TetraSOD®, a Unique Marine Microalgae Ingredient, Promotes an Antioxidant and Anti-Inflammatory Status in a Metabolic Syndrome-Induced Model in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment
2.2. Animals, Diets and Experimental Design
2.3. Body Composition Analyses
2.4. Analysis of Biochemical Parameters in Plasma
2.5. End Point Blood and Tissue Collection
2.6. Analysis of Oxidative Stress and Inflammatory Parameters in Plasma
2.7. Analyses in Liver
2.8. Analyses in WAT
2.9. Total RNA Isolation and cDNA Synthesis
2.10. Primer Design and RT-qPCR Synthesis
2.11. Statistical Analyses
3. Results
3.1. CAF Intake Induces MetS-Like Alterations
3.2. TetraSOD® Supplementation Modulates Oxidative Stress and Inflammatory Markers in the Plasma of CAF-Fed Rats
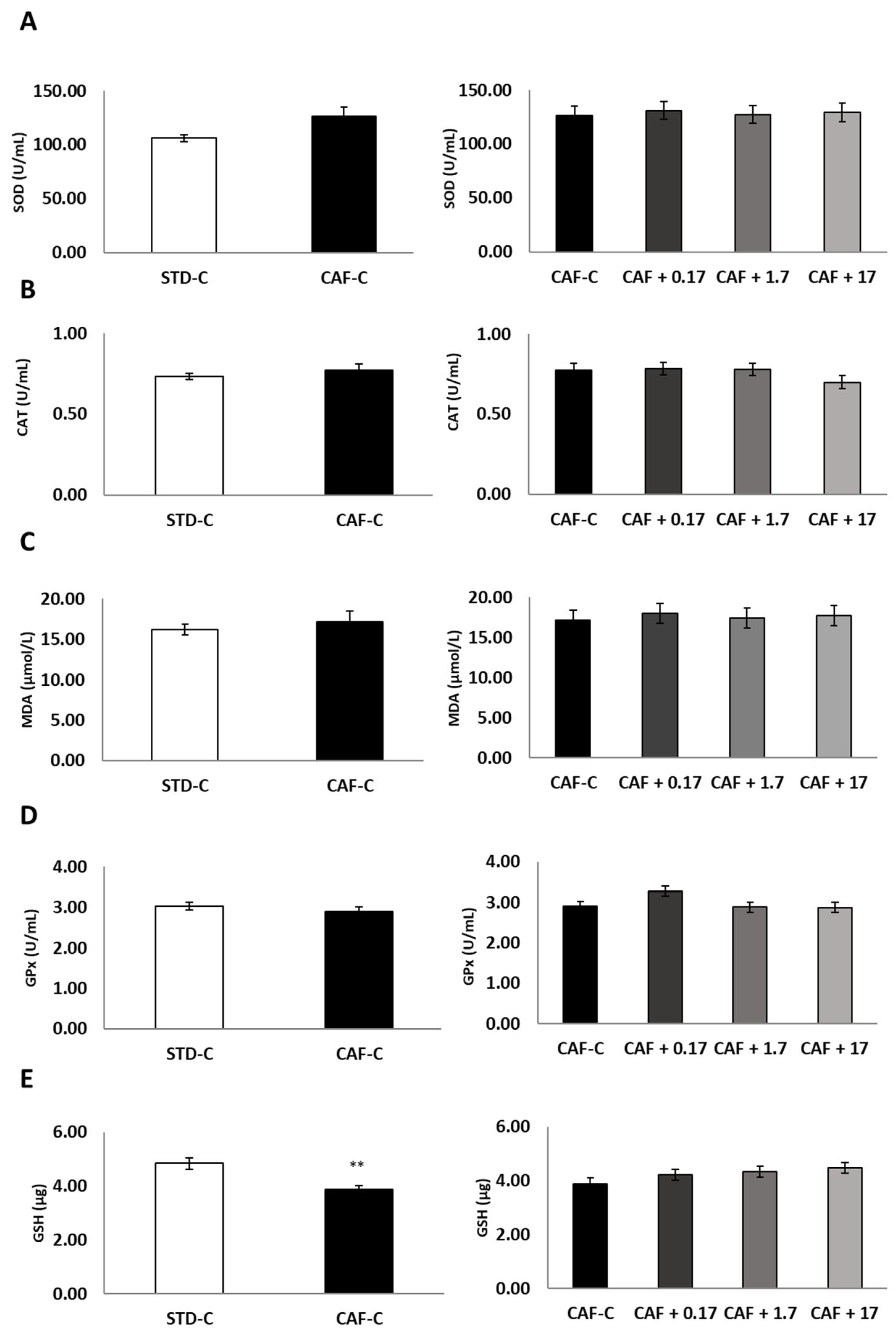
3.3. TetraSOD® Supplementation Promotes Antioxidant Enzymes and GSH in the Livers of CAF-Fed Rats
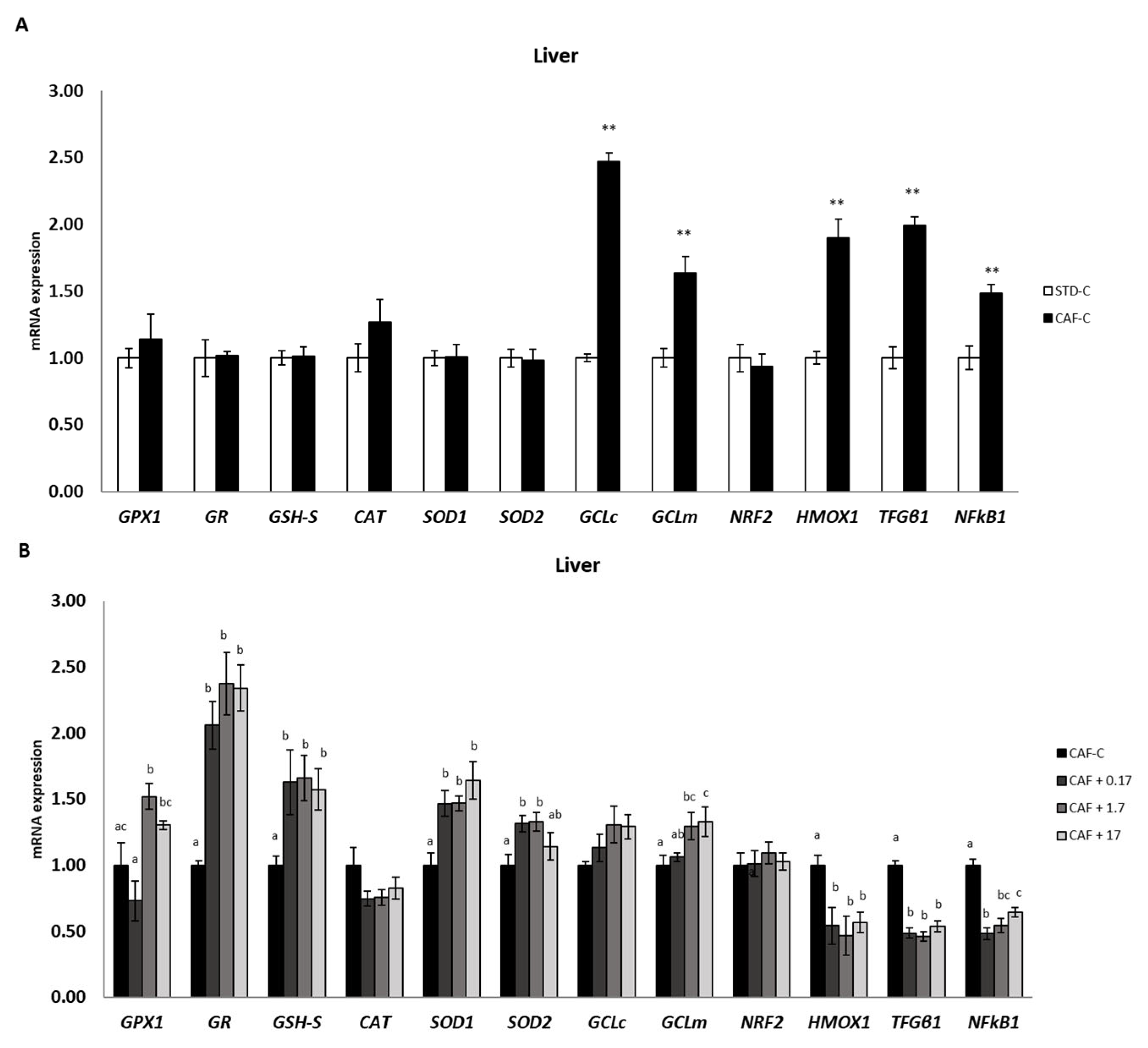
3.4. TetraSOD® Positively Modulates Genes Involved in Antioxidant and Anti-Inflammatory Pathways in the Liver
3.5. TetraSOD® Positively Modulates Genes Involved in Anti-Inflammatory Pathways in MWAT
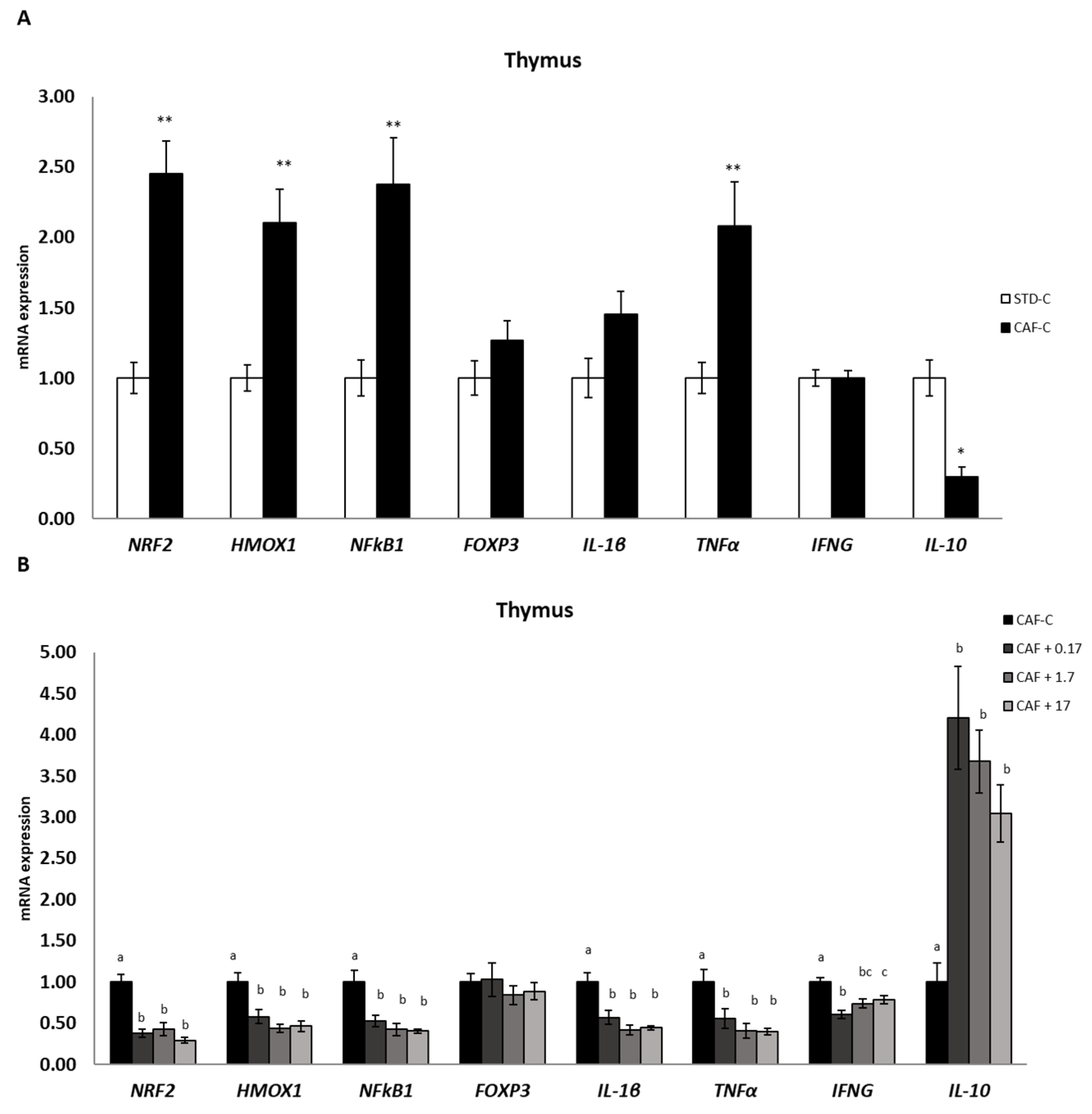
3.6. TetraSOD® Modulates Genes Involved in Immunity and Anti-Inflammatory Pathways in the Thymus
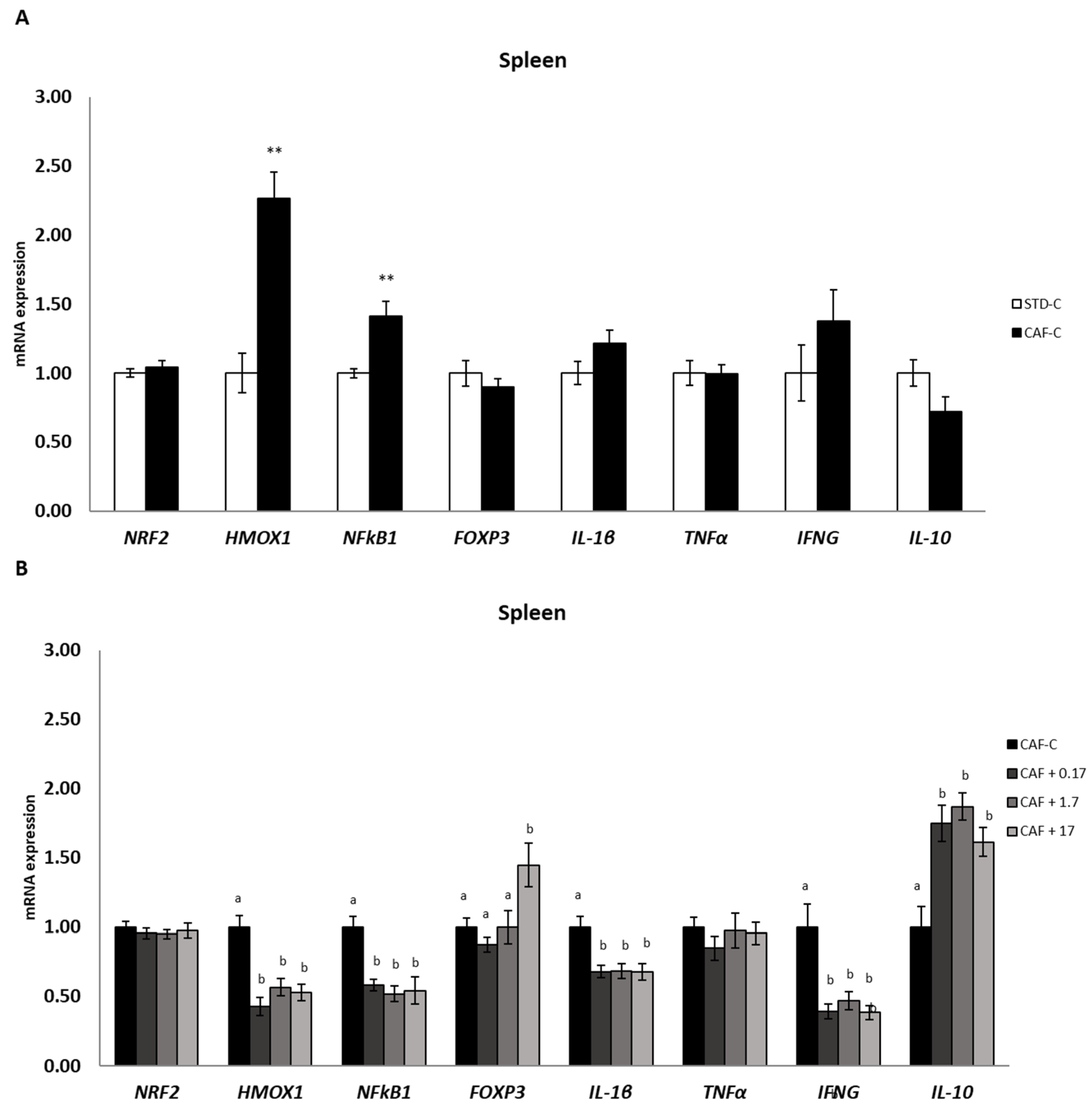
3.7. TetraSOD® Modulates Genes Involved in Immunity and Anti-Inflammatory Pathways in the Spleen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, J. A Comprehensive Review on Metabolic Syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef]
- Asrih, M.; Jornayvaz, F.R. Metabolic syndrome and nonalcoholic fatty liver disease: Is insulin resistance the link? Mol. Cell. Endocrinol. 2015, 418, 55–65. [Google Scholar] [CrossRef]
- Mishra, R.; Monica. Determinants of cardiovascular disease and sequential decision-making for treatment among women: A Heckman’s approach. SSM-Popul. Health 2019, 7, 100365. [Google Scholar] [CrossRef]
- Olusi, S. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int. J. Obes. 2002, 26, 1159–1164. [Google Scholar] [CrossRef]
- Vincent, H.K.; Taylor, A.G. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int. J. Obes. 2006, 30, 400–418. [Google Scholar] [CrossRef]
- Ulla, A.; Alam, M.A.; Sikder, B.; Sumi, F.A.; Rahman, M.M.; Habib, Z.F.; Mohammed, M.K.; Subhan, N.; Hossain, H.; Reza, H.M. Supplementation of Syzygium cumini seed powder prevented obesity, glucose intolerance, hyperlipidemia and oxidative stress in high carbohydrate high fat diet induced obese rats. BMC Complement. Altern. Med. 2017, 17, 289. [Google Scholar] [CrossRef]
- Carillon, J.; Romain, C.; Bardy, G.; Fouret, G.; Feillet-Coudray, C.; Gaillet, S.; Lacan, D.; Cristol, J.-P.; Rouanet, J.-M. Cafeteria diet induces obesity and insulin resistance associated with oxidative stress but not with inflammation: Improvement by dietary supplementation with a melon superoxide dismutase. Free Radic. Biol. Med. 2013, 65, 254–261. [Google Scholar] [CrossRef]
- Molina, N.; Bolin, A.; Otton, R. Green tea polyphenols change the profile of inflammatory cytokine release from lymphocytes of obese and lean rats and protect against oxidative damage. Int. Immunopharmacol. 2015, 28, 985–996. [Google Scholar] [CrossRef]
- Johnson, A.R.; Wilkerson, M.D.; Sampey, B.P.; Troester, M.A.; Hayes, D.N.; Makowski, L. Cafeteria diet-induced obesity causes oxidative damage in white adipose. Biochem. Biophys. Res. Commun. 2016, 473, 545–550. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Arola, L.; Blay, M.; Terra, X. Chronic supplementation with dietary proanthocyanidins protects from diet-induced intestinal alterations in obese rats. Mol. Nutr. Food Res. 2017, 61, 1601039. [Google Scholar] [CrossRef]
- Savini, I.; Catani, M.V.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Pedram, P.; Shahidi, M.; Du, J.; Yi, Y.; Gulliver, W.; Zhang, H.; Sun, G. Significant Beneficial Association of High Dietary Selenium Intake with Reduced Body Fat in the CODING Study. Nutrients 2016, 8, 24. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.-Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxidative Med. Cell. Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef]
