Vitamin D-Related Risk Factors for Maternal Morbidity and Mortality during Pregnancy: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. PICO Strategy
2.2. Literature Search
2.3. Study Inclusion/Exclusion Criteria and Data Extraction
- Review, narrative review, clinical review, systematic review or meta-analysis;
- Available in English or Spanish;
- Published between 2010 and January 2022;
- Study carried out on humans;
- Exposure of interest is vitamin D status or supplementation during pregnancy;
- Data on vitamin D or metabolite concentration in maternal blood during pregnancy are available;
- Main outcomes of interest are the incidence of maternal mortality and morbidity.
2.4. Quality Assessment
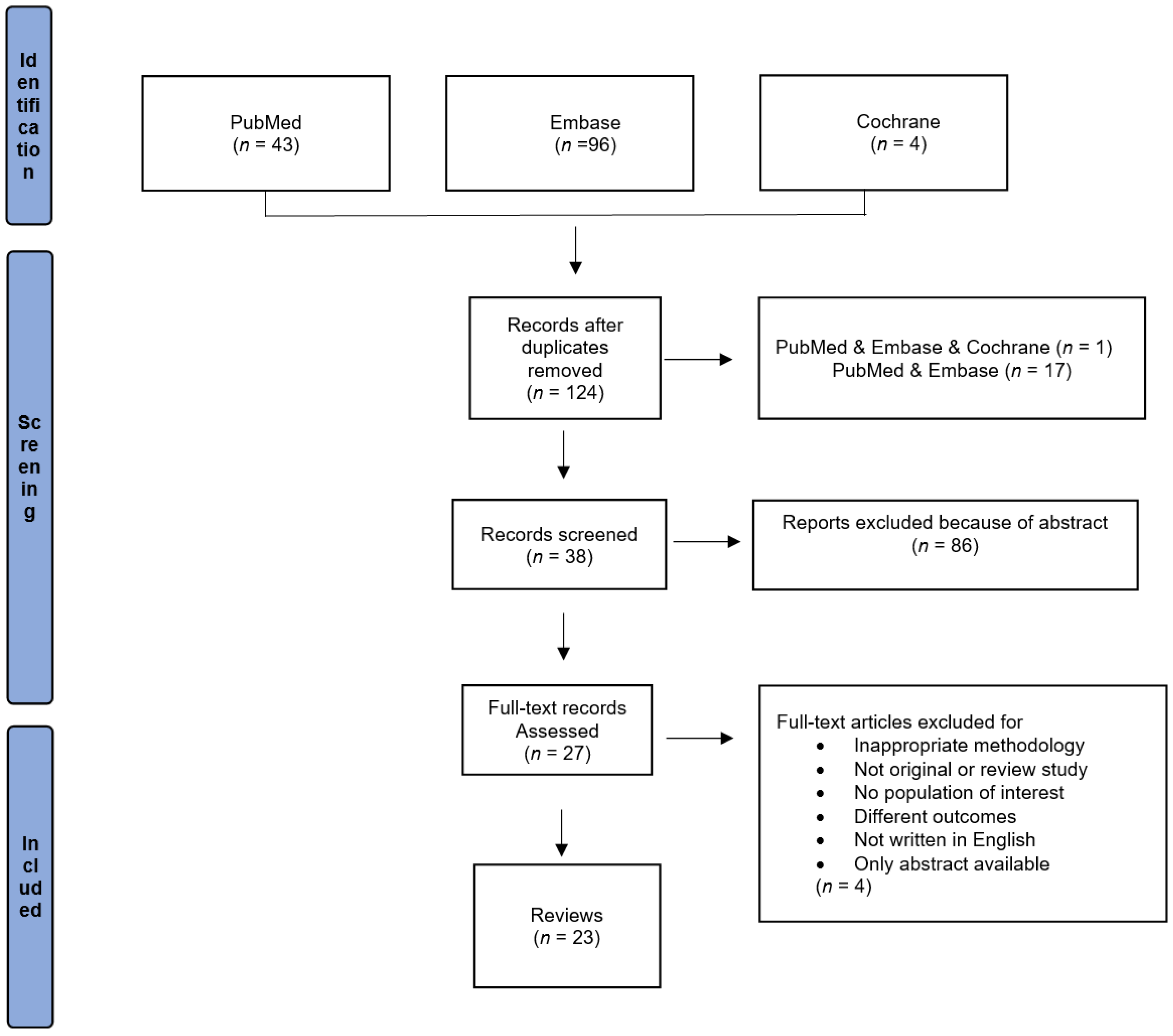
3. Results
3.1. Study Characteristics
3.2. Review and Meta-Analysis Studies
4. Discussion
4.1. Hemorrhage
4.2. Gestational Diabetes
4.3. Pulmonary Embolism or Other Embolism
4.4. Preterm Birth Risk
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, I.; Kim, S.S.; Song, J.I.; Yoon, S.H.; Park, G.Y.; Lee, Y.-W. Association between vitamin D level at birth and respiratory morbidities in very-low-birth-weight infants. Korean J. Pediatr. 2019, 62, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Eremkina, A.; Mokrysheva, N.; Pigarova, E.; Mirnaya, S. Vitamin D: Effects on pregnancy, maternal, fetal and postnatal outcomes. Ter. Arkhiv. 2018, 90, 115–127. [Google Scholar] [CrossRef]
- Baqai, S.; Siraj, A.; Imran, R. Association of vitamin-d insufficiency during pregnancy with maternal & perinatal morbidity and mortality. Pak. Armed. Forces Med. J. 2020, 70, 323–327. [Google Scholar]
- Thomas, D.J.; Khan, H.U.; Jaidev, S.P.; Hegde, P. A study on vitamin D levels in preterm and term neonates and their mothers. Int. J. Contemp. Pediatrics 2020, 7, 387–392. [Google Scholar] [CrossRef]
- Sankar, J.; Lotha, W.; Ismail, J.; Anubhuti, C.; Meena, R.S.; Sankar, M.J. Vitamin D deficiency and length of pediatric intensive care unit stay: A prospective observational study. Ann. Intensiv. Care 2016, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Gur, E.B.; Gokduman, A.; Turan, G.A.; Tatar, S.; Hepyilmaz, I.; Zengin, E.B.; Eskicioglu, F.; Guclu, S. Mid-pregnancy vitamin D levels and postpartum depression. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 110–116. [Google Scholar] [CrossRef]
- Song, S.J.; Si, S.; Liu, J.; Chen, X.; Zhou, L.; Jia, G.; Liu, G.; Niu, Y.; Wu, J.; Zhang, W.; et al. Vitamin D status in Chinese pregnant women and their newborns in Beijing and their relationships to birth size. Public Health Nutr. 2013, 16, 687–692. [Google Scholar] [CrossRef]
- Ates, S.; Sevket, O.; Ozcan, P.; Ozkal, F.; Kaya, M.O.; Dane, B. Vitamin D status in the first-trimester: Effects of Vitamin D deficiency on pregnancy outcomes. Afr. Health Sci. 2016, 16, 36–43. [Google Scholar] [CrossRef]
