Association between Plasma Omega-3 and Handgrip Strength According to Glycohemoglobin Levels in Older Adults: Results from NHANES 2011–2012
Abstract
1. Introduction
2. Methods
2.1. Data Source
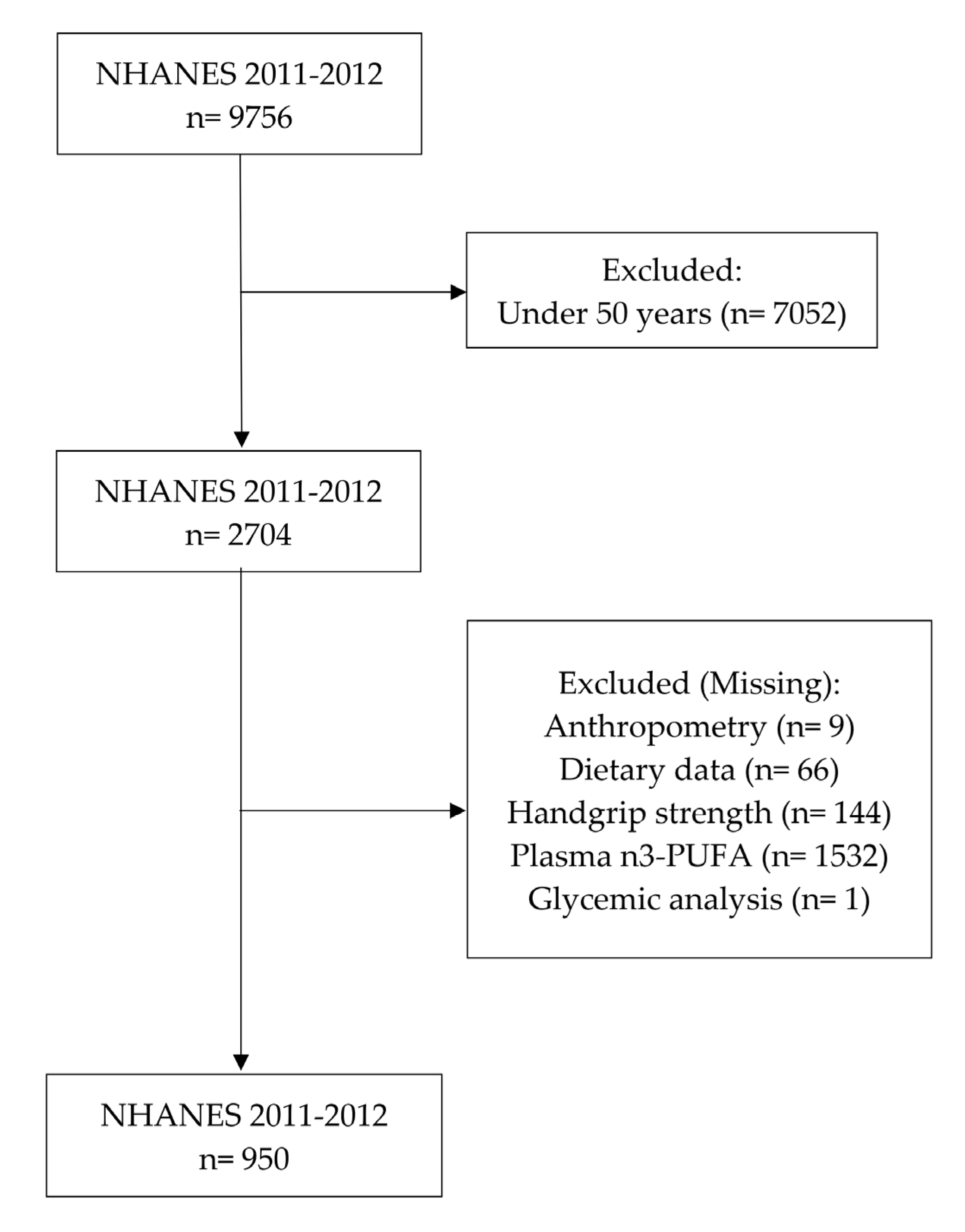
2.2. Sample Selection
2.3. Plasma ω-3 Levels
2.4. Plasma HbA1c Levels
2.5. Handgrip Strength
2.6. Other Variables of Interest
2.7. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaap, L.A.; Van Schoor, N.M.; Lips, P.; Visser, M. Associations of Sarcopenia Definitions, and Their Components, with the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- García-Hermoso, A.; Cavero-Redondo, I.; Ramírez-Vélez, R.; Ruiz, J.R.; Ortega, F.B.; Lee, D.C.; Martínez-Vizcaíno, V. Muscular Strength as a Predictor of All-Cause Mortality in an Apparently Healthy Population: A Systematic Review and Meta-Analysis of Data From Approximately 2 Million Men and Women. Arch. Phys. Med. Rehabil. 2018, 99, 2100–2113.e5. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.; Vellas, B.; Evans, W. Sarcopenia: An Undiagnosed Condition in Older Adults. Consensus Definition: Prevalence, Etiology, and Consequences. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Cermak, N.M.; Res, P.T.; De Groot, L.C.P.G.M.; Saris, W.H.M.; Van Loon, L.J.C. Protein Supplementation Augments the Adaptive Response of Skeletal Muscle to Resistance-Type Exercise Training: A Meta-Analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef]
- Peake, J.; Della Gatta, P.; Cameron-Smith, D. Aging and Its Effects on Inflammation in Skeletal Muscle at Rest and Following Exercise-Induced Muscle Injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1485–R1495. [Google Scholar] [CrossRef]
- Meng, S.J.; Yu, L.J. Oxidative Stress, Molecular Inflammation and Sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509. [Google Scholar] [CrossRef]
- Anagnostis, P.; Gkekas, N.K.; Achilla, C.; Pananastasiou, G.; Taouxidou, P.; Mitsiou, M.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. Type 2 Diabetes Mellitus Is Associated with Increased Risk of Sarcopenia: A Systematic Review and Meta-Analysis. Calcif. Tissue Int. 2020, 107, 453–463. [Google Scholar] [CrossRef]
- Mori, H.; Kuroda, A.; Matsuhisa, M. Clinical Impact of Sarcopenia and Dynapenia on Diabetes. Diabetol. Int. 2019, 10, 183–187. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Swiercz, A.; Pludowski, P.; Jaworski, M.; Szalecki, M. Skeletal Status, Body Composition, and Glycaemic Control in Adolescents with Type 1 Diabetes Mellitus. J. Diabetes Res. 2018, 2018, 8121634. [Google Scholar] [CrossRef]
- Mori, H.; Kuroda, A.; Yoshida, S.; Yasuda, T.; Umayahara, Y.; Shimizu, S.; Ryomoto, K.; Yoshiuchi, K.; Yamamoto, T.; Matsuoka, T.A.; et al. High Prevalence and Clinical Impact of Dynapenia and Sarcopenia in Japanese Patients with Type 1 and Type 2 Diabetes: Findings from the Impact of Diabetes Mellitus on Dynapenia Study. J. Diabetes Investig. 2021, 12, 1050–1059. [Google Scholar] [CrossRef]