- Entezari, M.H.; Khosravi, Z.S.; Kafeshani, M.; Tavasoli, P.; Zadeh, A.H. Effect of Vitamin D supplementation on weight loss, glycemic indices, and lipid profile in obese and overweight women: A clinical trial study. Int. J. Prev. Med. 2018, 9, 63. [Google Scholar] [CrossRef]
- Major, G.C.; Alarie, F.P.; Doré, J.; Tremblay, A. Calcium plus vitamin D supplementation and fat mass loss in female very low-calcium consumers: Potential link with a calcium-specific appetite control. Br. J. Nutr. 2008, 101, 659–663. [Google Scholar] [CrossRef]
- Décordé, K.; Teissèdre, P.-L.; Sutra, T.; Ventura, E.; Cristol, J.-P.; Rouanet, J.-M. Chardonnay grape seed procyanidin extract supplementation prevents high-fat diet-induced obesity in hamsters by improving adipokine imbalance and oxidative stress markers. Mol. Nutr. Food Res. 2008, 53, 659–666. [Google Scholar] [CrossRef]
- Décordé, K.; Ventura, E.; Lacan, D.; Ramos, J.; Cristol, J.-P.; Rouanet, J.-M. An SOD rich melon extract Extramel® prevents aortic lipids and liver steatosis in diet-induced model of atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 301–307. [Google Scholar] [CrossRef]
- Carillon, J.; Jover, B.; Cristol, J.-P.; Rouanet, J.-M.; Richard, S.; Virsolvy, A. Dietary supplementation with a specific melon concentrate reverses vascular dysfunction induced by cafeteria diet. Food Nutr. Res. 2016, 60, 32729. [Google Scholar] [CrossRef]
- Décordé, K.; Agne, A.; Lacan, D.; Ramos, J.; Fouret, G.; Ventura, E.; Feillet-Coudray, C.; Cristol, J.-P.; Rouanet, J.-M. Preventive Effect of a Melon Extract Rich in Superoxide Scavenging Activity on Abdominal and Liver Fat and Adipokine Imbalance in High-Fat-Fed Hamsters. J. Agric. Food Chem. 2009, 57, 6461–6467. [Google Scholar] [CrossRef]
- Carillon, J.; Rouanet, J.M.; Cristol, J.P.; Brion, R. Superoxide Dismutase Administration, a Potential Therapy against Oxidative Stress Related Diseases: Several Routes of Supplementation and Proposal of an Original Mechanism of Action. Pharm. Res. 2013, 30, 2718–2728. [Google Scholar] [CrossRef]
- Mantecón, L.; Moyano, R.; Cameán, A.; Jos, A. Safety assessment of a lyophilized biomass of Tetraselmis chuii (TetraSOD®) in a 90 day feeding study. Food Chem. Toxicol. 2019, 133, 110810. [Google Scholar] [CrossRef]
- Ramirez, P.; Torres, S.; Lama, C.; Mantecón, L.; Unamunzaga, C.; Infante, C. TetraSOD® activates the antioxidant response pathway in human cells: An in vitro approach. Afr. J. Biotechnol. 2020, 19, 367–373. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.; Pagès, T.; Viscor, G.; Torres, J. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Valenzuela, C.A.; De Souza, C.O.; Yaqoob, P.; Miles, E.; Calder, P.C. Comparative anti-inflammatory effects of plant- and marine-derived omega-3 fatty acids explored in an endothelial cell line. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158662. [Google Scholar] [CrossRef]
- Yu, J.; Ma, Y.; Sun, J.; Ran, L.; Li, Y.; Wang, N.; Yu, T.; Gao, W.; Jia, W.; Jiang, R.; et al. Microalgal Oil from Schizochytrium sp. Prevents HFD-Induced Abdominal Fat Accumulation in Mice. J. Am. Coll. Nutr. 2017, 36, 347–356. [Google Scholar] [CrossRef]
- Kokkali, M.; Martí-Quijal, F.J.; Taroncher, M.; Ruiz, M.-J.; Kousoulaki, K.; Barba, F.J. Improved Extraction Efficiency of Antioxidant Bioactive Compounds from Tetraselmis chuii and Phaedoactylum tricornutum Using Pulsed Electric Fields. Molecules 2020, 25, 3921. [Google Scholar] [CrossRef]
- Widowati, I.; Zainuri, M.; Kusumaningrum, H.P.; Susilowati, R.; Hardivillier, Y.; Leignel, V.; Bourgougnon, N.; Mouget, J.-L. Antioxidant activity of three microalgae Dunaliella salina, Tetraselmis chuii and Isochrysis galbana clone Tahiti. IOP Conf. Ser. Earth Environ. Sci. 2017, 55, 012067. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Sharp, M.; Sahin, K.; Stefan, M.; Orhan, C.; Gheith, R.; Reber, D.; Sahin, N.; Tuzcu, M.; Lowery, R.; Durkee, S.; et al. Phytoplankton Supplementation Lowers Muscle Damage and Sustains Performance across Repeated Exercise Bouts in Humans and Improves Antioxidant Capacity in a Mechanistic Animal. Nutrients 2020, 12, 1990. [Google Scholar] [CrossRef]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Raun, K.; Yan, F.-F.; O Larsen, M.; Tang-Christensen, M. Laboratory animals as surrogate models of human obesity. Acta Pharmacol. Sin. 2012, 33, 173–181. [Google Scholar] [CrossRef]