- Behjat Sasan, S.; Zandvakili, F.; Soufizadeh, N.; Baybordi, E. The Effects of Vitamin D Supplement on Prevention of Recurrence of Preeclampsia in Pregnant Women with a History of Preeclampsia. Obstet. Gynecol. Int. 2017, 2017, 8249264. [Google Scholar] [CrossRef]
- Rostami, M.; Tehrani, F.R.; Simbar, M.; Hosseinpanah, F.; Majd, H.A.; Khan, F.R. Rationale and Design of Khuzestan Vitamin D Deficiency Screening Program in Pregnancy: A Stratified Randomized Vitamin D Supplementation Controlled Trial. JMIR Res. Protoc. 2017, 6, e54. [Google Scholar] [CrossRef]
- Sepandi, M.; Esmailzadeh, S.; Hosseini, M.S.; Hashemi, S.R.; Abbaszadeh, S.; Alimohamadi, Y.; Taghdir, M. Prevalence of Vitamin D Deficiency Among Iranian Pregnant Women. Nutr. Diet. Suppl. 2020, 12, 97–102. [Google Scholar] [CrossRef]
- Saraf, R.; Morton, S.M.; Camargo, C.A., Jr.; Grant, C.C. Global summary of maternal and newborn vitamin D status–a systematic review. Matern. Child Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, A.; Sandström, H.; Stenlund, H.; Johansson, I.; Hultdin, J. Vitamin D Status during Pregnancy: A Longitudinal Study in Swedish Women from Early Pregnancy to Seven Months Postpartum. PLoS ONE 2016, 11, e0150385. [Google Scholar] [CrossRef]
- Perreault, M.; Moore, C.J.; Fusch, G.; Teo, K.K.; Atkinson, S.A. Factors Associated with Serum 25-Hydroxyvitamin D Concentration in Two Cohorts of Pregnant Women in Southern Ontario, Canada. Nutrients 2019, 11, 123. [Google Scholar] [CrossRef]
- Ochoa-Correa, E.D.C.; Garcia-Hernandez, P.A.; Villarreal-Perez, J.Z.; Treviño-Garza, C.; Villarreal, L.E.M.-D.; Zapata-Castilleja, C.A.; De La O-Cavazos, M.E. Vitamin D deficiency in Mexican mothers and their newborns. Gac. Médica México 2017, 153, 559–565. [Google Scholar] [CrossRef]
- Christakos, S. Mechanism of action of 1,25-dihydroxyvitamin D3 on intestinal calcium absorption. Rev. Endocr. Metab. Disord. 2012, 13, 39–44. [Google Scholar] [CrossRef]
- Kumar, R.; Thompson, J.R. The Regulation of Parathyroid Hormone Secretion and Synthesis. J. Am. Soc. Nephrol. 2011, 22, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Lederer, E. Regulation of serum phosphate. J. Physiol. 2014, 592, 3985–3995. [Google Scholar] [CrossRef]
- Eisman, A.J.; Bouillon, R. Vitamin D: Direct effects of vitamin D metabolites on bone: Lessons from genetically modified mice. BoneKEy Rep. 2014, 3, 499. [Google Scholar] [CrossRef]
- Ciresi, A.; Giordano, C. Vitamin D across growth hormone (GH) disorders: From GH deficiency to GH excess. Growth Horm. IGF Res. 2017, 33, 35–42. [Google Scholar] [CrossRef]