- de Freitas, M.M.; de Oliveira, V.L.P.; Grassi, T.; Valduga, K.; Miller, M.E.P.; Schuchmann, R.A.; Souza, K.L.A.; de Azevedo, M.J.; Viana, L.V.; de Paula, T.P. Difference in Sarcopenia Prevalence and Associated Factors According to 2010 and 2018 European Consensus (EWGSOP) in Elderly Patients with Type 2 Diabetes Mellitus. Exp. Gerontol. 2020, 132, 110835. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The Role of Omega-3 in the Prevention and Treatment of Sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Rossato, L.T.; de Branco, F.M.S.; Azeredo, C.M.; Rinaldi, A.E.M.; de Oliveira, E.P. Association between Omega-3 Fatty Acids Intake and Muscle Strength in Older Adults: A Study from National Health and Nutrition Examination Survey (NHANES) 1999–2002. Clin. Nutr. 2020, 39, 3434–3441. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish Oil-Derived n-3 PUFA Therapy Increases Muscle Mass and Function in Healthy Older Adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef]
- Rodacki, C.L.N.; Rodacki, A.L.F.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-Oil Supplementation Enhances the Effects of Strength Training in Elderly Women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef]
- Robinson, S.M.; Jameson, K.A.; Batelaan, S.F.; Martin, H.J.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Sayer, A.A. Diet and Its Relationship with Grip Strength in Community-Dwelling Older Men and Women: The Hertfordshire Cohort Study. J. Am. Geriatr. Soc. 2008, 56, 84–90. [Google Scholar] [CrossRef]
- Lalia, A.Z.; Dasari, S.; Robinson, M.M.; Abid, H.; Morse, D.M.; Klaus, K.A.; Lanza, I.R. Influence of Omega-3 Fatty Acids on Skeletal Muscle Protein Metabolism and Mitochondrial Bioenergetics in Older Adults. Aging 2017, 9, 1096–1129. [Google Scholar] [CrossRef]
- Da Boit, M.; Sibson, R.; Sivasubramaniam, S.; Meakin, J.R.; Greig, C.A.; Aspden, R.M.; Thies, F.; Jeromson, S.; Hamilton, D.L.; Speakman, J.R.; et al. Sex Differences in the Effect of Fish-Oil Supplementation on the Adaptive Response to Resistance Exercise Training in Older People: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2017, 105, 151–158. [Google Scholar] [CrossRef]
- Reinders, I.; Song, X.; Visser, M.; Eiriksdottir, G.; Gudnason, V.; Sigurdsson, S.; Aspelund, T.; Siggeirsdottir, K.; Brouwer, I.A.; Harris, T.B.; et al. Plasma Phospholipid PUFAs Are Associated with Greater Muscle and Knee Extension Strength but Not with Changes in Muscle Parameters in Older Adults. J. Nutr. 2015, 145, 105–112. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary Omega-3 Fatty Acid Supplementation Increases the Rate of Muscle Protein Synthesis in Older Adults: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef]