- Sampey, B.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria Diet Is a Robust Model of Human Metabolic Syndrome With Liver and Adipose Inflammation: Comparison to High-Fat Diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Terra, X.; Blay, M. A cafeteria diet triggers intestinal inflammation and oxidative stress in obese rats. Br. J. Nutr. 2017, 117, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Higa, T.S.; Spinola, A.V.; Fonseca-Alaniz, M.H.; Evangelista, F.S.A. Comparison between cafeteria and high-fat diets in the induction of metabolic dysfunction in mice. Int. J. Physiol. Pathophysiol. Pharmacol. 2014, 6, 47–54. [Google Scholar]
- Andersen, C.J.; E Murphy, K.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. Int. Rev. J. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Caimari, A.; del Bas, J.M.; Boqué, N.; Crescenti, A.; Puiggròs, F.; Chenoll, E.; Martorell, P.; Ramón, D.; Genovés, S.; Arola, L. Heat-killed Bifidobacterium animalis subsp. Lactis CECT 8145 increases lean mass and ameliorates metabolic syndrome in cafeteria-fed obese rats. J. Funct. Foods 2017, 38, 251–263. [Google Scholar] [CrossRef]
- Caimari, A.; Del Bas, J.M.; Crescenti, A.; Arola, L. Low doses of grape seed procyanidins reduce adiposity and improve the plasma lipid profile in hamsters. Int. J. Obes. 2012, 37, 576–583. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- García, Á.; Toro-Román, V.; Siquier-Coll, J.; Bartolomé, I.; Muñoz, D.; Maynar-Mariño, M. Effects of Tetraselmis chuii Microalgae Supplementation on Anthropometric, Hormonal and Hematological Parameters in Healthy Young Men: A Double-Blind Study. Environ. Res. Public Health 2022, 19, 6060. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Perseghin, G.; Caumo, A.; Caloni, M.; Testolin, G.; Luzi, L. Incorporation of the Fasting Plasma FFA Concentration into QUICKI Improves Its Association with Insulin Sensitivity in Nonobese Individuals. J. Clin. Endocrinol. Metab. 2001, 86, 4776–4781. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, M.; Sasaki, Y.; Giddings, J.C.; Yamamoto, J. Preventive Effects of Hesperidin, Glucosyl Hesperidin and Naringin on Hypertension and Cerebral Thrombosis in Stroke-prone Spontaneously Hypertensive Rats. Phytother. Res. 2012, 26, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Jankowski, J.; Tepel, M.; Giet, M.; Zidek, W.; Schlüter, H. Oxidized low density lipoprotein. J. Hypertens. 2000, 18, S20. [Google Scholar] [CrossRef]
- Maiolino, G.; Rossitto, G.; Caielli, P.; Bisogni, V.; Rossi, G.P.; Calò, L.A. The Role of Oxidized Low-Density Lipoproteins in Atherosclerosis: The Myths and the Facts. Mediat. Inflamm. 2013, 2013, 714653. [Google Scholar] [CrossRef]
- Modun, D.; Giustarini, D.; Tsikas, D. Nitric Oxide-Related Oxidative Stress and Redox Status in Health and Disease. Oxidative Med. Cell. Longev. 2014, 2014, 129651. [Google Scholar] [CrossRef]
- Lubos, E.; E Handy, D.; Loscalzo, J. Role of oxidative stress and nitric oxide in atherothrombosis. Front. Biosci. 2008, 13, 5323–5344. [Google Scholar] [CrossRef]
- Kim, W.; Kim, M. The Change of Lipid Metabolism and Immune Function Caused by Antioxidant Material in the Hypercholesterolemic Elderly Women in Korea. J. Nutr. Health 2005, 38, 67–75. [Google Scholar]
- Morel, D.W.; E DiCorleto, P.; Chisolm, G.M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arter. Off. J. Am. Heart Assoc. Inc. 1984, 4, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, U. Role of superoxide in endothelial-cell modification of low-density lipoproteins. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1988, 959, 20–30. [Google Scholar] [CrossRef]