- Larqué, E.; Morales, E.; Leis, R.; Blanco-Carnero, J.E. Maternal and Foetal Health Implications of Vitamin D Status during Pregnancy. Ann. Nutr. Metab. 2018, 72, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-Q.; Qi, H.-P.; Luo, Z.-C.; Fraser, W.D. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2013, 26, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- SIGN 50: A Guideline Developer’s Handbook; Scottish Intercollegiate Guidelines Network: Edinburgh, UK, 2015; Available online: https://www.sign.ac.uk/assets/sign50_2015.pdf (accessed on 29 September 2022).
- Sackett, D.L. Evidence-based medicine. Semin. Perinatol. 1997, 21, 3–5. [Google Scholar] [CrossRef]
- González-Wong, C.; Fuentes-Barría, H.; Aguilera-Eguía, R.; Urbano-Cerda, S.; Vera-Aguirre, V. The role of vitamin D in preeclampsia risk: A narrative review. Rev. Chil. Nutr. 2021, 48, 118–125. [Google Scholar] [CrossRef]
- Kinshella, M.-L.; Omar, S.; Scherbinsky, K.; Vidler, M.; Magee, L.; von Dadelszen, P.; Moore, S.; Elango, R.; The PRECISE Conceptual Framework Working Group. Effects of Maternal Nutritional Supplements and Dietary Interventions on Placental Complications: An Umbrella Review, Meta-Analysis and Evidence Map. Nutrients 2021, 13, 472. [Google Scholar] [CrossRef]
- Oh, C.; Keats, E.C.; Bhutta, Z.A. Vitamin and Mineral Supplementation During Pregnancy on Maternal, Birth, Child Health and Development Outcomes in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 491. [Google Scholar] [CrossRef]
- Aguilar-Cordero, M.; Lasserrot-Cuadrado, A.; Mur-Villar, N.; León-Ríos, X.; Rivero-Blanco, T.; Pérez-Castillo, I. Vitamin D, preeclampsia and prematurity: A systematic review and meta-analysis of observational and interventional studies. Midwifery 2020, 87, 102707. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Pasupuleti, V.; Mezones-Holguin, E.; Benites-Zapata, V.A.; Thota, P.; Deshpande, A.; Hernandez, A.V. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: A systematic review and meta-analysis of randomized controlled trials. Fertil. Steril. 2015, 103, 1278–1288.e1274. [Google Scholar] [CrossRef]
- Sibtain, S.; Sinha, P.; Manoharan, M.; Azeez, A. Controversies related to vitamin D deficiency effect on the maternal and feto-placental unit–an update. J. Obstet. Gynaecol. 2020, 40, 759–766. [Google Scholar] [CrossRef] [PubMed]
- De Souza, E.A.; Pisani, L.P. The relationship among vitamin D, TLR4 pathway and preeclampsia. Mol. Biol. Rep. 2020, 47, 6259–6267. [Google Scholar] [CrossRef] [PubMed]
- Weinert, L.S.; Silveiro, S.P. Maternal–Fetal Impact of Vitamin D Deficiency: A Critical Review. Matern. Child Health J. 2015, 19, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, CD008873. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W.; Kotsa, K.; Fakhoury, H.; Karras, S.N. Vitamin D administration during pregnancy as prevention for pregnancy, neonatal and postnatal complications. Rev. Endocr. Metab. Disord. 2017, 18, 307–322. [Google Scholar] [CrossRef]
