary fiber extracted from pomelo fruitlets promotes intestinal functions, both in vitro and in vivo. Carbohydr. Polym. 2021, 252, 117186. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Aderogba, M.A.; Amoo, S.O.; Stirk, W.A.; Van Staden, J. Potential antiradical and alpha-glucosidase inhibitors from Ecklonia maxima (Osbeck) Papenfuss. Food Chem. 2013, 141, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cao, Y.; Song, G.; Zhao, B.; Ma, Q.; Li, Z.; He, C. Anti-diabetic properties of genistein-chromium (III) complex in db/db diabetic mice and its sub-acute toxicity evaluation in normal mice. J. Trace Elem. Med. Biol. 2020, 62, 126606. [Google Scholar] [CrossRef]
- Liu, L.N.; He, Y.Y.; Wang, K.; Miao, J.L.; Zheng, Z. Metagenomics approach to the intestinal microbiome structure and function in high fat diet-induced obesity in mice fed with conjugated linoleic acid (CLA). Food Funct. 2020, 11, 9729–9739. [Google Scholar] [CrossRef]
- Sun, C.; Wu, X.; Chen, X.; Li, X.; Zheng, Z.; Jiang, S. Production and characterization of okara dietary fiber produced by fermentation with Monascus anka. Food Chem. 2020, 316, 126243. [Google Scholar] [CrossRef]
- Yan, X.; Ye, R.; Chen, Y. Blasting extrusion processing: The increase of soluble dietary fiber content and extraction of soluble-fiber polysaccharides from wheat bran. Food Chem. 2015, 180, 106–115. [Google Scholar] [CrossRef]
- Huang, J.Y.; Liao, J.S.; Qi, J.R.; Jiang, W.X.; Yang, X.Q. Structural and physicochemical properties of pectin-rich dietary fiber prepared from citrus peel. Food Hydrocoll. 2020, 110, 106140. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Zhong, F.; Mothibe, K.J. In vitro hypoglycemic and cholesterol lowering effects of dietary fiber prepared from cocoa (Theobroma cacao L.) shells. Food Funct. 2012, 3, 1044–1050. [Google Scholar] [CrossRef]
- Ding, Q.; Li, Z.; Wu, W.; Su, Y.; He, R. Physicochemical and functional properties of dietary fiber from Nannochloropsis oceanica: A comparison of alkaline and ultrasonic-assisted alkaline extractions. LWT-Food Sci. Technol. 2020, 113, 110080. [Google Scholar] [CrossRef]
- Du, X.; Wang, L.; Huang, X.; Jing, H.; Wang, H. Effects of different extraction methods on structure and properties of soluble dietary fiber from defatted coconut flour. LWT-Food Sci. Technol. 2021, 143, 111031. [Google Scholar] [CrossRef]
- Do Espirito Santo, B.L.S.; da Silva, É.C.; Jordão Cândido, C.; da Silva, A.F.; do Nascimento, V.A.; Reis Ballard, C.; Baú Betim Cazarin, C.; Roberto Maróstica Júnior, M.; Mach Côrtes Cordeiro, L.; Youssef Abboud, K.; et al. Dietary fiber chemical structures and physicochemical properties of edible Pouteria glomerata fruits, native from Brazilian Pantanal. Food Res. Int. 2020, 137, 109576. [Google Scholar] [CrossRef]
- Fan, S.Q.; Pang, J.L.; Chen, X.B.; Dai, Z.Y.; Fa, X.Q.; Dong, J.; Wang, X.Q. Functional properties of alginate and its application in gel products. Food Nutr. Sci. 2019, 8, 90–94. [Google Scholar]
- Sen, M. Effects of molecular weight and ratio of guluronic acid to mannuronic acid on the antioxidant properties of sodium alginate fractions prepared by radiation-induced degradation. Appl. Radiat. Isot. 2011, 69, 126–129. [Google Scholar] [CrossRef]
- Deng, M.; Lin, Y.; Dong, L.; Jia, X.; Zhang, R. Physicochemical and functional properties of dietary fiber from pummelo (Citrus grandis L. Osbeck) and grapefruit (Citrus paradisi Mcfad) cultivars. Food Biosci. 2021, 40, 100890. [Google Scholar] [CrossRef]
- Brownlee, I.A. The physiological roles of dietary fibre. Food Hydrocoll. 2011, 25, 238–250. [Google Scholar] [CrossRef]
- Cui, J.; Gu, X.; Zhang, Q.; Ou, Y.; Wang, J. Production and anti-diabetic activity of soluble dietary fiber from apricot pulp by Trichoderma viride fermentation. Food Funct. 2015, 6, 1635–1642. [Google Scholar] [CrossRef]