- Galle, J.; Schneider, R.; Heinloth, A.; Wanner, C.; Galle, P.R.; Conzelmann, E.; Dimmeler, S.; Heermeier, K. Lp(a) and LDL induce apoptosis in human endothelial cells and in rabbit aorta: Role of oxidative stress. Kidney Int. 1999, 55, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Mascher, D.; Paredes-Carbajal, M.C.; Torres-Durán, P.V.; Zamora-González, J.; Díaz-Zagoya, J.C.; Juárez-Oropeza, M.A. Ethanolic Extract of Spirulina maxima Alters the Vasomotor Reactivity of Aortic Rings from Obese Rats. Arch. Med. Res. 2006, 37, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, T.; Shimizu, K.; Maeda, S. Changes in arterial stiffness and nitric oxide production with Chlorella-derived multicomponent supplementation in middle-aged and older individuals. J. Clin. Biochem. Nutr. 2015, 57, 228–232. [Google Scholar] [CrossRef]
- McAllister, R.M.; Laughlin, M.H. Vascular nitric oxide: Effects of physical activity, -importance for health. Essays Biochem. 2006, 42, 119–131. [Google Scholar] [CrossRef]
- Yang, M.; Chan, H.; Yu, L. Glutathione peroxidase and glutathione reductase activities are partially responsible for determining the susceptibility of cells to oxidative stress. Toxicology 2006, 226, 126–130. [Google Scholar] [CrossRef]
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, S.B. Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, 132–141. [Google Scholar] [CrossRef]
- Kumar, S.M.; Dey, A. Regulation of Glutathione in Health and Disease with Special Emphasis on Chronic Alcoholism and Hyperglycaemia Mediated Liver Injury: A Brief Perspective. Springer Sci. Rev. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J. Glutathione! Integr. Med. (Encinitas) 2014, 13, 8–12. [Google Scholar] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Fernández-Iglesias, A.; Pajuelo, D.; Quesada, H.; Díaz, S.; Bladé, C.; Arola, L.; Salvadó, M.J.; Mulero, M. Grape seed proanthocyanidin extract improves the hepatic glutathione metabolism in obese Zucker rats. Mol. Nutr. Food Res. 2013, 58, 727–737. [Google Scholar] [CrossRef]
- Al-Rejaie, S.S.; Aleisa, A.M.; Sayed-Ahmed, M.M.; Al-Shabanah, O.A.; Abuohashish, H.M.; Ahmed, M.M.; Al-Hosaini, K.A.; Hafez, M.M. Protective effect of rutin on the antioxidant genes expression in hypercholestrolemic male Westar rat. BMC Complement. Altern. Med. 2013, 13, 136. [Google Scholar] [CrossRef]
- Silva, C.F.; Nardin, P.; Grings, M.; Uecker, J.N.; Cerqueira, J.H.; Alvarado-Rincón, J.A.; Abib, R.T.; Schneider, A.; Barros, C.C.; Andreazza, R.; et al. Evaluation of the Redox State of Wistar Rats Submitted to High-Fat Diet Supplemented with Infusion of Ilex paraguariensis. Braz. Arch. Biol. Technol. 2018, 61, e18161189. [Google Scholar] [CrossRef]
- Ide, T. Effect of dietary α-lipoic acid on the mRNA expression of genes involved in drug metabolism and antioxidation system in rat liver. Br. J. Nutr. 2014, 112, 295–308. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Ismail, M.; Al-Naqeep, G.; Chan, K.W. Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radic. Biol. Med. 2010, 48, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Yida, Z.; Imam, M.U.; Ismail, M.; Hou, Z.; Abdullah, M.A.; Ideris, A.; Ismail, N. Edible Bird’s Nest attenuates high fat diet-induced oxidative stress and inflammation via regulation of hepatic antioxidant and inflammatory genes. BMC Complement. Altern. Med. 2015, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of Cell Protection by Heme Oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Biswas, R.; Bagchi, A. NFkB pathway and inhibition: An overview. Comput. Mol. Biol. 2016, 6, 1–20. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Dooley, S.; Weng, H.; Mertens, P.R. Hypotheses on the Role of Transforming Growth Factor-β in the Onset and Progression of Hepatocellular Carcinoma. Dig. Dis. 2009, 27, 93–101. [Google Scholar] [CrossRef]
- Stanton, M.C.; Chen, S.-C.; Jackson, J.V.; Rojas-Triana, A.; Kinsley, D.; Cui, L.; Fine, J.S.; Greenfeder, S.; Bober, L.A.; Jenh, C.-H. Inflammatory Signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J. Inflamm. 2011, 8, 8. [Google Scholar] [CrossRef]