- Agarwal, S.; Kovilam, O.; Agrawal, D.K. Vitamin D and its impact on maternal-fetal outcomes in pregnancy: A critical review. Crit. Rev. Food Sci. Nutr. 2018, 58, 755–769. [Google Scholar] [CrossRef]
- Girling, J.; Sykes, L. Thyroid disorders and other endocrinological disorders in pregnancy. Obstet. Gynaecol. Reprod. Med. 2013, 23, 171–179. [Google Scholar] [CrossRef]
- Alzaim, M.; Wood, R.J. Vitamin D and gestational diabetes mellitus. Nutr. Rev. 2013, 71, 158–167. [Google Scholar] [CrossRef]
- Thorne-Lyman, A.; Fawzi, W.W. Vitamin D During Pregnancy and Maternal, Neonatal and Infant Health Outcomes: A Systematic Review and Meta-analysis. Paediatr. Périnat. Epidemiol. 2012, 26, 75–90. [Google Scholar] [CrossRef]
- Senti, J.; Thiele, D.K.; Anderson, C.M. Maternal Vitamin D Status as a Critical Determinant in Gestational Diabetes. J. Obstet. Gynecol. Neonatal Nurs. 2012, 41, 328–338. [Google Scholar] [CrossRef]
- Mulligan, M.L.; Felton, S.K.; Riek, A.E.; Bernal-Mizrachi, C. Implications of vitamin D deficiency in pregnancy and lactation. Am. J. Obstet. Gynecol. 2010, 202, 429.e1–429.e9. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Zittermann, A.; Obeid, R.; Hahn, A.; Pludowski, P.; Trummer, C.; Lerchbaum, E.; Pérez-López, F.R.; Karras, S.N.; März, W. The Role of Vitamin D in Fertility and during Pregnancy and Lactation: A Review of Clinical Data. Int. J. Environ. Res. Public Health 2018, 15, 2241. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.; Khodadadi, B.; Ahmadi, S.A.Y.; Abbaszadeh, S.; Shahsavar, F. Association of vitamin D level and vitamin D deficiency with risk of preeclampsia: A systematic review and updated meta-analysis. Taiwan. J. Obstet. Gynecol. 2018, 57, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, M.; Salehi-Abargouei, A.; Tabesh, M.; Esmaillzadeh, A. Maternal Vitamin D Status and Risk of Pre-Eclampsia: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2013, 98, 3165–3173. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.A.; Wadhwani, N.S.; Joshi, S.R. Altered metabolic homeostasis between vitamin D and long chain polyunsaturated fatty acids in preeclampsia. Med. Hypotheses 2017, 100, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; De-Regil, L.M.; Lombardo, L.K.; Peña-Rosas, J.P. Vitamin D supplementation during pregnancy: Updated meta-analysis on maternal outcomes. J. Steroid Biochem. Mol. Biol. 2016, 164, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality—A review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Barrett, H.; McElduff, A. Vitamin D and pregnancy: An old problem revisited. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 527–539. [Google Scholar] [CrossRef]
- CDC. Pregnancy Mortality Surveillance System. 22 June 2022. Available online: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-mortality-surveillance-system.htm (accessed on 28 September 2022).
