Trimester-Specific Serum Fructosamine in Association with Abdominal Adiposity, Insulin Resistance, and Inflammation in Healthy Pregnant Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements of Fructosamine and Albumin-Corrected Fructosamine
2.3. Measurements of Adiposity
2.4. Measurements of Glucose Homeostasis
2.5. Measurements of Inflammation
2.6. Other Variables
2.7. Statistical Analyses
3. Results
3.1. Participant Characteristics
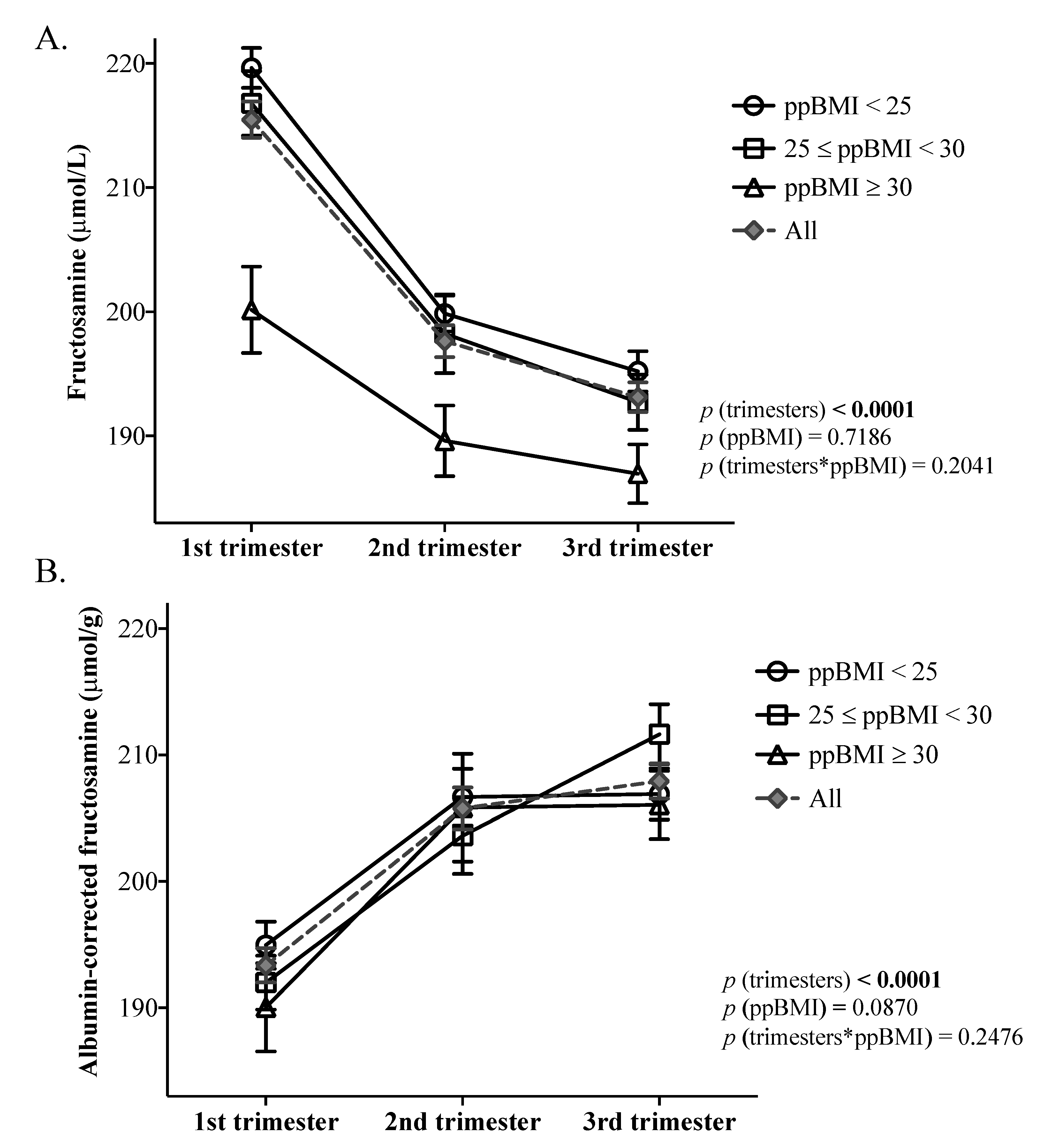
3.2. Variations in Fructosamine and Albumin-Corrected Fructosamine throughout Pregnancy
3.3. Associations between Fructosamine and Albumin-Corrected Fructosamine and Adiposity Measurements
3.4. Associations between Fructosamine and Albumin-Corrected Fructosamine and Glucose Homeostasis Measurements
3.5. Associations between Fructosamine and Albumin-Corrected Fructosamine and Inflammation Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poblete, J.A.; Olmos, P. Obesity and Gestational Diabetes in Pregnant Care and Clinical Practice. Curr. Vasc. Pharm. 2021, 19, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.G.; Souza, A.S.R.; Figueiroa, J.N.; de Araújo, C.A.L.; Guimarães, A.; Ray, J.G. Visceral Adipose Tissue Depth in Early Pregnancy and Gestational Diabetes Mellitus—A Cohort Study. Sci. Rep. 2020, 10, 2032. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosi, F.; Crovetto, F.; Colosi, E.; Fabietti, I.; Carbone, F.; Tassis, B.; Motta, S.; Bulfoni, A.; Fedele, L.; Rossi, G.; et al. Maternal Subcutaneous and Visceral Adipose Ultrasound Thickness in Women with Gestational Diabetes Mellitus at 24–28 Weeks’ Gestation. Fetal. Diagn. 2018, 43, 143–147. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.R.; Berger, H.; Retnakaran, R.; Maguire, J.L.; Nathens, A.B.; Connelly, P.W.; Ray, J.G. First-Trimester Maternal Abdominal Adiposity Predicts Dysglycemia and Gestational Diabetes Mellitus in Midpregnancy. Diabetes Care 2016, 39, 61–64. [Google Scholar] [CrossRef]
- De Souza, L.R.; Kogan, E.; Berger, H.; Alves, J.G.; Lebovic, G.; Retnakaran, R.; Maguire, J.L.; Ray, J.G. Abdominal adiposity and insulin resistance in early pregnancy. J. Obs. Gynaecol. Can. 2014, 36, 969–975. [Google Scholar] [CrossRef]
- Kung, W.J.; Kuo, H.Y.; Lee, C.H.; Zen, Y.H.; Kong, L.C.; Lin, C.C. Association between gestational abnormal glucose tolerance and maternal-fetal outcomes. J. Obs. Gynaecol. Res. 2022. advance online publication. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Juan, J.; Xu, Q.Q.; Su, R.N.; Hirst, J.E.; Yang, H.X. Increasing insulin resistance predicts adverse pregnancy outcomes in women with gestational diabetes mellitus. J. Diabetes 2020, 12, 438–446. [Google Scholar] [CrossRef]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Mirghani Dirar, A.; Doupis, J. Gestational diabetes from A to Z. World J. Diabetes 2017, 8, 489–511. [Google Scholar] [CrossRef]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Feig, D.S.; Berger, H.; Donovan, L.; Godbout, A.; Kader, T.; Keely, E.; Sanghera, R. Diabetes and Pregnancy. Can. J. Diabetes 2018, 42 (Suppl. 1), S255–S282. [Google Scholar] [CrossRef]