- Ghanbari, M.; Momen Maragheh, S.; Aghazadeh, A.; Mehrjuyan, S.R.; Hussen, B.M.; Abdoli Shadbad, M.; Dastmalchi, N.; Safaralizadeh, R. Interleukin-1 in obesity-related low-grade inflammation: From molecular mechanisms to therapeutic strategies. Int. Immunopharmacol. 2021, 96, 107765. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M.F. Tumour necrosis factor signalling in health and disease. F1000Research 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Riso, P.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium) consumption improves inflammatory status in the obese Zucker rat model of the metabolic syndrome. J. Nutr. Biochem. 2013, 24, 1508–1512. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; González-Abuín, N.; Mulero, M.; Pinent, M.; Ardévol, A.; Blay, M. Procyanidins and docosahexaenoic acid suppress inflammation and boost immune system in cafeteria diet-fed rats. J. Funct. Foods 2015, 15, 61–71. [Google Scholar] [CrossRef]
- Rivera, L.; Morón, R.; Sánchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin Ameliorates Metabolic Syndrome and Improves the Inflammatory Status in Obese Zucker Rats. Obesity 2008, 16, 2081–2087. [Google Scholar] [CrossRef]
- Garcés-Rimón, M.; González, C.; Uranga, J.; Lopez-Miranda, V.; López-Fandiño, R.; Miguel, M. Pepsin Egg White Hydrolysate Ameliorates Obesity-Related Oxidative Stress, Inflammation and Steatosis in Zucker Fatty Rats. PLoS ONE 2016, 11, e0151193. [Google Scholar] [CrossRef]
- Mayer, C.; Côme, M.; Ulmann, L.; Zittelli, G.C.; Faraloni, C.; Nazih, H.; Ouguerram, K.; Chénais, B.; Mimouni, V. Preventive Effects of the Marine Microalga Phaeodactylum tricornutum, Used as a Food Supplement, on Risk Factors Associated with Metabolic Syndrome in Wistar Rats. Nutrients 2019, 11, 1069. [Google Scholar] [CrossRef]
- Mayer, C.; Richard, L.; Côme, M.; Ulmann, L.; Nazih, H.; Chénais, B.; Ouguerram, K.; Mimouni, V. The Marine Microalga, Tisochrysis lutea, Protects against Metabolic Disorders Associated with Metabolic Syndrome and Obesity. Nutrients 2021, 13, 430. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Y.; Chen, X.; Xiong, W.; Tang, Y.; Lin, L. Spirulina platensis alleviates chronic inflammation with modulation of gut microbiota and intestinal permeability in rats fed a high-fat diet. J. Cell. Mol. Med. 2020, 24, 8603–8613. [Google Scholar] [CrossRef]
- Amine, H.; Benomar, Y.; Haimeur, A.; Messaouri, H.; Meskini, N.; Taouis, M. Odontella aurita-enriched diet prevents high fat diet-induced liver insulin resistance. J. Endocrinol. 2015, 228, 1–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nacer, W.; Ahmed, F.Z.B.; Merzouk, H.; Benyagoub, O.; Bouanane, S. Evaluation of the anti-inflammatory and antioxidant effects of the microalgae Nannochloropsis gaditana in streptozotocin-induced diabetic rats. J. Diabetes Metab. Disord. 2020, 19, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Sharp, M.; Wilson, J.; Stefan, M.; Gheith, R.; Lowery, R.; Ottinger, C.; Reber, D.; Orhan, C.; Sahin, N.; Tuzcu, M.; et al. Marine phytoplankton improves recovery and sustains immune function in humans and lowers proinflammatory immunoregulatory cytokines in a rat model. Phys. Act. Nutr. 2021, 25, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, Inflammation and Diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143–152. [Google Scholar] [CrossRef]
- Yang, H.; Youm, Y.-H.; Vandanmagsar, B.; Ravussin, A.; Gimble, J.M.; Greenway, F.; Stephens, J.M.; Mynatt, R.L.; Dixit, V.D. Obesity Increases the Production of Proinflammatory Mediators from Adipose Tissue T Cells and Compromises TCR Repertoire Diversity: Implications for Systemic Inflammation and Insulin Resistance. J. Immunol. 2010, 185, 1836–1845. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Folco, E.J.; Sukhova, G.; Shimizu, K.; Gotsman, I.; Vernon, A.H.; Libby, P. Interferon-γ, a Th1 Cytokine, Regulates Fat Inflammation. Circ. Res. 2008, 103, 467–476. [Google Scholar] [CrossRef]
- O’Rourke, R.W.; Metcalf, M.D.; White, A.E.; Madala, A.; Winters, B.R.; Maizlin, I.I.; A Jobe, B.A.; Roberts, C.T., Jr.; Slifka, M.K.; Marks, D.L. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int. J. Obes. 2009, 33, 978–990. [Google Scholar] [CrossRef]