- WHO. Maternal Mortality. 19 September 2019. Available online: https://www.who.int/en/news-room/fact-sheets/detail/maternal-mortality (accessed on 28 September 2022).
- Grant, W.B.; Boucher, B.J.; Al Anouti, F.; Pilz, S. Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin D. Nutrients 2022, 14, 3811. [Google Scholar] [CrossRef]
- Li, W.-J.; Chen, K.-H.; Huang, L.-W.; Tsai, Y.-L.; Seow, K.-M. Low Maternal Serum 25-Hydroxyvitamin D Concentration Is Associated with Postpartum Hemorrhage: A Retrospective Observational Study. Front. Endocrinol. 2022, 13, 816480. [Google Scholar] [CrossRef]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.M.; Haeri, S.; Camargo, A.C.; Stuebe, A.M.; Boggess, A.K. A Nested Case-Control Study of First-Trimester Maternal Vitamin D Status and Risk for Spontaneous Preterm Birth. Am. J. Perinatol. 2011, 28, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Rouse, D.J.; Momirova, V.; Peaceman, A.; Sciscione, A.; Spong, C.Y.; Varner, M.; Malone, F.D.; Iams, J.D.; Mercer, B.M.; et al. Maternal 25-Hydroxyvitamin D and Preterm Birth in Twin Gestations. Obstet. Gynecol. 2013, 122, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Thota, C.; Menon, R.; Fortunato, S.J.; Brou, L.; Lee, J.-E.; Al-Hendy, A. 1,25-Dihydroxyvitamin D Deficiency Is Associated with Preterm Birth in African American and Caucasian Women. Reprod. Sci. 2014, 21, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Liong, S.; Di Quinzio, M.K.W.; Fleming, G.; Permezel, M.; Georgiou, H.M. Is Vitamin D Binding Protein a Novel Predictor of Labour? PLoS ONE 2013, 8, e76490. [Google Scholar] [CrossRef]
- Zhou, S.-S.; Tao, Y.-H.; Huang, K.; Zhu, B.-B.; Tao, F.-B. Vitamin D and risk of preterm birth: Up-to-date meta-analysis of randomized controlled trials and observational studies. J. Obstet. Gynaecol. Res. 2017, 43, 247–256. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, K.A.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Baggerly, L.L.; Ebeling, M.D.; Rittenberg, C.S.; Goodier, C.G.; Niño, J.F.M.; et al. Maternal 25(OH)D concentrations ≥40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE 2017, 12, e0180483. [Google Scholar] [CrossRef]
- Morales-Suárez-Varela, M.M.; Uçar, N.; Peraita-Costa, I.; Huertas, M.F.; Soriano, J.M.; Llopis-Morales, A.; Grant, W.B. Vitamin D-Related Risk Factors for Maternal Morbidity during Pregnancy: A Systematic Review. Nutrients 2022, 14, 3166. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef]
| Ref. | Design | Location | Vitamin Concentration and Supplementation | Findings | SIGN | |
|---|---|---|---|---|---|---|
| LE | GR | |||||
| González-Wong et al. 2021 [27] | Narrative review | Chile | Supplementation during pregnancy | Vitamin D may lower risk of PE. | 3 | C |
| Kinshella et al. 2021 [28] | Review | Canada | Supplementation during pregnancy | Half of reviews reported that vitamin D supplementation had a significant protective effect on PE incidence; little evidence that vitamin D supplementation affected PTB or stillbirth. Pooled outcomes showed 38% reduced risk of developing PE among pregnant women who received vitamin D supplementation in comparison with those who did not, without heterogeneity between studies (RR = 0.62; 95% CI, 0.43–0.91; I2 = 0%; 12 studies, n = 1353), which did not change in direction or significance with sensitivity analyses. | 1 ++ | A |
| Aguliar-Cordero et al. 2020 [30] | Systematic review and meta-analysis of observational and interventional studies | Spain | Maternal concn during pregnancy | Interventional studies indicated that vitamin D supplementation acts as a prevention factor for PE and prematurity. Observational studies showed that vitamin D insufficiency and deficiency are associated with higher risk of developing PE. However, prematurity and vitamin D were associated only for maternal vitamin D concentrations < 75 nmol/L. Random-effects meta-analysis indicated no significant association between vitamin D, PE, and prematurity in either observational or interventional studies. | 2 ++ | A |