- Rasoamanana, R.; Even, P.C.; Darcel, N.; Tomé, D.; Fromentin, G. Dietary fibers reduce food intake by satiation without conditioned taste aversion in mice. Physiol. Behav. 2013, 110, 13–19. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Dai, Z.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Fuzhuan brick tea polysaccharides attenuate metabolic syndrome in high-fat diet induced mice in association with modulation in the gut microbiota. J. Agric. Food Chem. 2018, 66, 2783–2795. [Google Scholar] [CrossRef]
- Sanae, F.; Kamiyama, O.; Ikeda-Obatake, K.; Higashi, Y.; Asano, N.; Adachi, I.; Kato, A. Effects of eugenol-reduced clove extract on glycogen phosphorylase b and the development of diabetes in db/db mice. Food Funct. 2014, 5, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Q.; Huang, J.; Fang, D.; Zhuang, W.; Luo, X.; Zou, X.; Zheng, B.; Cao, H. Hypoglycemic effect of dietary fibers from bamboo shoot shell: An in vitro and in vivo study. Food Chem. Toxicol. 2019, 127, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Joo, H.; Kim, C.T.; Kim, I.H.; Kim, Y. High hydrostatic pressure extract of garlic increases the HDL cholesterol level via up-regulation of apolipoprotein A-I gene expression in rats fed a high-fat diet. Lipids Health Dis. 2012, 11, 77. [Google Scholar] [CrossRef] [Green Version]
- Ling, Z.; Kong, J.; Jia, P.; Wei, C.; Wang, Y.; Pan, Z.; Huang, W.; Li, L.; Chen, H.; Xiang, C. Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microb. Ecol. 2010, 60, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Jackson, M.A.; Pallister, T.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Gut microbiome diversity and high-fibre intake are related to lower long-term weight gain. Int. J. Obes. 2017, 41, 1099–1105. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Fu, Y.; Deng, Z.; Fan, Y.; Li, H. Effects of soluble dietary fiber from soybean residue fermented by Neurospora crassa on the intestinal flora in rats. Food Funct. 2020, 11, 7433–7445. [Google Scholar] [CrossRef]
- Kang, Y.; Li, Y.; Du, Y.; Guo, L.; Chen, M.; Huang, X.; Yang, F.; Hong, J.; Kong, X. Konjaku flour reduces obesity in mice by modulating the composition of the gut microbiota. Int. J. Obes. 2019, 43, 1631–1643. [Google Scholar] [CrossRef]
- Mou, D.; Li, S.; Yan, C.; Zhang, Q.; Wu, D. Dietary fiber sources for gestation sows: Evaluations based on combined in vitro and in vivo methodology. Anim. Feed. Sci. Technol. 2020, 269, 114636. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, C.; Zhai, Z.; Deng, Z.Y.; De Jonge, H.R.; Wu, X.; Ruan, Z. Uridine attenuates obesity, ameliorates hepatic lipid accumulation and modifies the gut microbiota composition in mice fed with a high-fat diet. Food Funct. 2021, 12, 1829–1840. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Hatziioanou, D.; Mayer, M.J.; Duncan, S.H.; Flint, H.J.; Narbad, A. A representative of the dominant human colonic Firmicutes, Roseburia faecis M72/1, forms a novel bacteriocin-like substance. Anaerobe 2013, 23, 5–8. [Google Scholar] [CrossRef]
- Brahe, L.K.; Astrup, A.; Larsen, L.H. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes. Rev. 2013, 14, 950–959. [Google Scholar] [CrossRef]
- Xing, J.; Li, X.; Sun, Y.; Zhao, J.; Miao, S.; Xiong, Q.; Zhang, Y.; Zhang, G. Comparative genomic and functional analysis of Akkermansia muciniphila and closely related species. Genes Genom. 2019, 41, 1253–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, E.C.; Lowe, E.C.; Chiang, H.; Pudlo, N.A.; Wu, M.; McNulty, N.P.; Abbott, D.W.; Henrissat, B.; Gilbert, H.J.; Bolam, D.N.; et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011, 9, e1001221. [Google Scholar] [CrossRef]