- de Waal Malefyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; De Vries, J.E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 Inhibits Cytokine Production by Activated Macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar]
- Wang, P.; Wu, P.; Siegel, M.I.; Egan, R.W.; Billah, M.M. Interleukin (IL)-10 Inhibits Nuclear Factor кB (NFкB) Activation in Human Monocytes: IL-10 AND IL-4 SUPPRESS CYTOKINE SYNTHESIS BY DIFFERENT MECHANISMS. J. Biol. Chem. 1995, 270, 9558–9563. [Google Scholar] [CrossRef]
- Tian, Y.; Mollo, S.B.; Harrington, L.E.; Zajac, A.J. IL-10 Regulates Memory T Cell Development and the Balance between Th1 and Follicular Th Cell Responses during an Acute Viral Infection. J. Immunol. 2016, 197, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; Galgani, M.; De Rosa, V.; Matarese, G. Intracellular metabolic pathways control immune tolerance. Trends Immunol. 2012, 33, 3815–3822. [Google Scholar] [CrossRef]
- Borghetti, P.; Saleri, R.; Mocchegiani, E.; Corradi, A.; Martelli, P. Infection, immunity and the neuroendocrine response. Veter-Immunol. Immunopathol. 2009, 130, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Youm, Y.-H.; Vandanmagsar, B.; Rood, J.; Kumar, K.G.; Butler, A.; Dixit, V.D. Obesity accelerates thymic aging. Blood 2009, 114, 3803–3812. [Google Scholar] [CrossRef] [PubMed]
- Harapko, T. Structural changes of the spleen in experimental obesity. Rep. Morphol. 2019, 25, 36–41. [Google Scholar] [CrossRef]
- Lamas, O.; Martinez, J.; Marti, A. T-helper lymphopenia and decreased mitogenic response in cafeteria diet-induced obese rats. Nutr. Res. 2002, 22, 497–506. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Landrier, J.; Kasiri, E.; Karkeni, E.; Mihály, J.; Béke, G.; Weiss, K.; Lucas, R.; Aydemir, G.; Salles, J.; Walrand, S.; et al. Reduced adiponectin expression after high-fat diet is associated with selective up-regulation of ALDH1A1 and further retinoic acid receptor signaling in adipose tissue. FASEB J. 2016, 31, 203–211. [Google Scholar] [CrossRef]
- Surendar, J.; Frohberger, S.J.; Karunakaran, I.; Schmitt, V.; Stamminger, W.; Neumann, A.-L.; Wilhelm, C.; Hoerauf, A.; Hübner, M.P. Adiponectin Limits IFN-γ and IL-17 Producing CD4 T Cells in Obesity by Restraining Cell Intrinsic Glycolysis. Front. Immunol. 2019, 10, 2555. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]

| STD Diet | CAF Diet | |
|---|---|---|
| Protein (%) | 20 | 10 |
| Fat (%) | 13 | 31.9 |
| Saturated fat (%) | 1.9 | 14.4 |
| Carbohydrate (%) | 67 | 58.1 |
| Sugar (%) | 39.8 |
| Gene Symbol | Gene Name | Primer Sequence | ENSEMBL Accession Number | Amplicon Length (bp) | Optimal Ta (°C) |
|---|---|---|---|---|---|
| ACDC | Adiponectin | F: 5′-ATTATGACGGCAGCACTGGCAA-3′ | ENSRNOG00000001821.6 | 149 | 59.2 |
| R: 5′-TTCCTGATACTGGTCGTAGGTGA-3′ | |||||
| FOXP3 | Forkhead box P3 | F: 5′-CTCTGCACCTTCCCACGCTCA-3′ | ENSRNOG00000011702.6 | 142 | 62.9 |
| R: 5′-GAAACTCTCCCGGCTCCTCGAA-3′ | |||||
| GCLc | Glutamate–cysteine ligase, catalytic subunit | F: 5′-GGAGCGAGATGCCGTCTTACAGG-3′ | ENSRNOG00000006302.4 | 102 | 60.2 |
| R: 5′-GAGCTGGTCTGGGCCTTGCTA-3′ | |||||
| GCLm | Glutamate–cysteine ligase, modifier subunit | F: 5′-CACAATGACCCAAAAGAACTGCTC-3′ | ENSRNOG00000013409.4 | 124 | 60.3 |
| R: 5′-TCACGATGACCGAGTACCTCAGC-3′ | |||||
| GPx1 | Glutathione peroxidase 1 | F: 5′-CGTCCCTCGGCACCACG-3′ | ENSRNOG00000048812.2 | 68 | 61.3 |
| R: 5′-ACGAGGCCCCAGACGCTT-3′ | |||||
| GR | Glutathione-disulfide reductase | F: 5′-CACTTCTCACCCCAGTTGCGA-3′ | ENSRNOG00000014915.6 | 103 | 60.2 |
| R: 5′-ACGGTAGGGATGTTGTCATAGTCCA-3′ | |||||
| GSH-S | Glutathione synthetase | F: 5′-CGCCTTCCTGGAGCAAACACT-3′ | ENSRNOG00000018964.2 | 125 | 59.8 |
| R: 5′-GATTGAGGCCCAGGAACACAGT-3′ | |||||
| HMOX1 | Heme oxygenase 1 | F: 5′-GACCGCCTTCCTGCTCAACA-3′ | ENSRNOG00000014117.7 | 103 | 60.6 |
| R: 5′-CTGGCGAAGAAACTCTGTCTGTGA-3′ | |||||
| IFNG | Interferon gamma | F: 5′-GACAACCAGGCCATCAGCAACAAC-3′ | ENSRNOG00000007468.2 | 118 | 58.7 |
| R: 5′-TCACCTCGAACTTGGCGATGCTCA-3′ | |||||
| IL-1β | Interleukin 1 beta | F: 5′-CTACCTATGTCTTGCCCGTGGA-3′ | ENSRNOG00000004649.4 | 124 | 59.9 |
| R: 5′-CATCACACACTAGCAGGTCGTC-3′ | |||||
| IL-10 | Interleukin 10 | F: 5′-TGGCCCAGAAATCAAGGAGCATT-3′ | ENSRNOG00000004647.5 | 116 | 59.7 |
