Initial Serum Magnesium Level Is Associated with Mortality Risk in Traumatic Brain Injury Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Statistical Analysis
3. Results
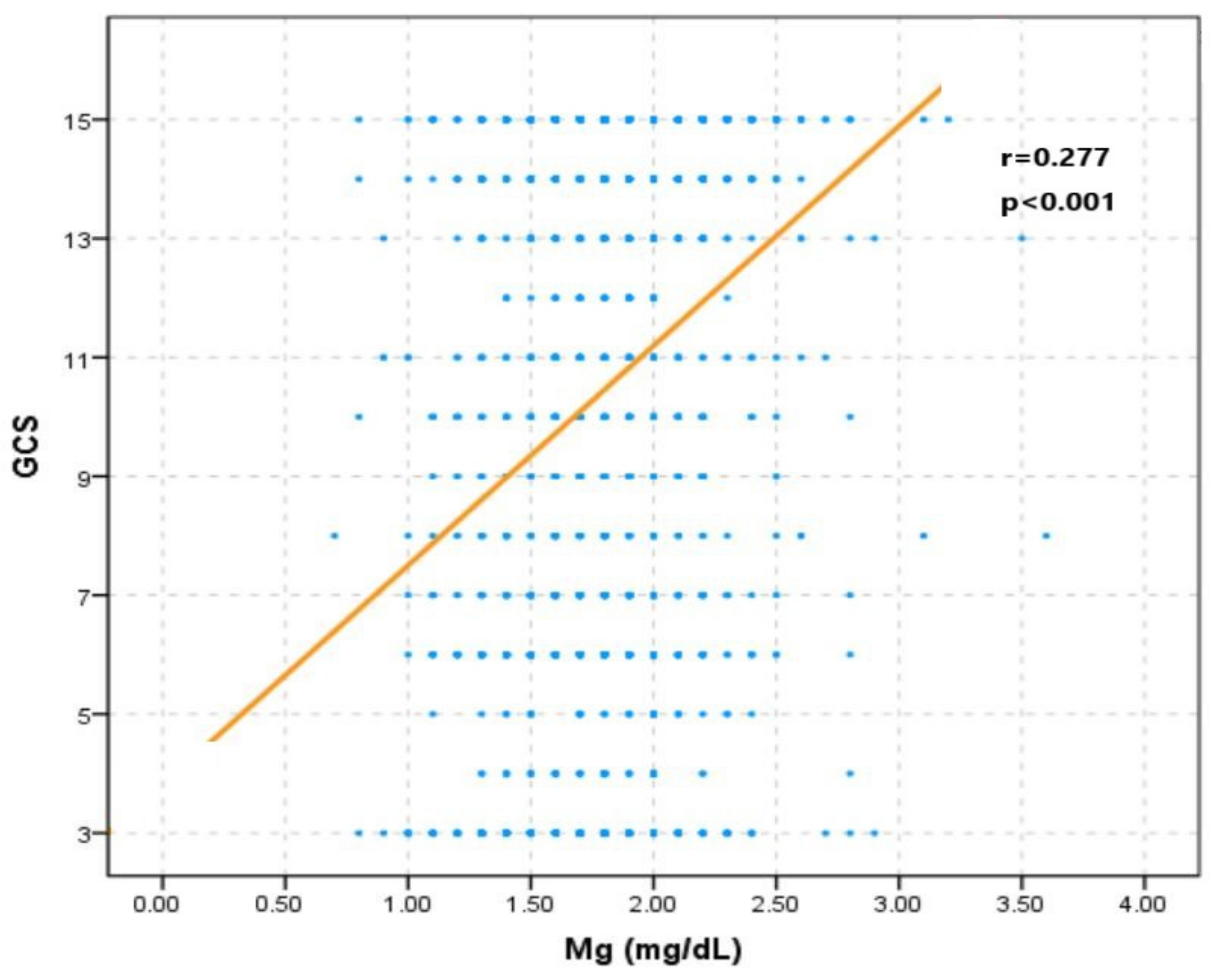
3.1. Baseline Characteristics of TBI Participants
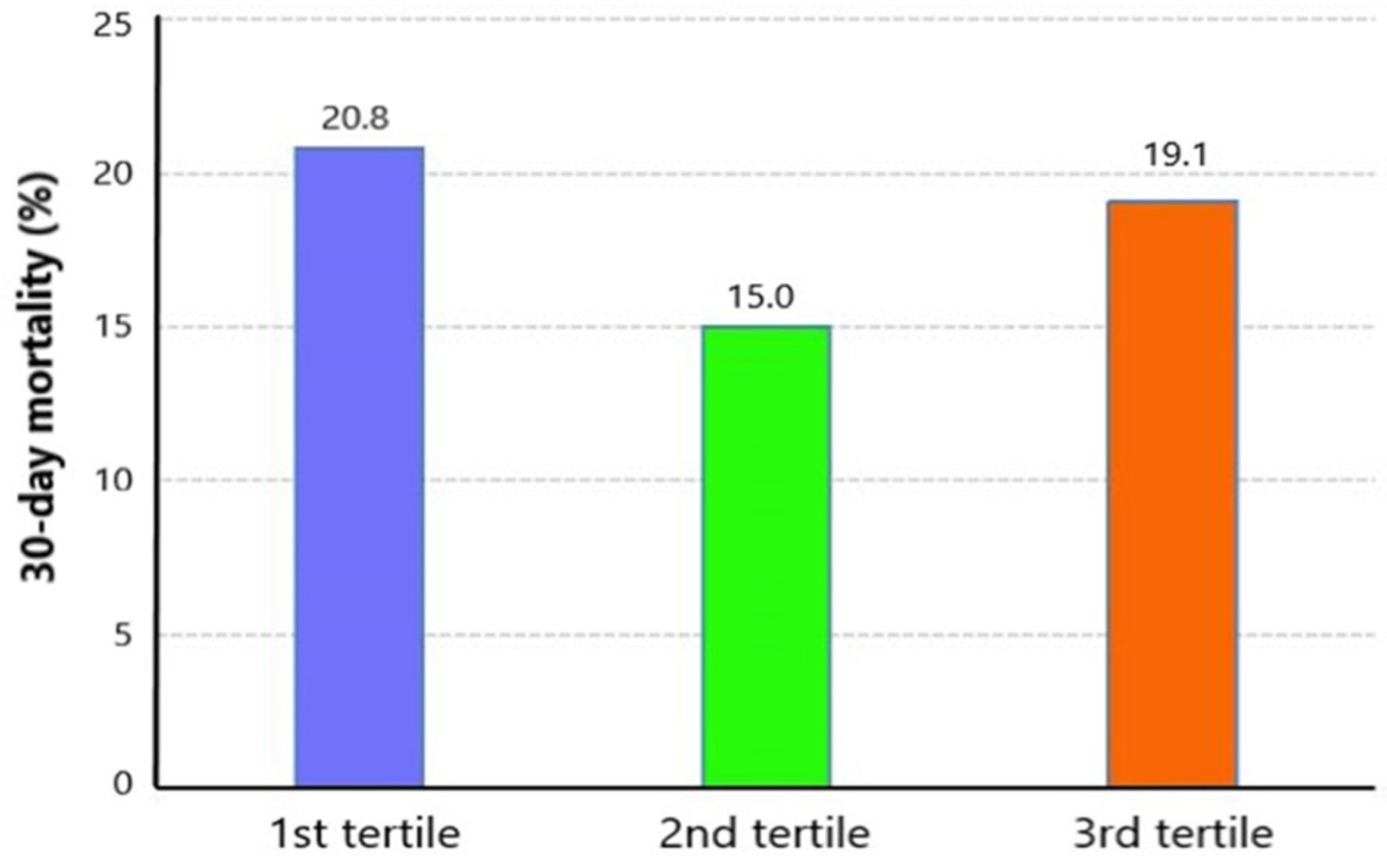
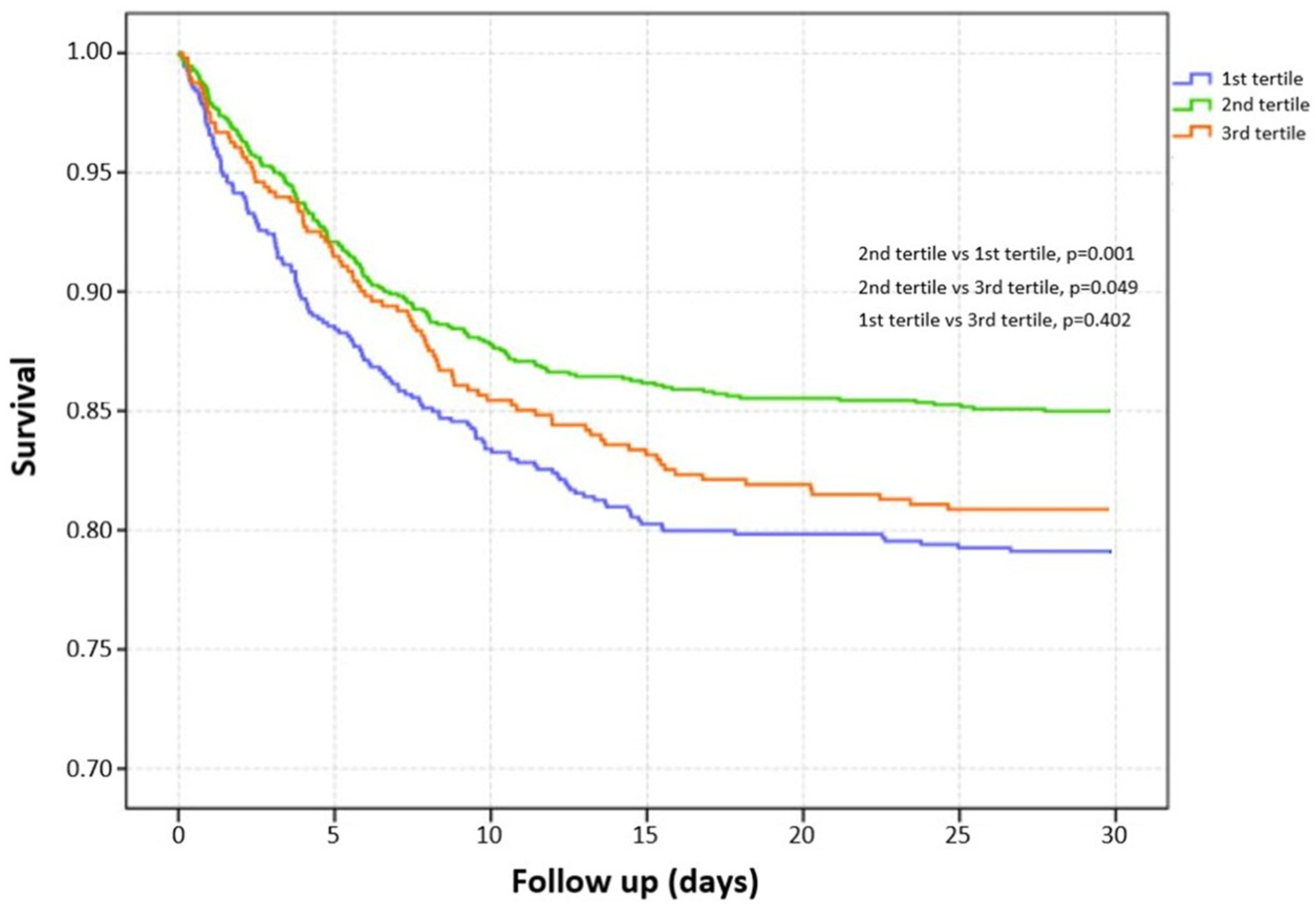
3.2. Unadjusted Association between Serum Magnesium Level and Risk of Mortality
3.3. Adjusted Association between Serum Magnesium Level and Risk of Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Fleminger, S.; Ponsford, J. Long term outcome after traumatic brain injury. BMJ 2005, 331, 1419–1420. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Q.; Shi, Y.C.; Lin, S.; Chen, X.R. Metabolic disorders on cognitive dysfunction after traumatic brain injury. Trends Endocrinol. Metab. 2022, 33, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Corral, L.; Javierre, C.F.; Ventura, J.L.; Marcos, P.; Herrero, J.I.; Manez, R. Impact of non-neurological complications in severe traumatic brain injury outcome. Crit. Care 2012, 16, R44. [Google Scholar] [CrossRef] [PubMed]
- Pin-On, P.; Saringkarinkul, A.; Punjasawadwong, Y.; Kacha, S.; Wilairat, D. Serum electrolyte imbalance and prognostic factors of postoperative death in adult traumatic brain injury patients: A prospective cohort study. Medicine 2018, 97, e13081. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Kumar, R.; Tarat, A. Evaluation of Electrolyte Imbalance in Patients with Traumatic Brain Injury Admitted in the Central ICU of a Tertiary Care Centre: A Prospective Observational Study. Cureus 2021, 13, e17517. [Google Scholar] [CrossRef]
- Altura, B.M. Basic biochemistry and physiology of magnesium: A brief review. Magnes. Trace Elem. 1991, 10, 167–171. [Google Scholar] [PubMed]
- Volpe, S.L. Magnesium in disease prevention and overall health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef]
