Potential Modulation of Inflammation by Probiotic and Omega-3 Supplementation in Elderly with Chronic Low-Grade Inflammation—A Randomized, Placebo-Controlled Trial
Abstract
1. Background
1.1. Low-Grade Inflammation in the Elderly and Inflammaging
1.2. The Use of Nutritional Supplements to Reduce Low-Grade Inflammation
1.2.1. Probiotics
1.2.2. Omega-3
1.2.3. Dual Supplementation
1.3. Significance and Aim
2. Materials and Methods
2.1. Ethics Approval
2.2. Design
2.3. Subjects
2.4. Study Intervention
2.5. Randomization and Blinding
2.6. Study Outcomes
2.6.1. Blood Sample Collection and Analysis
2.6.2. In Vivo Intestinal Permeability Test
2.7. Sample Preparation and Determination of Sugar Concentration
2.7.1. Short-Chain Fatty-Acids in Stool Samples
2.7.2. Sit-to-Stand Test (SST)
2.7.3. Western Ontario and McMaster Osteoathritis Index (WOMAC)
2.7.4. Food Frequency Questionnaire (FFQ)
2.8. Statistical Analysis
2.8.1. Power Calculation
2.8.2. Data Analysis
2.8.3. Compliance
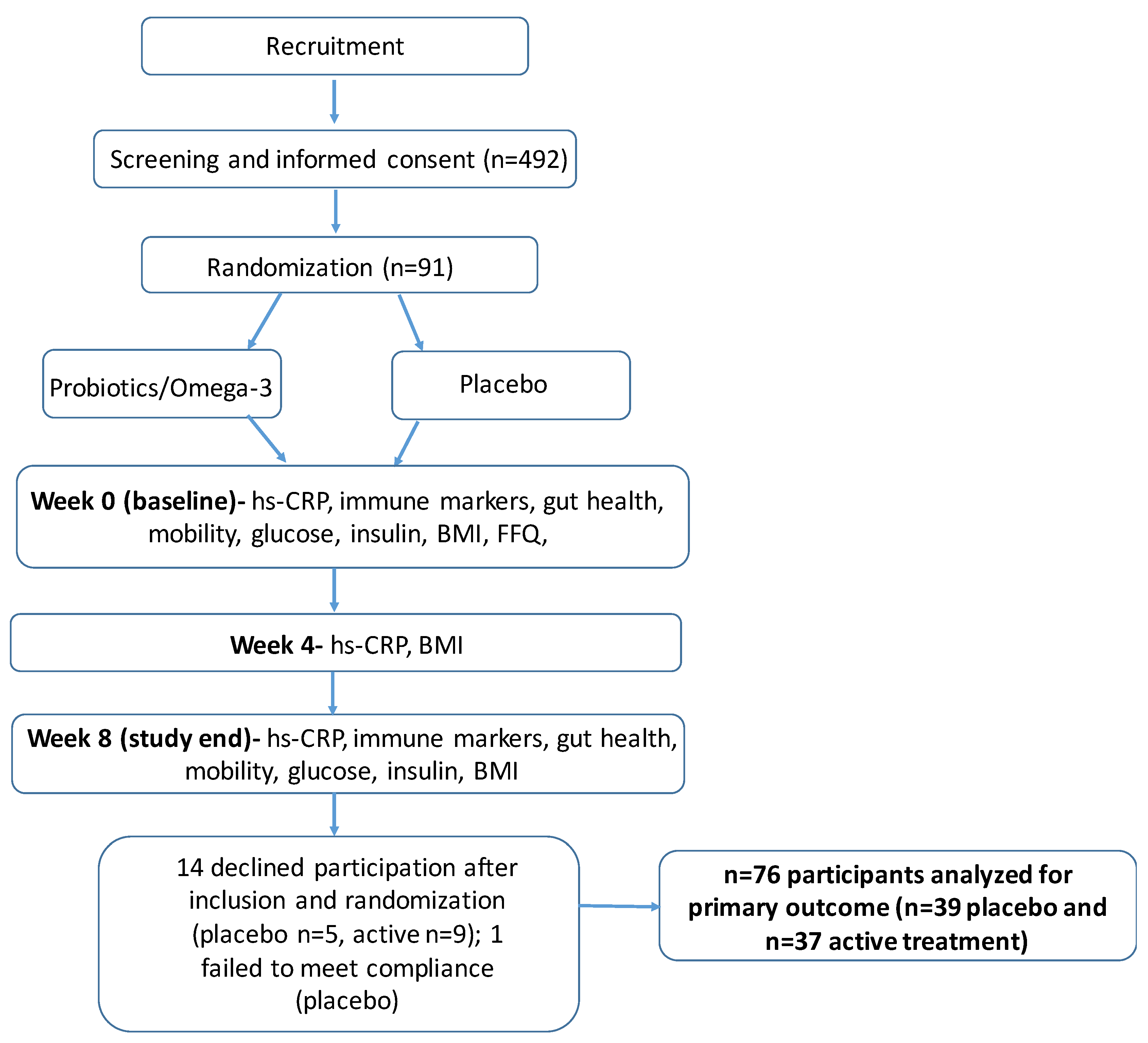
3. Results
3.1. Sample Characteristics
3.2. Effect of Dual Supplementation on hs-CRP Levels and Other Inflammatory Markers
3.3. Gut-Health
3.3.1. Effects on Intestinal Permeability
I-FABP
SCFA in Stool
3.4. Mobility
3.5. Health Monitoring
- -
- Mild: Transient symptoms, no interference with the subject’s daily activities
- -
- Moderate: Marked symptoms, moderate interference with the subject’s daily activities but still acceptable
- -
- Severe: Considerable interference with the subject’s daily activities, unacceptable.
3.6. Compliance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, J.E. Human population: The next half century. Science 2003, 302, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125 (Suppl. 2), S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef]
- Cohen, H.J.; Pieper, C.F.; Harris, T.; Rao, K.M.K.; Currie, M.S. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, M201–M208. [Google Scholar] [CrossRef]
- Ferrucci, L.; Semba, R.D.; Guralnik, J.M.; Ershler, W.B.; Bandinelli, S.; Patel, K.V.; Sun, K.; Woodman, R.C.; Andrews, N.; Cotter, R.J.; et al. Proinflammatory state, hepcidin, and anemia in older persons. Blood 2010, 115, 3810–3816. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Leggio, F.; Vacante, M.; Motta, M.; Giordano, M.; Biondi, A.; Basile, F.; Mastrojeni, S.; Mistretta, A.; Malaguarnera, M.; et al. Probiotics in the gastrointestinal diseases of the elderly. J. Nutr. Health Aging 2012, 16, 402–410. [Google Scholar] [CrossRef]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2018, 9, 3160. [Google Scholar] [CrossRef]
- Moro-García, M.A.; Alonso-Arias, R.; Baltadjieva, M.; Benítez, C.F.; Barrial, M.A.F.; Ruisánchez, E.D.; Santos, R.A.; Sánchez, M.; Miján, J.S.; López-Larrea, C. Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age 2013, 35, 1311–1326. [Google Scholar] [CrossRef]