| Oh, Keats, and Bhutta 2020 [29] | Systematic review and meta-analysis | Canada | Micronutrient and vitamin supplementation during pregnancy | Vitamin D supplementation may have reduced risk of PTB by 36% (average RR = 0.64; 95% CI, 0.40–1.04; studies = 7), though the upper limit of the confidence interval just crossed the line of no effect. | 1 ++ | A |
| Sibtain et al. 2020 [32] | Systematic review | Pakistan | Status during pregnancy and fetoplacental unit | Studies recommended substantial vitamin D supplementation during pregnancy. High-quality randomized controlled trials (RCTs) required to see optimal level of vitamin D. | 2 ++ | A |
| De Souza and Pisani 2020 [33] | Narrative review | Brazil | Status and risk of PE | Although studies showing relation between vitamin D and lower risk of PE are limited, maternal status of vitamin D seems to influence risk of developing PE. Therefore, vitamin D supplementation in women may improve pregnancy outcomes. | 3 | C |
| Palacios, Kostiuk, and Peña-Rosas 2019 [35] | Review | USA | Supplementation during pregnancy | Supplementation with vitamin D alone during pregnancy probably reduces risk of PE (RR = 0.48; 95% CI, 0.30–0.79; 4 trials, n = 499) and gestational diabetes (RR = 0.51; 95% CI, 0.27–0.97; 4 trials, n = 446) and probably reduces risk of low birthweight (<2500 g; RR = 0.55, 95% CI, 0.35–0.87; 5 trials, n = 697) compared with women who received placebo or no intervention. Vitamin D supplementation may make little or no difference in risk of PTB (<37 weeks) compared with no intervention or placebo (RR = 0.66; 95% CI, 0.34–1.30; 7 trials, n = 1640), and vitamin D supplementation may reduce risk of severe postpartum bleeding (RR = 0.68; 95% CI, 0.51–0.91; 1 trial, n = 1134). | 2 ++ | A |
| Pilz et al. 2018 [43] | Review of clinical data | Germany | Status during pregnancy and lactation | Based on available evidence derived from RCTs on vitamin D supplementation in pregnancy, this study reported that vitamin D is safe and improves vitamin D and calcium status, thereby protecting skeletal health. Data from RCTs and meta-analyses of RCTs suggest other beneficial effects but are inconsistent on whether vitamin D supplementation improves clinical neonatal or maternal outcomes such as SGA, fetal/infant growth, infant/neonatal mortality, asthma/wheeze, PE, or GDM. | 2 ++ | A |
| Akbari et al. 2018 [44] | Systematic review and updated meta-analysis | Iran | Status during pregnancy | Based on the forest plot, lower levels of 25(OH)D were significantly associated with risk of PE (fixed and random p < 0.001). Women with vitamin D deficiency (<20 ng/mL) at higher risk of PE. Association can be specific up to 90% at 10.60-ng/mL cutoff. | 2 ++ | A |
| Nandi, Wadhwani, and Joshi 2017 [46] | Narrative review | India | Vitamin D and LCPUFAs and their role in PE development | Vitamin D [1,25(OH)2D3] induces CBS gene expression while it can suppress the oxidative stress–induced COX-2 upregulation and thromboxane production. On that basis, it is hypothesized that disturbed vitamin D and LCPUFA metabolism influences regulation of the one-carbon cycle, which will trigger inflammation through oxidative stress in PE. That may lead to altered fetoplacental growth and development of PE. | 3 | C |
| Wagner et al. 2017 [36] | Review | USA | Status and supplementation during pregnancy | A growing body of observational studies indicated that maternal hypovitaminosis D (<20 ng/mL or <50 nmol/L) is a significant risk factor for adverse neonatal outcomes, including asthma, multiple sclerosis, and other neurological disorders. Results of RCTs of vitamin D supplementation during pregnancy recently showed decreased complications of pregnancy/birth and GDM. | 2 ++ | A |
| Agarwal, Kovilam, and Agrawal 2018 [37] | Critical review | USA | PE, PTL, GDM, GH | In pregnancy, vitamin D deficiency is associated with increased incidence of adverse maternal and fetal outcomes, primarily PE, GDM, low birth weight, and PTB. Other outcomes still under study; no definite conclusions drawn yet. | 2 ++ | A |