| R: 5′-CCACTGCCTTGCTTTTATTCTCACA-3′ | |||||
| NRF2 | Nuclear factor, erythroid 2-like 2 | F: 5′-TGCTGCCATTAGTCAGTCGCTCT-3′ | ENSRNOG00000001548.6 | 104 | 60.4 |
| R: 5′-CCGTGCCTTCAGTGTGCTTC-3′ | |||||
| NFkB1 | Nuclear factor kappa B subunit 1 | F: 5′-GAAGATGTGGTGGAGGACTTGCTGA-3′ | ENSRNOG00000023258.6 | 140 | 61.5 |
| R: 5′-GCTGCCTTGCTGTTCTTGAGT-3′ | |||||
| SOD1 | Superoxide dismutase 1 | F: 5′-ATTAACTGAAGGCGAGCATGGGT-3′ | ENSRNOG00000002115.6 | 137 | 60.2 |
| R: 5′-CTCCAACATGCCTCTCTTCATCCG-3′ | |||||
| SOD2 | Superoxide dismutase 2 | F: 5′-AGAACCCAAAGGAGAGTTGCTGGA-3′ | ENSRNOG00000019048.4 | 111 | 60.1 |
| R: 5′-CCCCAGCCTGAACCTTGGAC-3′ | |||||
| TGFβ1 | Transforming growth factor, beta 1 | F: 5′-CTACCAGAAATATAGCAACAATTCCT-3′ | ENSRNOG00000020652.4 | 141 | 60.0 |
| R: 5′-AAGCCCTGTATTCCGTCTCCT-3′ | |||||
| TNFα | Tumour necrosis factor | F: 5′-ATGGGCTGTACCTTATCTACTCC-3′ | ENSRNOG00000055156.1 | 100 | 59.4 |
| R: 5′-TATGAAATGGCAAATCGGCTGAC-3′ | |||||
| B2M | Beta-2 microglobulin | F: 5′-GCCCAACTTCCTCAACTGCTACGTG-3′ | ENSRNOG00000017123.5 | 148 | 59.5 |
| R: 5′-AGTGTGAGCCAGGATGTAGAAAGACC-3′ | |||||
| PPIA | Peptidylprolyl isomerase A | F: 5′-TATCTGCACTGCCAAGACTGA-3′ | ENSRNOG00000027864.5 | 122 | 59.3 |
| R: 5′-TGCTGGTCTTGCCATTCCTG-3′ | |||||
| RPLP1 | Ribosomal protein lateral stalk subunit P1 | F: 5′-CGGCAGTCCACAACATGGCT-3′ | ENSRNOG00000013874.5 | 100 | 60.6 |
| R: 5′-TTGATCTTATCCTCCGTGACCGT-3′ | |||||
| RPL32 | Ribosomal protein L32 | F: 5′-CCAAGAAGTTCATCAGGCACCAGT-3′ | ENSRNOG00000010746.5 | 101 | 59.6 |
| R: 5′-CTTGAATCTTCTCCGCACCCTGT-3′ | |||||
| SDHA | Succinate dehydrogenase complex flavoprotein subunit A | F: 5′-TGGACAGAGCCTCAAGTTCG-3′ | ENSRNOG00000013331.5 | 116 | 60.5 |
| R: 5′-TGTCATACCGCAGAGATCGTC-3′ |
| STD | CAF | p | |
|---|---|---|---|
| Body composition | |||
| Weight (g) | 379.5 ± 6.2 | 441.1 ± 6.8 * | <0.001 |
| Weight change (g) | 124.1 ± 5.4 | 189.1 ± 6.4 * | <0.001 |
| Fat (%) | 3.3 ± 0.5 | 12.2 ± 0.6 * | <0.001 |
| Lean (%) | 92.2 ± 0.5 | 83.6 ± 0.7 * | <0.001 |
| Dietary parameters | |||
| Cumulative Energy intake (kcal) | 536.2 ± 9.1 | 1130.5 ± 20.4 * | <0.001 |
| Cumulative Protein (g) | 32.2 ± 0.5 | 28.4 ± 0.5 * | 0.001 |
| Cumulative Carbohydrates (g) | 77.8 ± 1.3 | 177.9 ± 3.5 * | <0.001 |
| Cumulative Fat (g) | 10.7 ± 0.2 | 34.2 ± 0.6 * | <0.001 |
| Cumulative Fibre (g) | 25.4 ± 0.4 | 8.2 ± 0.4 * | <0.001 |
| Plasma parameters | |||
| TC (mmol/L) | 3.1 ± 0.1 | 2.9 ± 0.1 | 0.200 |
| HDL-C (mmol/L) | 1.8 ± 0.1 | 1.8 ± 0.0 | 0.655 |
| LDL/VLDL-C (mmol/L) | 1.2 ± 0.1 | 1.1 ± 0.0 | 0.409 |
| oxLDL (ngl/mL) | 31.9 ± 0.7 | 30.5 ± 0.5 | 0.224 |
| TG (mmol/L) | 0.7 ± 0.1 | 2.3 ± 0.1 * | <0.001 |
| NEFA (mmol/L) | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.275 |
| Glucose (mmol/L) | 6.3 ± 0.1 | 7.3 ± 0.1 * | <0.001 |
| Insulin (pmol/L) | 130.8 ± 12.7 | 366.7± 29.2 * | <0.001 |
| HOMA-IR | 5.1 ± 0.7 | 16.1 ± 1.5 * | <0.001 |
| R-QUICKI | 0.3 ± 0.0 | 0.2 ± 0.0 * | <0.001 |
| STD-C | CAF-C | p | CAF + 0.17 | CAF + 1.7 | CAF + 17 | p | |
|---|---|---|---|---|---|---|---|
| Body composition | |||||||
| Weight (g) | 420.7 ± 7.6 | 529.5 ± 18.1 * | <0.001 | 538.5 ± 20.9 | 535.8 ± 14.9 | 520.5 ± 14.5 | 0.826 |
| Weight change (g) | 165.3 ± 6.9 | 275.9 ± 17.4 * | <0.001 | 286.2 ± 20.1 | 283.9 ± 14.5 | 270.3 ± 13.6 | 0.782 |
| Fat (%) | 4.8 ± 0.7 | 17.9 ± 1.7 * | <0.001 | 16.9 ± 2.4 | 17.5 ± 1.4 | 16.8 ± 1.7 | 0.875 |
| Lean (%) | 90.9 ± 0.7 | 78.5 ± 1.7 * | <0.001 | 79.6 ± 2.3 | 78.9 ± 1.4 | 79.7 ± 1.7 | 0.817 |
| Lean/fat | 24.1 ± 4.3 | 5.1 ± 0.8 * | <0.001 | 5.9 ± 1.0 | 4.9 ± 0.5 | 4.6 ± 0.4 | 0.249 |
| Adiposity index (%) | 3.5 ± 0.3 | 8.1 ± 0.7 * | <0.001 | 8.1 ± 0.9 | 7.9 ± 0.5 | 7.8 ± 0.6 | 0.871 |
| RWAT (g) | 4.3 ± 0.5 | 13.8 ± 1.6 * | <0.001 | 14.9 ± 2.1 | 13.6 ± 1.1 | 12.8 ± 1.2 | 0.312 |
| MWAT (g) | 2.8 ± 0.2 | 8.2 ± 0.8 * | <0.001 | 8.5 ± 1.3 | 8.4 ± 0.7 | 7.7 ± 0.7 | 0.747 |
| EWAT (g) | 4.2 ± 0.4 | 12.1 ± 1.5 * | <0.001 | 12.7 ± 1.9 | 11.4 ± 1.1 | 11.1 ± 0.7 | 0.956 |
| IWAT (g) | 2.9 ± 0.3 | 8.4 ± 0.8 * | <0.001 | 8.4 ± 1.2 | 8.5 ± 0.7 | 7.9 ± 0.8 | 0.881 |
| BAT (g) | 0.4 ± 0.0 | 0.8 ± 0.1 * | <0.001 | 0.7 ± 0.1 | 0.8 ± 0.0 | 0.8 ± 0.1 | 0.400 |