- Costabile, A.; Bergillos-Meca, T.; Rasinkangas, P.; Korpela, K.; De Vos, W.M.; Gibson, G.R. Effects of Soluble Corn Fiber Alone or in Synbiotic Combination with Lactobacillus rhamnosus GG and the Pilus-Deficient Derivative GG-PB12 on Fecal Microbiota, Metabolism, and Markers of Immune Function: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Elderly (Saimes Study). Front. Immunol. 2017, 8, 1443. [Google Scholar] [CrossRef]
- Den, H.; Dong, X.; Chen, M.; Zou, Z. Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer’s disease or mild cognitive impairment- a meta-analysis of randomized controlled trials. Aging 2020, 12, 2010–4039. [Google Scholar] [CrossRef]
- Hutchinson, A.; Bergh, C.; Kruger, K.; Sűsserová, M.; Allen, J.; Améen, S.; Tingö, L. The Effect of Probiotics on Health Outcomes in the Elderly: A Systematic Review of Randomized, Placebo-Controlled Studies. Microorganisms 2021, 9, 1344. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Generoso, S.D.V.; Rodrigues, N.M.; Trindade, L.M.; Paiva, N.C.; Cardoso, V.N.; Carneiro, C.M.; Ferreira, A.V.D.M.; Faria, A.M.C.; Maioli, T.U. Dietary supplementation with omega-3 fatty acid attenuates 5-fluorouracil induced mucositis in mice. Lipids Health Dis. 2015, 14, 54. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef]
- Reinders, I.; Song, X.; Visser, M.; Eiriksdottir, G.; Gudnason, V.; Sigurdsson, S.; Aspelund, T.; Siggeirsdottir, K.; Brouwer, I.A.; Harris, T.B.; et al. Plasma phospholipid PUFAs are associated with greater muscle and knee extension strength but not with changes in muscle parameters in older adults. J. Nutr. 2015, 145, 105–112. [Google Scholar] [CrossRef]
- Rousseau, J.H.; Kleppinger, A.; Kenny, A.M. Self-reported dietary intake of omega-3 fatty acids and association with bone and lower extremity function. J. Am. Geriatr. Soc. 2009, 57, 1781–1788. [Google Scholar] [CrossRef]
- Robinson, S.M.; Jameson, K.A.; Batelaan, S.F.; Martin, H.J.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Sayer, A.A.; Hertfordshire Cohort Study Group. Diet and its relationship with grip strength in community-dwelling older men and women: The Hertfordshire cohort study. J. Am. Geriatr. Soc. 2008, 56, 84–90. [Google Scholar] [CrossRef]
- Takayama, M.; Arai, Y.; Sasaki, S.; Hashimoto, M.; Shimizu, K.; Abe, Y.; Hirose, N. Association of marine-origin n-3 polyunsaturated fatty acids consumption and functional mobility in the community-dwelling oldest old. J. Nutr. Health Aging 2013, 17, 82–89. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Custodero, C.; Mankowski, R.T.; Lee, S.A.; Chen, Z.; Wu, S.; Manini, T.M.; Echeverri, J.H.; Sabbà, C.; Beavers, D.P.; Cauley, J.A.; et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2018, 46, 42–59. [Google Scholar] [CrossRef]