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S73–S84. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Okumiya, T.; Saibara, T.; Park, K.; Sasaki, M. Abnormally decreased HbA1c can be assessed with erythrocyte creatine in patients with a shortened erythrocyte age. Diabetes Care 1998, 21, 1732–1735. [Google Scholar] [CrossRef] [PubMed]
- Kaiafa, G.; Veneti, S.; Polychronopoulos, G.; Pilalas, D.; Daios, S.; Kanellos, I.; Didangelos, T.; Pagoni, S.; Savopoulos, C. Is HbA1c an ideal biomarker of well-controlled diabetes? Postgrad. Med. J. 2021, 97, 380–383. [Google Scholar] [CrossRef]
- Mendes, N.; Tavares Ribeiro, R.; Serrano, F. Beyond self-monitored plasma glucose and HbA1c: The role of non-traditional glycaemic markers in gestational diabetes mellitus. J. Obs. Gynaecol. 2018, 38, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Benaiges, D.; Flores-Le Roux, J.A.; Marcelo, I.; Mañé, L.; Rodríguez, M.; Navarro, X.; Chillarón, J.J.; Llauradó, G.; Gortazar, L.; Pedro-Botet, J.; et al. Is first-trimester HbA1c useful in the diagnosis of gestational diabetes? Diabetes Res. Clin. Pract. 2017, 133, 85–91. [Google Scholar] [CrossRef]
- Cahill, A.G.; Tuuli, M.G.; Colvin, R.; Cade, W.T.; Macones, G.A. Markers of Glycemic Control and Neonatal Morbidity in High-Risk Insulin-Resistant Pregnancies. Am. J. Perinatol. 2016, 33, 151–156. [Google Scholar] [CrossRef]
- Ribeiro, R.T.; Macedo, M.P.; Raposo, J.F. HbA1c, Fructosamine, and Glycated Albumin in the Detection of Dysglycaemic Conditions. Curr. Diabetes Rev. 2016, 12, 14–19. [Google Scholar] [CrossRef]
- Selvin, E.; Rawlings, A.M.; Grams, M.; Klein, R.; Sharrett, A.R.; Steffes, M.; Coresh, J. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: A prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014, 2, 279–288. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Parker, T.B.; Johnson, C.R. Validity of serum fructosamine as index of short-term glycemic control in diabetic outpatients. Diabetes Care 1988, 11, 662–664. [Google Scholar] [CrossRef]