- Patten, G.S.; Abeywardena, M.Y.; McMurchie, E.J.; Jahangiri, A. Dietary Fish Oil Increases Acetylcholine- and Eicosanoid-Induced Contractility of Isolated Rat Ileum. J. Nutr. 2002, 132, 2506–2513. [Google Scholar] [CrossRef] [PubMed]
- Mcglory, C.; Wardle, S.L.; Macnaughton, L.S.; Witard, O.C.; Scott, F.; Dick, J.; Bell, J.G.; Phillips, S.M.; Galloway, S.D.R.; Hamilton, D.L.; et al. Fish Oil Supplementation Suppresses Resistance Exercise and Feeding-Induced Increases in Anabolic Signaling without Affecting Myofibrillar Protein Synthesis in Young Men. Physiol. Rep. 2016, 4, e12715. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 Polyunsaturated Fatty Acids Augment the Muscle Protein Anabolic Response to Hyperinsulinaemia-Hyperaminoacidaemia in Healthy Young and Middle-Aged Men and Women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Rossato, L.T.; Schoenfeld, B.J.; de Oliveira, E.P. Is There Sufficient Evidence to Supplement Omega-3 Fatty Acids to Increase Muscle Mass and Strength in Young and Older Adults? Clin. Nutr. 2020, 39, 23–32. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention National Health and Nutrition Examination Survey (NHANES). Available online: https://wwwn.cdc.gov/nchs/nhanes (accessed on 21 September 2021).
- Punia, S.; Sandhu, K.S.; Siroha, A.K.; Dhull, S.B. Omega 3-Metabolism, Absorption, Bioavailability and Health Benefits—A Review. PharmaNutrition 2019, 10, 100162. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Global Physical Activity Questionnaire (GPAQ) Analysis Guide. Available online: https://www.who.int/publications/m/item/global-physical-activity-questionnaire (accessed on 21 September 2021).
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, Interconversion, and Dose Response of n-3 Fatty Acids in Humans. Am. J. Clin. Nutr. 2006, 83, 1467S. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Is There a Relationship between Mean Blood Glucose and Glycated Hemoglobin? J. Diabetes Sci. Technol. 2011, 5, 1572–1583. [Google Scholar] [CrossRef]
| Variables | Total | Glycohemoglobin <5.7% | Glycohemoglobin ≥5.7% | p-Value |
|---|---|---|---|---|
| Age, years | 63.0 ± 8.9 | 61.1 ± 7.5 | 64.8 ± 9.8 | <0.001 |
| Non-Hispanic white, % | 76.5 (68.9–82.6) | 84.3 (77.0–89.7) | 68.5 (58.5–77.1) | 0.001 |
| Sex, % | 0.511 | |||
| Men | 45.8 (41.8–50.0) | 47.4 (43.0–52.0) | 44.2 (36.5–52.0) | |
| Women | 54.2 (50.0–58.2) | 52.6 (48.1–57.0) | 56.0 (48.0–63.5) | |
| Marital status, % | 0.800 | |||
| Single/divorced/widowed/never married | 31.5 (26.6–36.8) | 30.8 (22.9–39.9) | 32.2 (26.1–39.0) | |
| Married/living as married | 68.5 (63.2–73.4) | 69.2 (60.0–77.1) | 67.8 (61.1–74.0) | |
| Annual family income, % | 0.005 | |||
| USD 0–19.999 | 15.9 (12.1–20.7) | 11.6 (8.9–14.0) | 20.3 (14.1–28.5) | |
| USD 20.000–54.999 | 37.5 (30.1–45.6) | 32.0 (23.4–42.0) | 43.1 (35.3–51.3) | |