- Hutchinson, A.N.; Tingö, L.; Brummer, R.J. The Potential Effects of Probiotics and ω-3 Fatty Acids on Chronic Low-Grade Inflammation. Nutrients 2020, 12, 2402. [Google Scholar] [CrossRef]
- Costanzo, M.; Cesi, V.; Palone, F.; Pierdomenico, M.; Colantoni, E.; Leter, B.; Vitali, R.; Negroni, A.; Cucchiara, S.; Stronati, L. Krill oil, vitamin D and Lactobacillus reuteri cooperate to reduce gut inflammation. Benef. Microbes 2018, 9, 389–399. [Google Scholar] [CrossRef]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overwehight adults: A randomized, controlled trial. Mediat. Inflamm. 2014, 204, 348959. [Google Scholar] [CrossRef]
- van Wijck, K.; van Eijk, H.M.; Buurman, W.A.; Dejong, C.H.; Lenaerts, K. Novel analytical approach to a multi-sugar whole gut permeability assay. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2794–2801. [Google Scholar] [CrossRef]
- van Wijck, K.; Verlinden, T.J.; van Eijk, H.M.; Dekker, J.; Buurman, W.A.; Dejong, C.H.; Lenaerts, K. Novel multi-sugar assay for site-specific gastrointestinal permeability analysis: A randomized controlled crossover trial. Clin. Nutr. 2013, 32, 245–251. [Google Scholar] [CrossRef]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; Macdonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; De Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef] [PubMed]
- Mujagic, Z.; Ludidi, S.; Keszthelyi, D.; Hesselink, M.A.M.; Kruimel, J.W.; Lenaerts, K.; Hanssen, N.M.J.; Conchillo, J.M.; Jonkers, D.M.A.E.; Masclee, A.A.M. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment. Pharmacol. Ther. 2014, 40, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, I.R.; Lentle, R.G.; Kruger, M.C.; Hurst, R.D. Standardising the lactulose mannitol test of gut permeability to minimise error and promote comparability. PLoS ONE 2014, 9, e99256. [Google Scholar] [CrossRef]
- Rubio, M.F.R.; Eriksson, U.; Brummer, R.J.; König, J. Short intense psychological stress induced by skydiving does not impair intestinal barrier function. PLoS ONE 2021, 16, e0254280. [Google Scholar] [CrossRef]
- Jones, S.E.; Kon, S.S.C.; Canavan, J.L.; Patel, M.S.; Clark, A.L.; Nolan, C.M.; Polkey, M.I.; Man, W.D.-C. The five-repetition sit-to-stand test as a functional outcome measure in COPD. Thorax 2013, 68, 1015–1020. [Google Scholar] [CrossRef]
- McConnell, S.; Kolopack, P.; Davis, A.M. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): A review of its utility and measurement properties. Arthritis Rheumatol. 2001, 45, 453–461. [Google Scholar] [CrossRef]
- Christensen, S.E.; Möller, E.; Bonn, S.; Ploner, A.; Wright, A.; Sjölander, A.; Bälter, O.; Lissner, L.; Bälter, K. Two new meal- and web-based interactive food frequency questionnaires: Validation of energy and macronutrient intake. J. Med. Internet Res. 2013, 15, e109. [Google Scholar] [CrossRef]