| Muscle (g) | 2.9 ± 0.1 | 3.1 ± 0.1 | 0.420 | 3.1 ± 0.1 | 3.0 ± 0.1 | 3.0 ± 0.1 | 0.937 |
| Liver (g) | 11.1 ± 0.5 | 15.9 ± 0.6 * | <0.001 | 16.3 ± 0.7 | 16.8 ± 0.6 | 14.8 ± 0.5 | 0.194 |
| Thymus (g) | 0.3 ± 0.0 | 0.6 ± 0.1 * | 0.001 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.904 |
| Spleen (g) | 0.8 ± 0.0 | 0.9 ± 0.1 | 0.170 | 0.9 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.170 |
| Cecum (g) | 4.5 ± 0.3 | 3.7 ± 0.1 * | 0.042 | 4.5 ± 0.3 | 4.5 ± 0.3 | 3.7 ± 0.1 | 0.526 |
| Small intestine (cm) | 105.5 ± 2.2 | 115.2 ± 2.8 * | 0.015 | 112.8 ± 2.6 | 116.4 ± 2.4 | 111.6 ± 2.5 | 0.565 |
| Colon (cm) | 15.6 ± 0.5 | 17.3 ± 0.8 | 0.085 | 15.6 ± 0.8 | 15.6 ± 0.6 | 14.9 ± 0.6 | 0.126 |
| Dietary parameters | |||||||
| Cumulative Energy intake (kcal) | 1005.3 ± 16.5 | 2208.8 ± 88.8 * | <0.001 | 2236.5 ± 78.5 | 2280.6 ± 97.3 | 2158.5 ± 48.6 | 0.727 |
| Cumulative Protein (g) | 60.3 ± 1.0 | 53.7 ± 2.3 * | 0.022 | 54.2 ± 2.0 | 54.2 ± 2.1 | 51.8 ± 1.4 | 0.813 |
| Cumulative Carbohydrates (g) | 145.9 ± 2.4 | 346.4 ± 9.2 * | <0.001 | 358.7 ± 13.2 | 364.8 ± 15.4 | 342.7 ± 9.0 | 0.500 |
| Cumulative Fat (g) | 20.2 ± 0.3 | 64.1 ± 2.7 * | <0.001 | 66.3 ± 2.6 | 68.5 ± 3.9 | 65.5 ± 1.5 | 0.759 |
| Cumulative Fibre (g) | 47.7 ± 0.8 | 14.6 ± 1.5 * | <0.001 | 14.1 ± 1.2 | 13.1 ± 1.2 | 12.9 ± 1.3 | 0.682 |
| Plasma parameters | |||||||
| TC (mmol/L) | 3.4 ± 0.1 | 3.0 ± 0.2 | 0.062 | 3.1 ± 0.2 | 3.2 ± 0.1 | 3.2 ± 0.1 | 0.611 |
| HDL-C (mmol/L) | 1.9 ± 0.1 | 1.8 ± 0.1 | 0.363 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.1 | 0.781 |
| LDL/VLDL-C (mmol/L) | 1.2 ± 0.1 | 1.2 ± 0.1 | 0.724 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.0 | 0.171 |
| oxLDL (ngl/mL) | 26.1 ± 1.3 | 27.0 ± 1.0 | 0.577 | 26.5 ± 1.2 | 25.9 ± 1.0 | 23.8 ± 1.0 | 0.166 |
| TG (mmol/L) | 1.0 ± 0.1 | 1.8 ± 0.1 * | <0.001 | 2.2 ± 0.3 | 2.0 ± 0.1 | 2.3 ± 0.2 | 0.218 |
| NEFA (mmol/L) | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.813 | 0.6 ± 0.1 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.440 |
| Glucose (mmol/L) | 5.6 ± 0.2 | 7.1 ± 0.4 * | 0.003 | 6.4 ± 0.2 | 6.6 ± 0.3 | 6.4 ± 0.2 | 0.292 |
| Insulin (pmol/L) | 216.4 ± 17.4 | 414.3± 55.6 * | 0.001 | 429.8 ± 44.6 | 368.6 ± 36.7 | 420.9 ± 51.2 | 0.755 |
| HOMA-IR | 7.5 ± 0.8 | 18.0 ± 3.0 * | 0.008 | 17.2 ± 2.3 | 15.3 ± 1.9 | 16.4 ± 2.3 | 0.806 |
| R-QUICKI | 0.3 ± 0.0 | 0.2 ± 0.0 * | 0.001 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.642 |
| NOx (µmol/L) | 8.9 ± 0.4 | 8.5 ± 0.6 a | 0.551 | 13.3 ± 0.9 b | 9.6 ± 0.9 ab | 10.1 ± 0.9 ab | 0.048 |
| IL-10 (pg/mL) | 89.4 ± 18.3 | 39.4 ± 4.6 * a | 0.013 | 41.8 ± 11.1 a | 45.4 ± 10.5 a | 84.4 ± 10.5 b | 0.018 |
| MCP-1 (ng/mL) | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.432 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.434 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Cardoso, K.; Del Bas, J.M.; Caimari, A.; Lama, C.; Torres, S.; Mantecón, L.; Infante, C. TetraSOD®, a Unique Marine Microalgae Ingredient, Promotes an Antioxidant and Anti-Inflammatory Status in a Metabolic Syndrome-Induced Model in Rats. Nutrients 2022, 14, 4028. https://doi.org/10.3390/nu14194028
Gil-Cardoso K, Del Bas JM, Caimari A, Lama C, Torres S, Mantecón L, Infante C. TetraSOD®, a Unique Marine Microalgae Ingredient, Promotes an Antioxidant and Anti-Inflammatory Status in a Metabolic Syndrome-Induced Model in Rats. Nutrients. 2022; 14(19):4028. https://doi.org/10.3390/nu14194028
Chicago/Turabian StyleGil-Cardoso, Katherine, Josep M. Del Bas, Antoni Caimari, Carmen Lama, Sonia Torres, Lalia Mantecón, and Carlos Infante. 2022. "TetraSOD®, a Unique Marine Microalgae Ingredient, Promotes an Antioxidant and Anti-Inflammatory Status in a Metabolic Syndrome-Induced Model in Rats" Nutrients 14, no. 19: 4028. https://doi.org/10.3390/nu14194028
APA StyleGil-Cardoso, K., Del Bas, J. M., Caimari, A., Lama, C., Torres, S., Mantecón, L., & Infante, C. (2022). TetraSOD®, a Unique Marine Microalgae Ingredient, Promotes an Antioxidant and Anti-Inflammatory Status in a Metabolic Syndrome-Induced Model in Rats. Nutrients, 14(19), 4028. https://doi.org/10.3390/nu14194028