- Laupland, K.B.; Tabah, A.; Jacobs, N.; Ramanan, M. Determinants of serum magnesium abnormalities and outcome among admissions to the intensive care unit. Anaesth. Crit. Care Pain Med. 2020, 39, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Hansrivijit, P.; Thirunavukkarasu, S.; Chewcharat, A.; Medaura, J.; Mao, M.A.; Kashani, K.B. Association of serum magnesium level change with in-hospital mortality. BMJ Evid. Based Med. 2020, 25, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; You, S.; Huang, Z.; Han, Q.; Zhong, C.; Xu, J.; Shi, R.; Chen, D.; Zhang, Y.; Xiao, G.; et al. Prognostic Significance of Serum Magnesium in Acute Intracerebral Hemorrhage Patients. Curr. Neurovascular Res. 2019, 16, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Tsivgoulis, G.; Malhotra, K.; Houck, A.L.; Khorchid, Y.M.; Pandhi, A.; Inoa, V.; Alsherbini, K.; Alexandrov, A.V.; Arthur, A.S.; et al. Serum Magnesium Levels and Outcomes in Patients with Acute Spontaneous Intracerebral Hemorrhage. J. Am. Heart Assoc. 2018, 7, e008698. [Google Scholar] [CrossRef] [PubMed]
- Liotta, E.M.; Prabhakaran, S.; Sangha, R.S.; Bush, R.A.; Long, A.E.; Trevick, S.A.; Potts, M.B.; Jahromi, B.S.; Kim, M.; Manno, E.M.; et al. Magnesium, hemostasis, and outcomes in patients with intracerebral hemorrhage. Neurology 2017, 89, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Niu, X.; Hu, J.; Zhou, M.; Liang, H.; Zhang, Y.; Tong, W.; Xu, T. Relationship of serum magnesium concentration to risk of short-term outcome of acute ischemic stroke. Blood Press. 2013, 22, 297–301. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Zhong, C.; Du, H.; Zhang, Y.; Zheng, D.; Wang, X.; Qiu, C.; Zhao, H.; Cao, Y.; Liu, C.-F. Admission Low Magnesium Level Is Associated with In-Hospital Mortality in Acute Ischemic Stroke Patients. Cerebrovasc. Dis. 2017, 44, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Attry, S.; Ghosh, S.N. Serum Magnesium as a Marker of Neurological Outcome in Severe Traumatic Brain Injury Patients. Asian J. Neurosurg. 2018, 13, 685–688. [Google Scholar] [CrossRef]
- Stippler, M.; Fischer, M.R.; Puccio, A.M.; Wisniewski, S.R.; Carson-Walter, E.B.; Dixon, C.E.; Walter, K.A. Serum and cerebrospinal fluid magnesium in severe traumatic brain injury outcome. J. Neurotrauma 2007, 24, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Xu, Q.; Li, C.; Liu, J.; Shi, R. High-Normal Serum Magnesium and Hypermagnesemia Are Associated with Increased 30-Day In-Hospital Mortality: A Retrospective Cohort Study. Front. Cardiovasc. Med. 2021, 8, 625133. [Google Scholar] [CrossRef]