- Ding, C.M.; Wu, Y.H.; Liu, X.F. Diagnostic Accuracy of Intestinal Fatty Acid Binding Protein for Acute Intestinal Ischemia: A Systematic Review and Meta-Analysis. Clin. Lab. 2020, 66, 34371. [Google Scholar] [CrossRef]
- Adriaanse, M.P.M.; Tack, G.J.; Passos, V.L.; Damoiseaux, J.G.M.C.; Schreurs, M.W.J.; Van Wijck, K.; Riedl, R.G.; Masclee, A.A.M.; Buurman, W.A.; Mulder, C.J.J.; et al. Serum I-FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment. Pharmacol. Ther. 2013, 37, 482–490. [Google Scholar] [CrossRef]
- Heida, F.; Hulscher, J.; Schurink, M.; Timmer, A.; Kooi, E.; Bos, A.; Bruggink, J.; Kasper, D.; Pones, M.; Benkoe, T. Intestinal fatty acid-binding protein levels in Necrotizing Enterocolitis correlate with extent of necrotic bowel: Results from a multicenter study. J. Pediatr. Surg. 2015, 50, 1115–1118. [Google Scholar] [CrossRef]
- Finamore, A.; Roselli, M.; Donini, L.M.; Brasili, E.; Rami, R.; Carnevali, P.; Mistura, L.; Pinto, A.; Giusti, A.; Mengheri, E. Supplementation with Bifidobacterium longum Bar33 and Lactobacillus helveticus Bar13 mixture improves immunity in elderly humans (over 75 years) and aged mice. Nutrition 2019, 63–64, 184–192. [Google Scholar] [CrossRef]
- Bower, J.K.; Lazo, M.; Juraschek, S.; Selvin, E. Within-person variability in high-sensitivity C-reactive protein. Arch. Intern. Med. 2012, 172, 1519–1521. [Google Scholar] [CrossRef]
- Nasermoaddeli, A.; Sekine, M.; Kagamimori, S. Intra-individual variability of high-sensitivity C-reactive protein: Age-related variations over time in Japanese subjects. Circ. J. 2006, 70, 559–563. [Google Scholar] [CrossRef][Green Version]
- Wu, S.; Li, Y.; Jin, C.; Yang, P.; Li, D.; Li, H.; Shen, C. Intra-individual variability of high-sensitivity C-reactive protein in Chinese general population. Int. J. Cardiol. 2012, 157, 75–79. [Google Scholar] [CrossRef]
- Larsen, O.F.A.; Claassen, E.; Brummer, R.J. On the importance of intraindividual variation in nutritional research. Benef. Microbes 2020, 11, 511–517. [Google Scholar] [CrossRef]
- Spaiser, S.J.; Culpepper, T.; Nieves, C., Jr.; Ukhanova, M.; Mai, V.; Percival, S.S.; Christman, M.C.; Langkamp-Henken, B. Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and Bifidobacterium longum MM-2 Ingestion Induces a Less Inflammatory Cytokine Profile and a Potentially Beneficial Shift in Gut Microbiota in Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. J. Am. Coll. Nutr. 2015, 34, 459–469. [Google Scholar] [CrossRef]
- Nyangale, E.P.; Farmer, S.; Cash, H.A.; Keller, D.; Chernoff, D.; Gibson, G.R. Bacillus coagulans GBI-30, 6086 Modulates Faecalibacterium prausnitzii in Older Men and Women. J. Nutr. 2015, 145, 1446–1452. [Google Scholar] [CrossRef]