| USD 55.000–74.999 | 13.7 (9.6–19.3) | 14.0 (9.3–19.4) | 14.0 (8.2–22.6) | |
| Over USD 75.000 | 29.8 (20.6–41.0) | 40.4 (30.0–51.8) | 19.0 (11.7–29.3) | |
| Missing | 3.0 (1.7–5.4) | 2.5 (1.0–6.0) | 4.0 (2.0–6.4) | |
| Educational level, % | <0.001 | |||
| High school graduate or under | 39.1 (32.4–46.2) | 28.0 (21.0–36.2) | 50.3 (41.3–59.3) | |
| Some college or above | 60.9 (53.8–67.6) | 72.0 (63.8–79.0) | 50.0 (40.7–58.7) | |
| Health conditions and habits, % | ||||
| Hypertension | 51.8 (40.9–55.5) | 44.4 (36.6–52.6) | 59.3 (48.9–68.9) | 0.008 |
| Diabetes | <0.001 | |||
| Pre-diabetes | 2.9 (1.6–5.1) | 1.9 (0.5–6.2) | 4.0 (2.0–8.6) | |
| Yes | 15.7 (11.0–21.9) | 1.3 (0.5–3.2) | 30.3 (22.7–39.2) | |
| No | 81.4 (76.1–85.8) | 96.8 (92.8–98.6) | 65.8 (58.6–72.4) | |
| Missing | 0.02 (0.00–0.19) | 0.04 (0.00–0.38) | - | |
| Smoking | 0.025 | |||
| Yes | 16.6 (13.0–21.1) | 13.0 (9.6–17.4) | 20.3 (14.9–27.0) | |
| No | 83.3 (78.9–87.0) | 86.9 (82.6–90.3) | 79.7 (73.0–85.1) | |
| Missing | 0.03 (0.00–0.26) | 0.07 (0.01–0.55) | - | |
| Arthritis | 0.130 | |||
| Yes | 41.1 (36.0–46.5) | 37.0 (29.7–45.0) | 45.3 (39.0–51.9) | |
| No | 58.6 (53.3–63.8) | 62.5 (55.0–69.8) | 54.7 (48.1–61.2) | |
| Missing | 0.3 (0.2–0.4) | 0.5 (0.3–0.8) | - | |
| Physical activity, % | 0.035 | |||
| Yes | 46.9 (40.1–53.9) | 53.3 (45.9–60.6) | 40.5 (31.1–50.6) | |
| No | 53.1 (46.1–60.0) | 46.7 (39.4–54.1) | 59.5 (49.4–68.9) | |
| Moderate PA | 0.144 | |||
| Yes | 44.2 (37.4–51.3) | 48.7 (40.4–56.9) | 39.7 (30.4–49.8) | |
| No | 55.8 (48.7–62.6) | 51.3 (43.1–59.6) | 60.3 (50.2–69.7) | |
| Vigorous PA | 0.002 | |||
| Yes | 11.5 (6.8–18.7) | 16.5 (9.5–27.0) | 6.4 (3.6–11.0) | |
| No | 88.5 (81.3–93.2) | 83.5 (73.0–90.5) | 93.6 (89.0–96.4) | |
| Anthropometrics | ||||
| Weight, kg | 82.7 ± 20.7 | 78.9 ± 15.0 | 86.5 ± 25.7 | 0.002 |
| Height, m | 1.68 ± 0.1 | 1.69 ± 0.1 | 1.67 ± 0.1 | 0.040 |
| Body mass index, kg/m2 | 29.3 ± 6.5 | 27.6 ± 4.4 | 31.0 ± 8.2 | <0.001 |
| Strength | ||||
| Sum of handgrip strength, kg | 65.7 ± 20.6 | 68.2 ± 18.8 | 63.1 ± 21.7 | 0.005 |
| Laboratory data | ||||
| Fasting glucose, mg/dL | 110.6 ± 30.0 | 99.5 ± 8.7 | 121.8 ± 42.2 | <0.001 |
| Glycohemoglobin, % | 5.9 ± 1.0 | 5.3 ± 0.2 | 6.4 ± 1.2 | <0.001 |
| Plasma Fatty Acids | ||||
| Total plasma ω-3, µmol/L | 363.2 ± 177.2 | 357.2 ± 158.2 | 369.3 ± 194.1 | 0.425 |
| ALA, µmol/L | 96.1 ± 55.8 | 91.3 ± 38.5 | 101.0 ± 72.3 | 0.097 |
| EPA, µmol/L | 87.7 ± 87.9 | 91.0 ± 93.9 | 84.4 ± 71.7 | 0.465 |
| DHA, µmol/L | 179.4 ± 83.5 | 174.9 ± 71.2 | 183.9 ± 95.4 | 0.179 |
| Linoleic acid, µmol/L | 3895.8 ± 937.6 | 3845.8 ± 704.3 | 3946.5 ± 1169.8 | 0.204 |
| Dietary intake | ||||
| Energy, kcal | 1960.9 ± 672.1 | 2038.6 ± 597.9 | 1882.2 ± 729.5 | 0.012 |
| Carbohydrate, g | 237.8 ± 91.4 | 249.2 ± 85.4 | 226.3 ± 93.5 | 0.001 |