- Broman, M.; Hansson, F.; Klarin, B. Analysis of hypo- and hypermagnesemia in an intensive care unit cohort. Acta Anaesthesiol. Scand. 2018, 62, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Corbi, G.; Acanfora, D.; Iannuzzi, G.L.; Longobardi, G.; Cacciatore, F.; Furgi, G.; Filippelli, A.; Rengo, G.; Leosco, D.; Ferrara, N. Hypermagnesemia predicts mortality in elderly with congestive heart disease: Relationship with laxative and antacid use. Rejuvenation Res. 2008, 11, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.S.; Burdmann, E.A.; Vieira, E.A.; Ferreira, M.L.; Ferreira, A.P.; Inda-Filho, A.J. Association of magnesium abnormalities at intensive care unit admission with kidney outcomes and mortality: A prospective cohort study. Clin. Exp. Nephrol. 2022, 26, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Morooka, H.; Tanaka, A.; Kasugai, D.; Ozaki, M.; Numaguchi, A.; Maruyama, S. Abnormal magnesium levels and their impact on death and acute kidney injury in critically ill children. Pediatr. Nephrol. 2022, 37, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Haider, D.G.; Lindner, G.; Ahmad, S.S.; Sauter, T.; Wolzt, M.; Leichtle, A.B.; Fiedler, G.-M.; Exadaktylos, A.K.; Fuhrmann, V. Hypermagnesemia is a strong independent risk factor for mortality in critically ill patients: Results from a cross-sectional study. Eur. J. Intern. Med. 2015, 26, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.C.; Tomasi, C.D.; Constantino, L.; Giombelli, V.; Candal, R.; Bristot, M.D.L.; Topanotti, M.F.; Burdmann, E.A.; Dal-Pizzol, F.; Fraga, C.M.; et al. Hypomagnesemia as a risk factor for the non-recovery of the renal function in critically ill patients with acute kidney injury. Nephrol. Dial. Transplant. 2013, 28, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Saleem, A.F. On admission hypomagnesemia in critically ill children: Risk factors and outcome. Indian J. Pediatr. 