| Palacios et al. 2016 [47] | Meta-analysis | Puerto Rico | Oral supplementation (alone or with other vitamins and minerals); maternal levels and risk of developing PE, GDM, PTB, impaired glucose tolerance, Cesarean delivery, GH, and other adverse conditions | Data suggest that pregnant women supplemented with vitamin D had significantly higher vitamin D levels than controls (mean difference, 54.7 nmol/L; 95% CI, 36.6–72.9). Two trials showed lower risk of PE (8.9% vs. 15.5%; average risk ratio, 0.52; 95% CI, 0.25–1.05) and two others showed no difference in risk of GDM with vitamin D supplementation. Additionally, three trials showed that supplementation with vitamin D plus calcium reduced risk of PE (5% vs. 9%; average risk ratio, 0.51; 95% CI, 0.32–0.80). | 2 ++ | A |
| Pérez-López et al. 2015 [31] | Systematic review and meta-analysis of RCTs | Spain | Circulating levels, PE, GDM, SGA, low birth weight, PTB, birth weight, birth length, Cesarean delivery | Circulating vitamin D levels significantly higher at term than in control group (mean difference, 66.5 nmol/L; 95% CI, 66.2–66.7). Birth weight and birth length were significantly greater in vitamin D group; mean difference, 107.6 g (95% CI, 59.9–155.3) and 0.3 cm (95% CI, 0.10–0.41), respectively. Incidence of PE, GDM, SGA, low birth weight, PTB, and Cesarean delivery not influenced by vitamin D supplementation. Across RCTs, doses and types of vitamin D supplements, gestational age at first administration, and outcomes were diverse. | 2 ++ | A |
| Weinert and Silveiro 2015 [34] | Critical review | Brazil | Low birth weight, growth restriction, respiratory tract infection, altered glucose homeostasis, increased incidence of GDM, PE, bacterial vaginosis | Current state of evidence is controversial for some other endpoints, and actual benefit of vitamin D supplementation in pregnancy remains unclear. | 3 | D |
| Pludowski et al. 2013 [48] | Review of recent evidence | Poland | Status during pregnancy | Various health effects of vitamin D deficiency during pregnancy continue to be reported, notably with increased risk of PE, infection, PTL and PTB, Cesarean delivery, GDM. | 2 ++ | A |
| Girling and Sykes 2013 [38] | Narrative review | USA | Physiology and current management of thyroid dysfunction and the rarer endocrine disorders in pregnancy and includes current guidance on supplementation | Over recent years, awareness of potential adverse effects of vitamin D deficiency has driven guidance for vitamin D supplementation for pregnant and lactating women. | 3 | D |
| Tabesh, Salehi-Abargouei, and Esmaillzadeh 2013 [45] | Systematic review and meta-analysis | Iran | Maternal serum and risk of PE | Overall significant association between vitamin D deficiency and risk of PE; however, significant between-study heterogeneity evident (I2 = 52.7%; p = 0.039). In subgroup analysis, overall effect was significant for studies defining vitamin D deficiency as ≤50 nmol/L (20 ng/mL) but not for those that used <38 nmol/L (15.2 ng/mL). Association was seen for “cohort or nested case–control studies” as well as for “cross-sectional or case–control studies” (2.78; 95% CI, 1.45–5.33; p = 0.002). For analysis by study location, associations remained significant only for U.S. studies. | 2 ++ | A |
| Alzaim and Wood 2013 [39] | Critical review | USA | Status during pregnancy and GDM | Suggested that vitamin D deficiency in pregnant women increases risk for GDM. However, that determination is based largely on only six published observational studies and one short-term intervention study with an active analog form of 1,25(OH)2 vitamin D. Effect of treating existing vitamin D deficiency on later development of GDM in pregnant women unknown. | 2 ++ | A |
| Thorne-Lyman and Fawzi 2012 [40] | Systematic review and meta-analysis | USA | Supplementation during pregnancy for maternal, perinatal, or infant health outcomes | Only low-level evidence relates vitamin D supplementation or intake during pregnancy to perinatal and infant health-related outcomes. Emerging evidence suggesting plausible effects on intrauterine growth restriction, PE, and both maternal and infant infections as important outcomes in need of further research in low-income settings. | 2 ++ | A |