- Sierra, S.; Lara-Villoslada, F.; Olivares, M.; Jiménez, J.; Boza, J.; Xaus, J. IL-10 expression is involved in the regulation of the immune response by omega 3 fatty acids. Nutr. Hosp. 2004, 1986, 376–382. [Google Scholar]
- Dagdeviren, S.; Jung, D.Y.; Friedline, R.H.; Noh, H.L.; Kim, J.H.; Patel, P.R.; Tsitsilianos, N.; Inashima, K.; Tran, D.A.; Hu, X.; et al. IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle. FASEB J. 2017, 31, 701–710. [Google Scholar] [CrossRef]
- Meador, B.M.; Krzyszton, C.P.; Johnson, R.W.; Huey, K.A. Effects of IL-10 and age on IL-6, IL-1beta, and TNF-alpha responses in mouse skeletal and cardiac muscle to an acute inflammatory insult. J. Appl. Physiol. 2008, 104, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Sansoni, P.; Vescovini, R.; Fagnoni, F.; Biasini, C.; Zanni, F.; Zanlari, L.; Telera, A.; Lucchini, G.; Passeri, G.; Monti, D.; et al. The immune system in extreme longevity. Exp. Gerontol. 2008, 43, 61–65. [Google Scholar] [CrossRef]
- Rink, L.; Cakman, I.; Kirchner, H. Altered cytokine production in the elderly. Mech. Ageing Dev. 1998, 102, 199–209. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Arunachalam, K.; Gill, H.S.; Chandra, R.K. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). Eur. J. Clin. Nutr. 2000, 54, 263–267. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Barker, H.A.; D’Ari, L.; Kahn, J. Enzymatic reactions in the degradation of 5-aminovalerate by Clostridium aminovalericum. J. Biol. Chem. 1987, 262, 8994–9003. [Google Scholar] [CrossRef]
- Valerio, F.; Russo, F.; de Candia, S.; Riezzo, G.; Orlando, A.; Lonigro, S.L.; Lavermicocca, P. Effects of probiotic Lactobacillus paracasei-enriched artichokes on constipated patients: A pilot study. J. Clin. Gastroenterol. 2010, 44 (Suppl. 1), S49–S53. [Google Scholar] [CrossRef]
- Lai, Z.; Shan, W.; Li, J.; Min, J.; Zeng, X.; Zuo, Z. Appropriate exercise level attenuates gut dysbiosis and valeric acid increase to improve neuroplasticity and cognitive function after surgery in mice. Mol. Psychiatry 2021, 26, 7167–7187. [Google Scholar] [CrossRef]
- Yamashiro, K.; Tanaka, R.; Urabe, T.; Ueno, Y.; Yamashiro, Y.; Nomoto, K.; Takahashi, T.; Tsuji, H.; Asahara, T.; Hattori, N. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS ONE 2017, 12, e0171521. [Google Scholar] [CrossRef]
- Wei, D.; Ma, P.; Fan, Q.; Yu, H.; Peng, Y.; Li, X. Yanning Syrup ameliorates the lipopolysaccharide-induced inflammation: Adjusting the gut microbiota, short-chain fatty acids, and the CD4(+) T cell balance. J. Ethnopharmacol. 2022, 283, 114729. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Sun, Y.; Pan, D.; Chang, B.; Sang, L.-X. Nicotinamide Ameliorates Dextran Sulfate Sodium-Induced Chronic Colitis in Mice through Its Anti-Inflammatory Properties and Modulates the Gut Microbiota. J. Immunol. Res. 2021, 2021, 5084713. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Liang, H.; Ji, X.; Zhou, Z.; Liu, Y.; Sun, T.; Zhang, L. Effects of fucoidan on gut flora and tumor prevention in 1,2-dimethylhydrazine-induced colorectal carcinogenesis. J. Nutr. Biochem. 2020, 82, 108396. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, X.; Wei, W.; Li, R.; Hu, K.; Liu, X.; Jiang, W.; Liu, S.; Wang, W.; Sun, H.; et al. The Association of Fried Meat Consumption with the Gut Microbiota and Fecal Metabolites and Its Impact on Glucose Homoeostasis, Intestinal Endotoxin Levels, and Systemic Inflammation: A Randomized Controlled-Feeding Trial. Diabetes Care 2021, 44, 1970–1979. [Google Scholar] [CrossRef]
| Placebo (n = 39) | Treatment (n = 37) | p-Value | |||
|---|---|---|---|---|---|
| women:men [%] | 64.1 | 35.9 | 64.9 | 35.1 | |
| count | 25 | 14 | 24 | 13 | |
| age [years] | 70.0 | 65–81 | 71.0 | 65–80 | 0.261 |
| height [cm] | 169.2 | 158–186 | 169.5 | 151–186 | 0.925 |
| weight [kg] | 72.2 | 47–91 | 70.6 | 50–92 | 0.938 |
| BMI [kg/m2] | 24.6 | 18–27 | 24.7 | 20–27 | 0.767 |
| Fiber [g] | 25.7 | 4.6–133.5 | 24.6 | 11.0–95.9 | 0.618 |
| EPA [g] | 0.10 | 0.01–0.34 | 0.10 | 0.01–0.41 | 0.912 |
| DHA [g] | 0.21 | 0.02–0.65 | 0.20 | 0.01–0.85 | 0.679 |
| Vitamin D [µg] | 8.05 | 2.48–34.2 | 7.35 | 3.43–16.1 | 0.791 |
| Placebo | Active Treatment | Significance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Pre-Treatment | Post- Treatment | % Change Pre-Post | n | Pre- Treatment | Post- Treatment | % Change Pre-Post | p: Post-Treatment | p: % Change Pre-Post | |
| hs-CRP [g/mL] | 39 | 1.77 (1.04–3.240) | 1.79 (1.06–3.75) | 5.56 (−70.53–32.39) | 37 | 1.43 (0.94–1.89) | 1.49 (1.15–2.16) | 3.40 (−18.41–35.76) | 0.095 * | 0.50 |
| IL-12p70 [pg/mL] | 37 | 0.069 (0.038–0.11) | 0.074 (0.044–0.12) | 0.064 (−25.52–63.47) | 35 | 0.065 (0.041–0.086) | 0.075 (0.041–0.092) | −0.74 (−15.76–13.67) | 0.55 | 0.39 |
| IL-6 [pg/mL] | 39 | 0.51 (0.34–1.23) | 0.65 (0.42–1.06) | 10.15 (−16.76–55.51) | 37 | 0.44 (0.35–0.80) | 0.53 (0.40–0.84) | 8.18 (−18.97–36.61) | 0.16 | 0.36 |
| IFN-gamma [pg/mL] | 39 | 5.53 (3.34–8.95) | 5.68 (3.23–10.30) | 0.31 (−29.66–40.61) | 37 | 5.87 (3.84–7.88) | 6.16 (3.22–8.65) | −2.65 (−28.03–33.87) | 0.87 | 0.94 |
| TNF [pg/mL] | 39 | 1.25 (0.93–1.98) | 1.24 (0.89–1.93) | −1.69 (−14.43–13.11) | 37 | 1.19 (0.66–0.11) | 1.14 (0.83–1.52) | −3.05 (−10.02–5.47) | 0.65 | 0.80 |
| IL-8 [pg/mL] | 39 | 13.51 (11.30–16.83) | 12.87 (10.42–171.2) | −2.66 (−16.58–12.74) | 37 | 12.42 (9.48–16.25) | 11.53 (9.35–17.57) | −5.35 (−17.93–8.69) | 0.50 | 0.66 |
| IL-10 [pg/mL] | 39 | 0.21 (0.12–0.27) | 0.24 (0.11–0.29) | 14.79 (−9.96–32.81) | 37 | 0.23 (0.14–0.34) | 0.26 (0.16–0.36) | 18.23 (−0.38–32.71) | 0.050 ** | 0.54 |