| Protein, g | 77.9 ± 29.7 | 79.6 ± 27.0 | 76.3 ± 31.9 | 0.193 |
| Protein, g/kg | 1.0 ± 0.4 | 1.0 ± 0.3 | 0.9 ± 0.4 | 0.002 |
| Lipids, g | 74.0 ± 32.0 | 75.9 ± 26.9 | 72.2 ± 37.0 | 0.157 |
| Saturated fat, g | 23.6 ± 11.2 | 24.2 ± 9.4 | 23.0 ± 13.0 | 0.139 |
| Monounsaturated fat, g | 26.6 ± 12.8 | 27.1 ± 10.8 | 26.0 ± 14.7 | 0.292 |
| Polyunsaturated fat, g | 17.8 ± 8.7 | 18.4 ± 7.9 | 17.2 ± 9.4 | 0.122 |
| Total ω-3, g | 1.77 ± 0.97 | 1.87 ± 0.94 | 1.68 ± 0.94 | 0.030 |
| ALA, g | 1.69 ± 0.93 | 1.78 ± 0.91 | 1.60 ± 0.89 | 0.034 |
| EPA, g | 0.03 ± 0.07 | 0.03 ± 0.05 | 0.02 ± 0.07 | 0.113 |
| DHA, g | 0.06 ± 0.11 | 0.06 ± 0.01 | 0.06 ± 0.12 | 0.456 |
| Linoleic acid, g | 15.7 ± 7.9 | 16.2 ± 7.1 | 15.2 ± 8.5 | 0.148 |
| Fiber, g | 18.2 ± 10.4 | 19.6 ± 10.9 | 16.7 ± 8.6 | 0.001 |
| Alcohol, g | 8.8 ± 20.2 | 10.9 ± 18.0 | 6.7 ± 21.9 | 0.114 |
| Medicines, % | ||||
| Oral hypoglycemic agents Yes No Not eligible Insulin Yes No | 13.7 (9.0–20.4) 4.8 (3.1–7.4) 81.5 (76.1–85.8) 5.30 (3.55–7.83) 94.70 (92.17–96.45) | 0.7 (0.2–2.8) 2.4 (0.9–6.3) 96.9 (92.9–98.6) 0.03 (0.00–0.31) 99.96 (99.69–100.00) | 26.9 (19.0–36.4) 7.3 (4.0–13.0) 65.8 (58.6–72.4) 10.62 (7.37–15.08) 89.37 (84.92–92.63) | 0.079 <0.001 |
| Variables | Glycohemoglobin < 5.7% (50.3%) | Glycohemoglobin ≥ 5.7% (49.7%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Tertile 1 96.8–268.8 µmol/L | Tertile 2 270–385.9 µmol/L | Tertile 3 387.6–1803.7 µmol/L | p-Trend | Tertile 1 115.1–281.2 µmol/L | Tertile 2 281.6–383.2 µmol/L | Tertile 3 385.4–1561 µmol/L | p-Trend | |
| Age, years | 59.5 ± 7.6 | 61.9 ± 8.8 | 62.2 ± 8.7 | 0.013 | 63.8 ± 8.4 | 65.1 ± 9.4 | 65.7 ± 8.9 | 0.149 |
| Non-Hispanic white, % | 85.9 (76.1–92.1) | 82.0 (69.5–90.1) | 85.0 (76.4–90.8) | 0.833 | 73.8 (61.4–83.3) | 63.6 (50.5–74.9) | 67.5 (57.8–75.9) | 0.201 |
| Sex, % | 0.084 | 0.004 | ||||||
| Men | 57.8 (50.9–64.5) | 37.4 (28.4–47.4) | 46.1 (36.4–56.1) | 49.2 (37.3–61.3) | 54.3 (42.6–65.6) | 29.3 (20.7–39.6) | ||
| Women | 42.2 (35.5–49.1) | 62.6 (52.6–71.6) | 53.9 (43.9–63.6) | 50.8 (38.7–62.7) | 45.7 (34.4–57.4) | 70.7 (60.4–79.3) | ||
| Marital status, % | 0.702 | 0.139 | ||||||
| Single/divorced/widowed/never married | 70.2 (54.9–82.0) | 71.5 (55.6–83.4) | 65.9 (49.4–79.4) | 73.1 (65.3–79.6) | 63.7 (50.2–75.4) | 66.1 (55.9–75.1) | ||
| Married/ living as married | 29.8 (18.0–45.1) | 28.5 (16.7–44.4) | 34.1 (20.6–50.6) | 26.9 (20.4–34.7) | 36.3 (24.6–49.8) | 33.9 (24.9–44.1) | ||
| Annual family income, % | 0.021 | 0.217 | ||||||
| $0–19.999 | 15.3 (10.7–21.3) | 11.9 (6.0–21.9) | 7.3 (3.7–14.1) | 22.4 (12.0–38.0) | 21.9 (16.0–29.1) | 16.6 (11.1–24.1) | ||
| $20.000–54.999 | 35.5 (25.9–46.4) | 29.7 (17.2–46.3) | 30.5 (18.6–45.8) | 41.6 (31.7–52.3) | 42.0 (29.5–55.7) | 45.8 (32.3–59.8) | ||
| $55.000–74.999 | 18.9 (11.6–29.3) | 8.3 (3.4–18.9) | 13.0 (5.4–28.4) | 13.9 (6.3–27.9) | 12.1 (6.2–22.5) | 15.6 (9.7–24.1) | ||