| Senti et al. 2012 [41] | Systematic review | USA | Status during pregnancy and GDM | Study findings consist solely of level 2 evidence for associating maternal vitamin D deficiency with risk of GDM. Five (83%) studies reported inverse relationship between circulating vitamin D levels and markers of glucose homeostasis associated with GDM or increased risk for GDM associated with reduced maternal levels of vitamin D. In one study, researchers did not identify association between vitamin D and GDM but did identify association between higher vitamin D levels and lower fasting glucose and insulin levels. | 3 | D |
| Barrett and McElduff 2010 [50] | Narrative review | Australia | Supplementation during pregnancy | Vitamin D’s role in multiple nonclassical metabolic processes, though shown primarily by association studies in human populations, has a possible physiological basis. Considerable evidence associates low maternal vitamin D levels with worse outcomes for both mother and fetus in pregnancy and for the neonate. Whether association between vitamin D status and a wide range of adverse health outcomes is because vitamin D acts as a marker for some other health parameter such as obesity or occurs because of a direct causal relationship usually remains to be determined. Optimal concentration of vitamin D is unclear or at least controversial. RCTs of vitamin D supplementation with measurement of vitamin D to determine baseline status, level achieved on supplementation with appropriate documentation of possible confounders, and assessment of various health outcomes are required. Trying to achieve a vitamin D concentration of >50 nmol/L seems reasonable in most populations, including pregnant women. | 3 | B |
| Mulligan et al. 2010 [42] | Narrative review | USA | Metabolism and implications of deficiency in pregnancy and lactation | Vitamin D deficiency is associated with increased prevalence of PE, a common cause of increased mortality rates in pregnancy. Current recommendations for daily vitamin D intake (200 IU) are inadequate to maintain serum levels of vitamin D in the recommended range during pregnancy and lactation. More studies are needed to determine serum levels and degree of supplementation necessary to optimize maternal and fetal outcomes. However, because vitamin D supplementation is simple and cost-effective with a low likelihood of toxicity, we recommend increased supplementation in all pregnant women to keep serum levels of vitamin D in the reference range for adults (>32 ng/mL). | 4 | D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Suárez-Varela, M.; Uçar, N.; Soriano, J.M.; Llopis-Morales, A.; Sanford, B.S.; Grant, W.B. Vitamin D-Related Risk Factors for Maternal Morbidity and Mortality during Pregnancy: Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4124. https://doi.org/10.3390/nu14194124
Morales-Suárez-Varela M, Uçar N, Soriano JM, Llopis-Morales A, Sanford BS, Grant WB. Vitamin D-Related Risk Factors for Maternal Morbidity and Mortality during Pregnancy: Systematic Review and Meta-Analysis. Nutrients. 2022; 14(19):4124. https://doi.org/10.3390/nu14194124
Chicago/Turabian StyleMorales-Suárez-Varela, María, Nazlı Uçar, José Miguel Soriano, Agustín Llopis-Morales, Beth S. Sanford, and William B. Grant. 2022. "Vitamin D-Related Risk Factors for Maternal Morbidity and Mortality during Pregnancy: Systematic Review and Meta-Analysis" Nutrients 14, no. 19: 4124. https://doi.org/10.3390/nu14194124
APA StyleMorales-Suárez-Varela, M., Uçar, N., Soriano, J. M., Llopis-Morales, A., Sanford, B. S., & Grant, W. B. (2022). Vitamin D-Related Risk Factors for Maternal Morbidity and Mortality during Pregnancy: Systematic Review and Meta-Analysis. Nutrients, 14(19), 4124. https://doi.org/10.3390/nu14194124