2009, 76, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Ji, M.J.; Wang, X.M.; Wang, S.Q.; Sun, J.; Ma, C.M. Hospital-acquired dysmagnesemia and mortality in critically ill patients: Data from MIMIC-III database. Magnes. Res. 2021, 34, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xun, P.; Unverzagt, F.; McClure, L.A.; Irvin, M.R.; Judd, S.; Cushman, M.; He, K. Serum magnesium concentration and incident cognitive impairment: The reasons for geographic and racial differences in stroke study. Eur. J. Nutr. 2021, 60, 1511–1520. [Google Scholar] [CrossRef]
- Behrouz, R.; Hafeez, S.; Mutgi, S.A.; Zakaria, A.; Miller, C.M. Hypomagnesemia in Intracerebral Hemorrhage. World Neurosurg. 2015, 84, 1929–1932. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, W.M.; Algra, A.; van der Sprenkel, J.W.; Tulleken, C.A.; Rinkel, G.J. Hypomagnesemia after aneurysmal subarachnoid hemorrhage. Neurosurgery 2003, 52, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, S.; Ozgurtas, T.; Kayali, H.; Atabey, C.; Kutluay, T.; Timurkaynak, E. Monitoring of serum ionized magnesium in neurosurgical intensive care unit: Preliminary results. Clin. Chim. Acta Int. J. Clin. Chem. 2003, 334, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Mendez, D.R.; Corbett, R.; Macias, C.; Laptook, A. Total and ionized plasma magnesium concentrations in children after traumatic brain injury. Pediatr. Res. 2005, 57, 347–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bareyre, F.M.; Saatman, K.E.; Raghupathi, R.; McIntosh, T.K. Postinjury treatment with magnesium chloride attenuates cortical damage after traumatic brain injury in rats. J. Neurotrauma 2000, 17, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Bareyre, F.M.; Saatman, K.E.; Helfaer, M.A.; Sinson, G.; Weisser, J.D.; Brown, A.L.; McIntosh, T.K. Alterations in ionized and total blood magnesium after experimental traumatic brain injury: Relationship to neurobehavioral outcome and neuroprotective efficacy of magnesium chloride. J. Neurochem. 