| Over $75.000 | 27.6 (17.1–41.4) | 48.7 (31.6–66.0) | 46.0 (33.6–59.0) | 18.1 (9.3–32.5) | 19.0 (9.7–33.8) | 20.0 (10.2–35.3) | ||
| Missing | 2.7 (0.6–11.9) | 1.5 (0.6–3.4) | 3.2 (07–13.1) | 3.9 (1.2–12.4) | 5.0 (2.0–12.0) | 2.1 (0.9–4.9) | ||
| Educational level, % | 0.870 | 0.910 | ||||||
| High school graduate or under | 25.0 (16.2–36.7) | 35.4 (22.7–50.5) | 23.8 (15.7–34.4) | 48.8 (36.4–61.2) | 54.5 (41.8–66.6) | 48.1 (36.8–59.5) | ||
| Some college or above | 75.0 (63.3–83.8) | 64.6 (49.5–77.3) | 76.2 (65.6–84.3) | 51.2 (38.8–63.6) | 45.5 (33.4–58.2) | 51.9 (40.5–63.2) | ||
| Health conditions and habits, % | ||||||||
| Hypertension | 47.6 (33.4–62.2) | 42.9 (29.9–56.9) | 42.6 (30.0–56.3) | 0.509 | 57.7 (37.8–75.4) | 59.4 (49.7–68.5) | 60.9 (50.3–70.6) | 0.749 |
| Diabetes | 0.926 | 0.144 | ||||||
| Pre-diabetes | 2.9 (0.5–15.4) | 0.7 (0.2–2.3) | 2.0 (0.2–16.4) | 1.6 (0.5–4.7) | 3.0 (0.9–10.2) | 7.0 (2.5–18.0) | ||
| Yes | 0.7 (0.2–3.5) | 1.0 (0.3–3.5) | 2.1 (0.5–8.7) | 37.7 (28.0–48.4) | 26.2 (21.4–31.7) | 26.3 (15.2–41.5) | ||
| No | 96.3 (85.9–99.1) | 98.4 (95.8–99.4) | 95.7 (84.9–98.7) | 60.7 (49.8–70.7) | 70.8 (65.7–75.4) | 66.6 (53.6–77.6) | ||
| Missing | 0.1 (0.0–1.1) | - | - | - | - | - | ||
| Smoking | 0.336 | 0.033 | ||||||
| Yes | 17.0 (8.9–30.2) | 12.0 (6.0–22.4) | 9.7 (4.6–19.4) | 29.3 (19.4–41.6) | 19.7 (11.3–32.1) | 11.1(5.3–21.8) | ||
| No | 83.0 (69.8–91.1) | 87.8 (77.6–93.8) | 90.3 (80.6–95.4) | 70.7 (58.4–80.6) | 80.3 (67.9–88.7) | 88.9 (78.2–94.7) | ||
| Missing | - | 0.2 (0.0–1.7) | - | - | - | - | ||
| Arthritis | 0.430 | 0.613 | ||||||
| Yes | 42.3 (26.6–59.8) | 34.7 (23.4–48.0) | 33.7 (21.4–48.6) | 50.4 (36.8–63.9) | 38.9 (32.6–45.6) | 45.8 (32.9–59.4) | ||
| No | 57.7 (40.2–73.4) | 63.7 (50.3–75.3) | 66.3 (51.4–78.6) | 49.6 (36.1–63.2) | 61.1 (54.4–67.4) | 54.2 (40.6–67.1) | ||
| Missing | - | 1.6 (1.0–2.6) | - | - | - | - | ||
| Physical activity, % | 0.090 | 0.026 | ||||||
| Yes | 46.0 (32.5–60.1) | 52.5 (40.6–64.2) | 61.9 (51.2–71.5) | 32.3 (22.0–44.5) | 40.9 (30.2–52.5) | 49.1 (34.7–63.6) | ||
| No | 54.0 (40.0–67.5) | 47.5 (35.7–59.4) | 38.1 (28.5–48.8) | 67.7 (55.5–78.0) | 59.1 (47.5–69.8) | 50.9 (36.4–65.3) | ||
| Moderate PA | 0.403 | 0.028 | ||||||
| Yes | 44.9 (31.7–59.2) | 49.7 (36.6–62.8) | 51.7 (41.1–62.2) | 31.7 (21.6–43.8) | 40.0 (29.3–51.7) | 48.2 (33.9–62.8) | ||
| No | 55.1 (40.8–68.6) | 50.3 (37.2–63.4) | 48.3 (37.8–58.9) | 68.3 (56.2–78.4) | 60.0 (48.3–70.7) | 51.8 (37.2–66.2) | ||
| Vigorous PA | <0.001 | 0.659 | ||||||
| Yes | 6.9 (2.4–18.4) | 16.0 (7.7–30.4) | 27.3 (17.0–40.7) | 4.3 (1.4–12.7) | 9.3 (3.8–21.6) | 5.9 (1.9–16.6) | ||
| No | 93.1 (81.6–97.6) | 84.0 (69.6–92.3) | 72.7 (59.3–83.0) | 95.7 (87.3–98.6) | 90.7 (78.4–96.3) | 94.1 (83.4–98.1) | ||
| Anthropometrics | ||||||||