- Patnode, M.L.; Beller, Z.W.; Han, N.D.; Cheng, J.; Peters, S.L.; Terrapon, N.; Henrissat, B.; Le Gall, S.; Saulnier, L.; Hayashi, D.K.; et al. Interspecies competition impacts targeted manipulation of human gut bacteria by fiber-derived glycans. Cell 2019, 179, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Bradlow, H.L. Obesity and the gut microbiome: Pathophysiological aspects. Horm. Mol. Biol. Clin. Investig. 2014, 17, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, F.J.; Guan, Z.C.; Dong, F.T.; Cheng, J.H.; Gao, Y.P.; Li, D.; Yan, J.; Liu, C.H.; Han, D.P.; et al. The extracellular domain of Staphylococcus aureus LtaS binds insulin and induces insulin resistance during infection. Nat. Microbiol. 2018, 3, 622–631. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Hase, K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 2014, 10, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Waki, H.; Park, K.W.; Mitro, N.; Pei, L.; Damoiseaux, R.; Wilpitz, D.C.; Reue, K.; Saez, E.; Tontonoz, P. The small molecule harmine is an antidiabetic cell-type-specific regulator of PPARgamma expression. Cell Metab. 2007, 5, 357–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, J.M.; Moore, L.B.; Smith-Oliver, T.A.; Wilkison, W.O.; Willson, T.M.; Kliewer, S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ). J. Biol. Chem. 1995, 270, 12953–12956. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Alvarez-Perez, J.C.; Felsenfeld, D.P.; Liu, H.; Sivendran, S.; Bender, A.; Kumar, A.; Sanchez, R.; Scott, D.K.; Garcia-Ocaña, A.; et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat. Med. 2015, 21, 383–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Li, D.; Yu, S. Pharmacological effects of harmine and its derivatives: A review. Arch. Pharm. Res. 2020, 43, 1259–1275. [Google Scholar] [CrossRef]
- Demeure, C.E.; Yang, L.P.; Desjardins, C.; Raynauld, P.; Delespesse, G. Prostaglandin E2 primes naive T cells for the production of anti-inflammatory cytokines. Eur. J. Immunol. 1997, 27, 3526–3531. [Google Scholar] [CrossRef]
- James, J.; Mair, S.; Doll, W.; Sandefer, E.; Wurtman, D.; Maurer, A.; Deane, A.M.; Harris, M.S. The effects of ulimorelin, a ghrelin agonist, on liquid gastric emptying and colonic transit in humans. Neurogastroenterol. Motil. 2020, 32, e13784. [Google Scholar] [CrossRef]
- Akamatsu, H.; Komura, J.; Asada, Y.; Miyachi, Y.; Niwa, Y. Inhibitory effect of azelaic acid on neutrophil functions: A possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch. Dermatol. Res. 1991, 283, 162–166. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, F.; Hao, L.; Wang, N. Effects of magnoline on P-selectin’s expression in diabetic rats and its reno-protection. Kidney Blood Press. Res. 2013, 37, 211–220. [Google Scholar] [CrossRef] [PubMed]

| WHC (g/g) | OHC (g/g) | WSC (mL/g) | GAC (mmol/g) | CAC (mg/g) pH = 7 | CAC (mg/g) pH = 2 |
|---|---|---|---|---|---|
| 6.00 ± 0.3 | 1.70 ± 0.2 | 9.80 ± 0.2 | 24.70 ± 0.56 | 5.07 ± 0.2 | 2.42 ± 0.5 |
| Molecular Weight Distribution (MWD) | Proportion |
|---|---|
| 31,519–54,000 | 73.50% |
| 54,000–115,000 | 22.70% |
| 115,000–185,000 | 3.00% |
| 185,000–41,497 | 0.80% |
| 31,519–54,000 | 73.50% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, L.; Qin, L.; Wang, Y.; Chen, F.; Qu, C.; Miao, J. Physicochemical Properties of the Soluble Dietary Fiber from Laminaria japonica and Its Role in the Regulation of Type 2 Diabetes Mice. Nutrients 2022, 14, 329. https://doi.org/10.3390/nu14020329
Wang X, Zhang L, Qin L, Wang Y, Chen F, Qu C, Miao J. Physicochemical Properties of the Soluble Dietary Fiber from Laminaria japonica and Its Role in the Regulation of Type 2 Diabetes Mice. Nutrients. 2022; 14(2):329. https://doi.org/10.3390/nu14020329
Chicago/Turabian StyleWang, Xixi, Liping Zhang, Ling Qin, Yanfeng Wang, Fushan Chen, Changfeng Qu, and Jinlai Miao. 2022. "Physicochemical Properties of the Soluble Dietary Fiber from Laminaria japonica and Its Role in the Regulation of Type 2 Diabetes Mice" Nutrients 14, no. 2: 329. https://doi.org/10.3390/nu14020329
APA StyleWang, X., Zhang, L., Qin, L., Wang, Y., Chen, F., Qu, C., & Miao, J. (2022). Physicochemical Properties of the Soluble Dietary Fiber from Laminaria japonica and Its Role in the Regulation of Type 2 Diabetes Mice. Nutrients, 14(2), 329. https://doi.org/10.3390/nu14020329