1999, 73, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Vink, R.; O’Connor, C.A.; Nimmo, A.J.; Heath, D.L. Magnesium attenuates persistent functional deficits following diffuse traumatic brain injury in rats. Neurosci. Lett. 2003, 336, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Esen, F.; Erdem, T.; Aktan, D.; Kalayci, R.; Çakar, N.; Kaya, M.; Telci, L. Effects of magnesium administration on brain edema and blood-brain barrier breakdown after experimental traumatic brain injury in rats. J. Neurosurg. Anesthesiol. 2003, 15, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Temkin, N.R.; Anderson, G.D.; Winn, H.R.; Ellenbogen, R.G.; Britz, G.W.; Schuster, J.; Lucas, T.; Newell, D.W.; Mansfield, P.N.; Machamer, J.E.; et al. Magnesium sulfate for neuroprotection after traumatic brain injury: A randomised controlled trial. Lancet Neurol. 2007, 6, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Arango, M.F.; Bainbridge, D. Magnesium for acute traumatic brain injury. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Dhandapani, S.S.; Gupta, A.; Vivekanandhan, S.; Sharma, B.S.; Mahapatra, A.K. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. Indian J. Neurotrauma 2008, 5, 27–33. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, W.; Zhong, J.; Li, Y.; Cheng, Y.; Su, Z.; Zheng, W.; Guan, X.-D. The effects of magnesium sulfate therapy after severe diffuse axonal injury. Ther. Clin. Risk Manag. 2016, 12, 1481–1486. [Google Scholar] [CrossRef]
- Lee, J.S.; Han, Y.M.; Yoo, D.S.; Choi, S.J.; Choi, B.H.; Kim, J.H.; Kim, Y.H.; Huh, P.W.; Ko, Y.J.; Rha, H.K.; et al. A molecular basis for the efficacy of magnesium treatment following traumatic brain injury in rats. J. Neurotrauma 2004, 21, 549–561. [Google Scholar] [CrossRef]
- Lee, J.S.; Han, Y.M.; Yoo, D.S.; Choi, S.J.; Choi, B.H.; Kim, J.H.; Kim, Y.H.; Huh, P.W.; Ko, Y.J.; Rha, H.K.; et al. Plasma magnesium concentrations and clinical outcomes in aneurysmal subarachnoid hemorrhage patients: Post hoc analysis of intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage trial. Stroke 2010, 41, 1841–1844. [Google Scholar] [CrossRef] [PubMed]