| IL-4 [pg/mL] | 39 | 0.019 (0.013–0.028) | 0.015 (0.012–0.026) | −10.91 (−26.67–22.57) | 37 | 0.016 (0.0091–0.026) | 0.014 (0.0085–0.020) | −27.23 (−45.72–52.56) | 0.38 | 0.42 |
| Placebo | Active Treatment | Significance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Pre-Treatment | Post- Treatment | % Change Pre-Post | n | Pre- Treatment | Post- Treatment | % Change Pre-Post | p: Post-Treatment | p: % Change Pre-Post | |
| In vivo intestinal permeability | ||||||||||
| Sugar excretion: | ||||||||||
| 0–5 h: | ||||||||||
| L/R ratio | 37 | 0.03 (0.02–0.06) | 0.04 (0.02–0.06) | 13.2 (−11–59.7) | 34 | 0.03 (0.01–0.04) | 0.03 (0.02–0.05) | 4.2 (−25.9–44.5) | 0.27 | 0.33 |
| Sucrose [μg/mL] | 38 | 55.5 (13.8–181.6) | 41.8 (12.8–381.1) | 0.05 (−40.82–46.4) | 34 | 41.1 (17.8–188.3) | 36.2 (18.1–275.7) | −4.9 (−39.8–29.4) | 0.92 | 0.58 |
| 6–24 h: | ||||||||||
| S/E ratio | 35 | 0.03 (0.02–0.03) | 0.02 (0.02–0.03) | −2.8 (−27.1–24.4) | 31 | 0.02 (0.02–0.03) | 0.02 (0.02–0.03) | −1.4 (−20.8–22.2) | 0.31 | 0.98 |
| Intestinal damage marker | ||||||||||
| I-FABP [pg/mL] | 39 | 382.8 (264–545.6) | 329 (256.4–565.5) | −6 (−34.3–55.8) | 37 | 352.3 (242.6–621.7) | 381.2 (218.3–608.6) | −0.4 (−29.6–40.4) | 0.56 | 0.94 |
| Stool short-chain fatty-acids | ||||||||||
| [µmol/g in fecal dry mass] | ||||||||||
| Acetic acid | 19 | 87.1 (72.7–113.8) | 89.3 (75.7–120) | 4.1 (−18.7–29.8) | 20 | 95.2 (74.1–140.5) | 97.6 (79.4–143.6) | −6.4 (−27.6–51.3) | 0.55 | 0.81 |
| Propionic acid | 19 | 18.7 (15.2–24.4) | 20.5 (12.8–25.3) | 7.2 (−37.9–40) | 20 | 17.1 (12.2–45.8) | 18.1 (14.6–33.7) | −2.5 (−40.1–75.8) | 0.99 | 0.86 |
| Iso-butyric acid | 19 | 2.9 (2.4–3.8) | 2.9 (2–3.8) | −9 (−30.4–31.5) | 20 | 2.3 (1.8–2.9) | 3.4 (2.2–5) | 24.7 (−26.6–130.5) | 0.32 | 0.0948 * |
| Butyric acid | 19 | 16.2 (8.7–24.9) | 14.9 (7.6–26.1) | 24.2 (−50.3–95) | 20 | 14.9 (9.2–24.3) | 15.8 (11.83–29.8) | 19.8 (−44.3–131.4) | 0.53 | 0.61 |
| Iso-valeric acid | 19 | 4.1 (3.1–5.5) | 4.3 (3–5.4) | −6.9 (−19.2–38.9) | 20 | 3.4 (2.3–4.5) | 5.1 (3–7.1) | 20.5 (−22.7–100.5) | 0.19 | 0.18 |
| Valeric acid | 19 | 2.3 (1.9–3.5) | 2.5 (1.8–3.5) | 2.9 (−31.2–48.6) | 20 | 2.9 (2.1–5.1) | 3.7 (2.4–4.8) | 10.9 (−36.7–94.4) | 0.0436 ** | 0.56 |
| Iso-caproic acid | 19 | 0.2 (0.1–0.5) | 0.2 (0.2–0.5) | 12.8 (−49.6–117.8) | 20 | 0.4 (0.2–0.5) | 0.4 (0.3–0.6) | 25.1 (−21.5–198.6) | 0.20 | 0.73 |
| Hexanoic acid | 19 | 0.5 (0.3–1.79 | 0.7 (0.4–2) | 14.4 (−24.5–112.49 | 20 | 1.1 (0.6–1.7) | 1.4 (0.8–2.4) | 3.6 (−35.6–78.99) | 0.24 | 0.86 |
| Heptanoic acid | 19 | 0.5 (0.4–0.6) | 0.4 (0.3–0.5) | −11.9 (−68.6–27.5) | 20 | 0.4 (0.2–0.5) | 0.5 (0.3–0.69 | 7.8 (−25.9–170) | 0.17 | 0.12 |
| Placebo | Active Treatment | Significance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Pre- Treatment | Post- Treatment | % Change Pre-Post | n | Pre- Treatment | Post- Treatment | % Change Pre-Post | p: Post-Treatment | p: % Change Pre-Post | |