- Armbruster, D.A. Fructosamine: Structure, analysis, and clinical usefulness. Clin. Chem. 1987, 33, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Gingras, V.; Rifas-Shiman, S.L.; Switkowski, K.M.; Oken, E.; Hivert, M.F. Mid-Pregnancy Fructosamine Measurement-Predictive Value for Gestational Diabetes and Association with Postpartum Glycemic Indices. Nutrients 2018, 10, 2003. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Koga, M. Indicators of glycemic control in patients with gestational diabetes mellitus and pregnant women with diabetes mellitus. World J. Diabetes 2015, 6, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Segade, S.; Rodríguez, J.; Camiña, F. Corrected Fructosamine improves both correlation with HbA(1C) and diagnostic performance. Clin. Biochem. 2017, 50, 110–115. [Google Scholar] [CrossRef]
- Nasrat, H.A.; Ajabnoor, M.A.; Ardawi, M.S. Fructosamine as a screening-test for gestational diabetes mellitus: A reappraisal. Int. J. Gynaecol. Obs. 1991, 34, 27–33. [Google Scholar] [CrossRef]
- Durr, J. Maternal fluid adaptation to pregnancy. Reprod. Perinat. Med. 1989, 11, 227–270. [Google Scholar]
- Hartland, A.J.; Smith, J.M.; Dunne, F. Correcting serum fructosamine concentration for total protein or albumin concentration is not appropriate during Asian pregnancy. Clin. Chim. Acta 2000, 292, 175–180. [Google Scholar] [CrossRef]
- Goldstein, B.J.; Jabbour, S.; Ahmed, I. Glycated Hemoglobin and Serum Proteins as Tools for Monitoring; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Gillery, P. Assays of HbA1c and Amadori products in human biology. Ann. Pharm. Fr. 2014, 72, 330–336. [Google Scholar] [CrossRef]
- Shipman, K.E.; Jawad, M.; Sullivan, K.M.; Ford, C.; Gama, R. HbA1c is a reliable test for type 2 diabetes in primary care irrespective of chronic kidney disease. BMJ 2014, 348, g3780. [Google Scholar] [CrossRef]
- Panduranga Shenoy, R.; Hanumaiah, G.; Bolar Suryakanth, V.; Shridharan, P. Comparison of Serum Levels of Fructosamine and Erythrocyte Sodium Potassium ATPase (Na(+)/K(+) ATPase) in Gestational Diabetes Mellitus (GDM) and non-Gestational Diabetes Mellitus (non GDM) Patients. Rep. Biochem. Mol. Biol. 2019, 8, 253–259. [Google Scholar]
- Maitland, R.A.; Seed, P.T.; Briley, A.L.; Homsy, M.; Thomas, S.; Pasupathy, D.; Robson, S.C.; Nelson, S.M.; Sattar, N.; Poston, L. Prediction of gestational diabetes in obese pregnant women from the UK Pregnancies Better Eating and Activity (UPBEAT) pilot trial. Diabet. Med. 2014, 31, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.M.; Dhatt, G.S.; Othman, Y.; Ljubisavljevic, M.R. Gestational diabetes: An evaluation of serum fructosamine as a screening test in a high-risk population. Gynecol. Obs. Investig. 2011, 71, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Sobki, S.H.; Alhomida, A.S.; Khan, S.A. Paired values of serum fructosamine and blood glucose for the screening of gestational diabetes mellitus: A retrospective study of 165 Saudi pregnant women. Indian J. Clin. Biochem. 2007, 22, 65–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, K.; Yang, H.X. Value of fructosamine measurement in pregnant women with abnormal glucose tolerance. Chin. Med. J. (Engl.) 2006, 119, 1861–1865. [Google Scholar] [CrossRef]