- Celi, L.A.; Scott, D.J.; Lee, J.; Nelson, R.; Alper, S.L.; Mukamal, K.J.; Mark, R.G.; Danziger, J. Association of hypermagnesemia and blood pressure in the critically ill. J. Hypertens. 2013, 31, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- McKee, J.A.; Brewer, R.P.; Macy, G.E.; Phillips-Bute, B.; Campbell, K.A.; Borel, C.O.; Reynolds, J.D.; Warner, D.S. Analysis of the brain bioavailability of peripherally administered magnesium sulfate: A study in humans with acute brain injury undergoing prolonged induced hypermagnesemia. Crit. Care Med. 2005, 33, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Takasu, A.; Saitoh, D.; Kaneko, N.; Yanagawa, Y.; Okada, Y. Ionized magnesium in the cerebrospinal fluid of patients with head injuries. J. Trauma 2005, 58, 1103–1109. [Google Scholar] [CrossRef] [PubMed]



| Variables | Overall Patients (n = 2280) | Survivors (n = 1876, 83.0%) | Non-Survivors (n = 404, 17.0%) | p |
|---|---|---|---|---|
| Age (years) | 65 (44–81) | 61 (41–79) | 78 (61–86) | <0.001 |
| Male gender (%) | 1400 (61.4%) | 1162 (61.9%) | 238 (58.9%) | 0.281 |
| Comorbidities | ||||
| Diabetes (%) | 351 (15.4%) | 269 (14.3%) | 82 (20.3%) | 0.003 |
| Hypertension (%) | 844 (37.0%) | 685 (36.5%) | 159 (39.4%) | 0.309 |
| Hyperlipidemia (%) | 298 (13.1%) | 243 (13.0%) | 55 (13.6%) | 0.783 |
| Chronic heart disease (%) | 293 (12.9%) | 235 (12.5%) | 58 (14.4%) | 0.360 |
| History of myocardial infarction (%) | 83 (3.6%) | 70 (3.7%) | 13 (3.2%) | 0.724 |
| Cerebral vascular disease (%) | 41 (1.8%) | 32 (1.7%) | 9 (2.2%) | 0.610 |
| Chronic liver disease (%) | 94 (4.1%) | 78 (4.2%) | 16 (4.0%) | 0.966 |
| Chronic renal disease (%) | 153 (6.7%) | 103 (5.5%) | 50 (12.4%) | <0.001 |
| Vital signs on admission | ||||
| Systolic blood pressure (mmHg) | 132 (117–147) | 132 (118–147) | 132 (113–148) | 0.165 |
| Diastolic blood pressure (mmHg) | 67 (56–77) | 67 (57–78) | 66 (53–75) | 0.003 |
| Heart rate (s−1) | 83 (72–96) | 84 (72–96) | 83 (70–96) | 0.217 |
| SpO2 (%) | 99 (97–100) | 99 (97–100) | 100 (98–100) | 0.015 |
| GCS | 12 (6–15) | 14 (7–15) | 6 (3–11) | <0.001 |
| ISS | 16 (16–25) | 16 (16–22) | 20 (16–25) | <0.001 |
| Intracranial injury types | ||||
| Epidural hematoma (%) | 543 (23.8%) | 443 (23.6%) | 100 (24.8%) | 0.672 |
| Subdural hematoma (%) | 1319 (57.9%) | 1085 (57.8%) | 234 (57.9%) | 1.000 |
| Subarachnoid hemorrhage (%) | 958 (42.0%) | 775 (41.3%) | 183 (45.3%) | 0.157 |