| Vitamin D [ng/mL] | 36 | 25.00 (19.78–28.28) | 28.85 (25.43–32.73) | 16.04 (9.44–32.62) | 34 | 24.50 (20.90–30.53) | 31.55 (27.20–37.15) | 23.98 (11.75–38.43) | 0.018 ** | 0.12 |
| EPA [mg/100 mL] | 39 | 4.64 (3.27–5.87) | 3.79 (2.21–5.68) | −13.64 (−37.57–17.45) | 37 | 3.53 (2.61–5.95) | 7.56 (5.13–9.64) | 46.23 (24.82–61.93) | 0.0001 ** | 0.0001 ** |
| DHA [mg/100 mL plasma] | 39 | 13.29 (10.14–14.79) | 11.64 (4.48–15.63) | −20.20 (−77.20–5.62) | 37 | 9.48 (5.91–13.37) | 12.16 (8.17–14.47) | 13.80 (−13.58–36.25) | Baseline difference | 0.0003 ** |
| EPA + DHA [mg/100 mL] | 39 | 17.60 (14.42–20.92) | 14.92 (6.29–21.35) | −21.64 (−74.06–6.31) | 37 | 13.86 (9.12–23.76) | 19.68 (12.85–23.76) | 28.69 (3.91–44.22) | Baseline difference | 0.0001 ** |
| n-3 PUFA [mg/100 mL] | 39 | 23.55 (20.02–26.75) | 19.76 (9.07–29.19) | −19.03 (−79.45–8.22) | 37 | 18.42 (11.57–23.98) | 24.82 (17.27–28.38) | 28.50 (−2.78–41.06) | Baseline difference | 0.0001 ** |
| n-6:n-3 ratio [mg/100 mL] | 39 | 4.89 (4.13–5.48) | 4.66 (4.11–5.91) | 1.37 (−19.20–13.18) | 37 | 4.40 (3.85–5.11) | 3.23 (2.81–3.75) | −40.67 (−61.36–20.74) | 0.0001 ** | 0.0001 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tingö, L.; Hutchinson, A.N.; Bergh, C.; Stiefvatter, L.; Schweinlin, A.; Jensen, M.G.; Krüger, K.; Bischoff, S.C.; Brummer, R.J. Potential Modulation of Inflammation by Probiotic and Omega-3 Supplementation in Elderly with Chronic Low-Grade Inflammation—A Randomized, Placebo-Controlled Trial. Nutrients 2022, 14, 3998. https://doi.org/10.3390/nu14193998
Tingö L, Hutchinson AN, Bergh C, Stiefvatter L, Schweinlin A, Jensen MG, Krüger K, Bischoff SC, Brummer RJ. Potential Modulation of Inflammation by Probiotic and Omega-3 Supplementation in Elderly with Chronic Low-Grade Inflammation—A Randomized, Placebo-Controlled Trial. Nutrients. 2022; 14(19):3998. https://doi.org/10.3390/nu14193998
Chicago/Turabian StyleTingö, Lina, Ashley N. Hutchinson, Cecilia Bergh, Lena Stiefvatter, Anna Schweinlin, Morten G. Jensen, Kirsten Krüger, Stephan C. Bischoff, and Robert J. Brummer. 2022. "Potential Modulation of Inflammation by Probiotic and Omega-3 Supplementation in Elderly with Chronic Low-Grade Inflammation—A Randomized, Placebo-Controlled Trial" Nutrients 14, no. 19: 3998. https://doi.org/10.3390/nu14193998
APA StyleTingö, L., Hutchinson, A. N., Bergh, C., Stiefvatter, L., Schweinlin, A., Jensen, M. G., Krüger, K., Bischoff, S. C., & Brummer, R. J. (2022). Potential Modulation of Inflammation by Probiotic and Omega-3 Supplementation in Elderly with Chronic Low-Grade Inflammation—A Randomized, Placebo-Controlled Trial. Nutrients, 14(19), 3998. https://doi.org/10.3390/nu14193998