| Weight, kg | 82.2 ± 16.4 | 77.7 ± 16.5 | 76.7 ± 17.1 | 0.050 | 89.7 ± 27.8 | 86.9 ± 19.9 | 82.5 ± 19.3 | 0.080 |
| Height, m | 1.71 ± 0.1 | 1.66 ± 0.1 | 1.68 ± 0.1 | 0.013 | 1.68 ± 0.1 | 1.67 ± 0.1 | 1.65 ± 0.1 | 0.017 |
| Body mass index, kg/m2 | 27.8 ± 4.5 | 28.1 ± 5.3 | 27.0 ± 4.9 | 0.331 | 31.6 ± 8.9 | 31.1 ± 6.5 | 30.3 ± 6.1 | 0.340 |
| Strength | ||||||||
| Sum of handgrip strength, kg | 73.6 ± 20.8 | 63.8 ± 20.4 | 66.6 ± 21.0 | 0.019 | 63.6 ± 19.0 | 66.8 ± 20.3 | 59.3 ± 19.1 | 0.187 |
| Laboratory data | ||||||||
| Fasting glucose, mg/dL | 97.5 ± 9.0 | 99.9 ± 10.9 | 101.3 ± 8.8 | 0.002 | 125.1 ± 37.0 | 116.3 ± 28.2 | 123.7 ± 46.4 | 0.795 |
| Glycohemoglobin, % | 5.3 ± 0.2 | 5.4 ± 0.2 | 5.3 ± 0.3 | 0.764 | 6.6 ± 1.2 | 6.3 ± 1.0 | 6.4 ± 1.1 | 0.167 |
| Plasma Fatty Acids | ||||||||
| Total plasma ω-3, µmol/L | 214.5 ± 39.0 | 324.2 ± 31.3 | 543.4 ± 194.2 | <0.001 | 227.3 ± 34.4 | 334.9 ± 28.6 | 556.0 ± 188.4 | <0.001 |
| ALA, µmol/L | 66.0 ± 18.6 | 90.8 ± 26.8 | 118.9 ± 57.5 | <0.001 | 68.7 ± 23.4 | 94.0 ± 32.3 | 142.7 ± 94.3 | <0.001 |
| EPA, µmol/L | 46.2 ± 19.1 | 66.6 ± 22.9 | 163.4 ± 161.2 | 0.001 | 42.8 ± 14.6 | 70.3 ± 26.2 | 143.0 ± 82.1 | <0.001 |
| DHA, µmol/L | 102.3 ± 27.8 | 166.8 ± 29.3 | 261.1 ± 70.2 | <0.001 | 115.8 ± 27.8 | 170.7 ± 33.5 | 270.4 ± 92.2 | <0.001 |
| Linoleic acid, µmol/L | 3451.3 ± 549.2 | 3966.6 ± 789.9 | 4149.9 ± 851.4 | <0.001 | 3471.5 ± 738.3 | 3963.1 ± 897.9 | 4445.5 ± 1272.5 | <0.001 |
| Dietary intake | ||||||||
| Energy, kcal | 2190.5 ± 761.8 | 1946.1 ± 633.5 | 1967.0 ± 561.1 | 0.019 | 1918.9 ± 676.2 | 1908.6 ± 664.3 | 1817.3 ± 636.2 | 0.331 |
| Carbohydrate, g | 259.4 ± 99.0 | 235.8 ± 94.9 | 251.5 ± 92.1 | 0.593 | 225.0 ± 84.9 | 234.7 ± 91.4 | 219.8 ± 77.2 | 0.689 |
| Protein, g | 85.9 ± 36.9 | 76.1 ± 24.8 | 76.2 ± 25.4 | 0.076 | 78.1 ± 30.9 | 76.8 ± 26.9 | 73.9 ± 28.1 | 0.410 |
| Protein, g/kg | 1.1 ± 0.4 | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.419 | 0.9 ± 0.4 | 0.9 ± 0.3 | 0.9 ± 0.4 | 0.985 |
| Lipids, g | 85.2 ± 35.4 | 70.8 ± 27.7 | 70.9 ± 23.0 | 0.014 | 77.9 ± 34.3 | 71.1 ± 33.9 | 67.1 ± 31.3 | 0.106 |
| Saturated fat, g | 28.4 ± 11.6 | 21.6 ± 8.8 | 22.1 ± 9.3 | 0.004 | 24.9 ± 11.7 | 22.3 ± 11.6 | 21.6 ± 11.8 | 0.096 |
| Monounsaturated fat, g | 30.7 ± 13.5 | 25.5 ± 12.8 | 25.0 ± 8.7 | 0.010 | 28.3 ± 13.9 | 25.9 ± 13.2 | 23.5 ± 12.3 | 0.104 |
| Polyunsaturated fat, g | 19.4 ± 10.1 | 17.9 ± 7.5 | 18.0 ± 8.6 | 0.397 | 18.1 ± 8.7 | 17.2 ± 9.0 | 16.3 ± 7.8 | 0.276 |
| Total ω-3, g | 1.81 ± 0.96 | 1.79 ± 0.80 | 2.01 ± 1.35 | 0.325 | 1.72 ± 0.84 | 1.63 ± 0.86 | 1.70 ± 0.86 | 0.911 |
| ALA, g | 1.73 ± 0.90 | 1.74 ± 0.78 | 1.88 ± 1.33 | 0.418 | 1.66 ± 0.80 | 1.56 ± 0.82 | 1.58 ± 0.80 | 0.617 |
| EPA, g | 0.03 ± 0.06 | 0.02 ± 0.03 | 0.05 ± 0.10 | 0.078 | 0.02 ± 0.03 | 0.02 ± 0.05 | 0.04 ± 0.10 | 0.004 |
| DHA, g | 0.10 ± 0.10 | 0.04 ± 0.06 | 0.09 ± 0.15 | 0.185 | 0.04 ± 0.06 | 0.05 ± 0.08 | 0.08 ± 0.17 | 0.022 |