| Intraparenchymal hemorrhage (%) | 447 (19.6%) | 377 (20.1%) | 70 (17.3%) | 0.229 |
| Laboratory tests | ||||
| WBC (×109/L) | 11.60 (8.40–15.70) | 11.40 (8.30–15.43) | 12.80 (9.50–17.12) | <0.001 |
| Platelet (×109/L) | 230 (183–285) | 234 (188–288) | 213 (162–263) | <0.001 |
| RBC (×109/L) | 4.13 (3.67–4.57) | 4.17 (3.73–4.61) | 3.87 (3.41–4.36) | <0.001 |
| RDW (%) | 13.5 (12.9–14.4) | 13.4 (12.9–14.3) | 13.9 (13.2–15.2) | <0.001 |
| Hemoglobin (g/dL) | 12.8 (11.4–14.1) | 12.9 (11.6–14.3) | 12.0 (10.5–13.4) | <0.001 |
| Glucose | 132 (110–165) | 127 (107–156) | 159 (129–193) | <0.001 |
| Blood urea nitrogen | 16 (12–23) | 16 (12–22) | 19.50 (14–27) | <0.001 |
| Serum creatinine | 0.9 (0.7–1.1) | 0.9 (0.7–1.1) | 1.0 (0.8–1.3) | <0.001 |
| Magnesium (mg/dL) | 1.8 (1.6–2.0) | 1.8 (1.6–2.0) | 1.80 (1.6–2.0) | 0.430 |
| Sodium (mmol/L) | 139 (137–141) | 139 (137–141) | 139 (137–142) | 0.522 |
| Potassium (mmol/L) | 4.0 (3.7–4.4) | 4.0 (3.7–4.3) | 4.0 (3.6–4.5) | 0.561 |
| RBC transfusion during the first 24 hours (%) | 178 (7.8%) | 117 (6.2%) | 61 (15.1%) | <0.001 |
| Platelet transfusion during the first 24 hours (%) | 223 (9.8%) | 155 (8.3%) | 68 (16.8%) | <0.001 |
| Coagulopathy (%) | 743 (32.6%) | 543 (28.9%) | 200 (49.5%) | <0.001 |
| Neurosurgery (%) | 572 (25.1%) | 445 (23.7%) | 127 (31.4%) | 0.001 |
| Length of ICU stay (days) | 2 (1–6) | 2 (1–5) | 3 (2–7) | <0.001 |
| Length of hospital stay (days) | 6 (4–12) | 7 (4–13) | 5 (2–9) | <0.001 |
| Variables | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.025 | 1.020–1.031 | <0.001 |
| Gender | 1.135 | 0.912–1.413 | 0.257 |
| Diabetes | 1.521 | 1.156–2.002 | 0.003 |
| Hypertension | 1.128 | 0.905–1.407 | 0.283 |
| Hyperlipidemia | 1.059 | 0.773–1.451 | 0.721 |
| Chronic heart disease | 1.171 | 0.859–1.596 | 0.319 |
| History of myocardial infarction | 0.858 | 0.470–1.566 | 0.617 |
| Cerebral vascular disease | 1.313 | 0.622–2.772 | 0.475 |
| Chronic liver disease | 0.951 | 0.549–1.646 | 0.856 |
| Chronic renal disease | 2.431 | 1.702–3.473 | <0.001 |
| Systolic blood pressure | 0.997 | 0.992–1.001 | 0.139 |
| Diastolic blood pressure | 0.989 | 0.983–0.996 | 0.002 |
| Heart rate | 0.998 | 0.992–1.004 | 0.434 |
| SpO2 | 0.995 | 0.976–1.015 | 0.652 |
| GCS | 0.819 | 0.798–0.841 | <0.001 |
| ISS | 1.047 | 1.036–1.059 | <0.001 |
| Epidural hematoma | 1.064 | 0.829–1.366 | 0.626 |
| Subdural hematoma | 1.003 | 0.807–1.248 | 0.975 |
| Subarachnoid hemorrhage | 1.176 | 0.947–1.461 | 0.141 |
| Intraparenchymal hemorrhage | 0.833 | 0.629–1.104 | 0.204 |
| WBC | 1.039 | 1.023–1.055 | <0.001 |
| Platelet | 0.997 | 0.996–0.999 | <0.001 |
| RBC | 0.572 | 0.491–0.668 | <0.001 |
| RDW | 1.229 | 1.159–1.303 | <0.001 |
| Hemoglobin | 0.816 | 0.776–0.859 | <0.001 |
| Glucose | 1.008 | 1.007–1.010 | <0.001 |
| Blood urea nitrogen | 1.024 | 1.016–1.032 | <0.001 |
| Serum creatinine | 1.260 | 1.116–1.422 | <0.001 |
| Magnesium | 0.908 | 0.650–1.269 | 0.573 |
| Sodium | 1.020 | 0.994–1.046 | 0.135 |
| Potassium | 0.972 | 0.831–1.137 | 0.721 |
| RBC transfusion | 2.674 | 1.921–3.721 | <0.001 |
| Platelet transfusion | 2.247 | 1.651–3.058 | <0.001 |
| Coagulopathy | 2.407 | 1.933–2.996 | <0.001 |
| Neurosurgery | 1.474 | 1.165–1.866 | 0.001 |
| OR | 95% CI | p | |
|---|---|---|---|
| Model 1 | 0.845 | 0.584–1.222 | 0.370 |
| Model 2 | 1.540 | 1.029–2.305 | 0.036 |
| Model 3 | 1.620 | 1.044–2.514 | 0.032 |
| Model 4 | 1.661 | 1.068–2.582 | 0.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; He, M.; Xu, J. Initial Serum Magnesium Level Is Associated with Mortality Risk in Traumatic Brain Injury Patients. Nutrients 2022, 14, 4174. https://doi.org/10.3390/nu14194174
Wang R, He M, Xu J. Initial Serum Magnesium Level Is Associated with Mortality Risk in Traumatic Brain Injury Patients. Nutrients. 2022; 14(19):4174. https://doi.org/10.3390/nu14194174
Chicago/Turabian StyleWang, Ruoran, Min He, and Jianguo Xu. 2022. "Initial Serum Magnesium Level Is Associated with Mortality Risk in Traumatic Brain Injury Patients" Nutrients 14, no. 19: 4174. https://doi.org/10.3390/nu14194174
APA StyleWang, R., He, M., & Xu, J. (2022). Initial Serum Magnesium Level Is Associated with Mortality Risk in Traumatic Brain Injury Patients. Nutrients, 14(19), 4174. https://doi.org/10.3390/nu14194174