| Linoleic acid, g | 17.1 ± 9.1 | 15.7 ± 6.8 | 15.6 ± 7.7 | 0.299 | 16.1 ± 7.8 | 15.2 ± 8.2 | 14.3 ± 7.0 | 0.222 |
| Fiber, g | 17.2 ± 8.2 | 19.4 ± 11.6 | 22.5 ± 15.9 | 0.062 | 15.2 ± 7.0 | 17.8 ± 8.2 | 17.4 ± 8.1 | 0.054 |
| Alcohol, g | 10.3 ± 15.9 | 13.9 ± 27.4 | 8.7 ± 15.6 | 0.699 | 3.8 ± 10.8 | 7.1 ± 16.7 | 9.5 ± 28.6 | 0.242 |
| Medicines, % | ||||||||
| Oral hypoglycemic agents Yes No Not eligible Insulin No Yes | 0.3 (0.0–2.5) 3.3 (0.7–14.5) 96.4 (85.8–99.2) - - | 0.7 (0.1–3.3) 1.0 (0.3–2.8) 98.4 (95.8–99.4) 99.88 (99.04–99.98) 0.12 (0.02–1.00) | 1.3 (0.2–9.7) 2.9 (0.5–14.2) 95.9 (84.9–99.0) - - | 0.884 0.755 | 31.6 (21.3–44.2) 7.6 (3.6–15.6) 60.7 (49.8–70.7) 85.30 (75.33–91.68) 14.71 (8.32–24.67) | 21.8 (17.2–27.3) 7.4 (3.7–14.3) 70.8 (65.7–75.4) 93.09 (87.20–96.40) 6.90 (3.61–12.80) | 26.5 (15.0–42.4) 6.9 (2.8–15.6) 66.7 (53.6–77.6) 90.26 (82.51–94.79) 9.74 (5.21–17.49) | 0.800 0.110 |
| Glycohemoglobin < 5.7% | Glycohemoglobin ≥ 5.7% | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Total plasma ω-3, µmol/L | −0.02 (−0.03; −0.00) | 0.018 | −0.00 (−0.01; 0.01) | 0.936 | −0.01 (−0.03; 0.01) | 0.272 | 0.00 (−0.00; 0.01) | 0.525 |
| ALA, µmol/L | −0.01 (−0.10; 0.08) | 0.819 | 0.01 (−0.02; 0.06) | 0.429 | 0.01 (−0.03; 0.06) | 0.614 | 0.01 (−0.00; 0.03) | 0.148 |
| EPA, µmol/L | −0.01 (−0.03; 0.00) | 0.094 | −0.00 (−0.01; 0.01) | 0.895 | −0.03 (−0.06; 0.01) | 0.105 | −0.00 (−0.01; 0.01) | 0.415 |
| DHA, µmol/L | −0.05 (−0.07; −0.02) | 0.001 | −0.01 (−0.02; 0.01) | 0.485 | −0.03 (−0.05; −0.01) | 0.017 | 0.00 (−0.01; 0.01) | 0.750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, R.A.B.; de Branco, F.M.S.; Nehme, R.; de Oliveira, E.P.; Pena, G.d.G. Association between Plasma Omega-3 and Handgrip Strength According to Glycohemoglobin Levels in Older Adults: Results from NHANES 2011–2012. Nutrients 2022, 14, 4060. https://doi.org/10.3390/nu14194060
Batista RAB, de Branco FMS, Nehme R, de Oliveira EP, Pena GdG. Association between Plasma Omega-3 and Handgrip Strength According to Glycohemoglobin Levels in Older Adults: Results from NHANES 2011–2012. Nutrients. 2022; 14(19):4060. https://doi.org/10.3390/nu14194060
Chicago/Turabian StyleBatista, Raíssa A. B., Flávia M. S. de Branco, Rafaela Nehme, Erick P. de Oliveira, and Geórgia das G. Pena. 2022. "Association between Plasma Omega-3 and Handgrip Strength According to Glycohemoglobin Levels in Older Adults: Results from NHANES 2011–2012" Nutrients 14, no. 19: 4060. https://doi.org/10.3390/nu14194060
APA StyleBatista, R. A. B., de Branco, F. M. S., Nehme, R., de Oliveira, E. P., & Pena, G. d. G. (2022). Association between Plasma Omega-3 and Handgrip Strength According to Glycohemoglobin Levels in Older Adults: Results from NHANES 2011–2012. Nutrients, 14(19), 4060. https://doi.org/10.3390/nu14194060