- Adeshara, K.A.; Bangar, N.S.; Doshi, P.R.; Diwan, A.; Tupe, R.S. Action of metformin therapy against advanced glycation, oxidative stress and inflammation in type 2 diabetes patients: 3 months follow-up study. Diabetes Metab. Syndr. 2020, 14, 1449–1458. [Google Scholar] [CrossRef]
- Bavkar, L.N.; Patil, R.S.; Rooge, S.B.; Nalawade, M.L.; Arvindekar, A.U. Acceleration of protein glycation by oxidative stress and comparative role of antioxidant and protein glycation inhibitor. Mol. Cell Biochem. 2019, 459, 61–71. [Google Scholar] [CrossRef]
- Palma-Duran, S.A.; Kontogianni, M.D.; Vlassopoulos, A.; Zhao, S.; Margariti, A.; Georgoulis, M.; Papatheodoridis, G.; Combet, E. Serum levels of advanced glycation end-products (AGEs) and the decoy soluble receptor for AGEs (sRAGE) can identify non-alcoholic fatty liver disease in age-, sex- and BMI-matched normo-glycemic adults. Metabolism 2018, 83, 120–127. [Google Scholar] [CrossRef]
- Panezai, J.; Altamash, M.; Engstrӧm, P.E.; Larsson, A. Association of Glycated Proteins with Inflammatory Proteins and Periodontal Disease Parameters. J. Diabetes Res. 2020, 2020, 6450742. [Google Scholar] [CrossRef]
- Morisset, A.S.; Dubé, M.C.; Côté, J.A.; Robitaille, J.; Weisnagel, S.J.; Tchernof, A. Circulating interleukin-6 concentrations during and after gestational diabetes mellitus. Acta Obs. Gynecol. Scand. 2011, 90, 524–530. [Google Scholar] [CrossRef]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Martinez-Santibañez, G.; Lumeng, C.N. Macrophages and the regulation of adipose tissue remodeling. Ann. Rev. Nutr. 2014, 34, 57–76. [Google Scholar] [CrossRef]
- Vergès, B.; Rouland, A.; Baillot-Rudoni, S.; Brindisi, M.C.; Duvillard, L.; Simoneau, I.; Legris, P.; Petit, J.M.; Bouillet, B. Increased body fat mass reduces the association between fructosamine and glycated hemoglobin in obese type 2 diabetes patients. J Diabetes Investig. 2021, 12, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Nouya, A.Y.; Nansseu, J.R.; Moor, V.J.; Pieme, C.A.; Noubiap, J.J.; Tchoula, C.M.; Mokette, B.M.; Takam, R.D.; Tankeu, F.; Ngogang, J.Y.; et al. Determinants of fructosamine levels in a multi-ethnic Sub-Saharan African population. Diabetes Res. Clin. Pract. 2015, 107, 123–129. [Google Scholar] [CrossRef]
- Poon, A.K.; Juraschek, S.P.; Ballantyne, C.M.; Steffes, M.W.; Selvin, E. Comparative associations of diabetes risk factors with five measures of hyperglycemia. BMJ Open Diabetes Res. Care 2014, 2, e000002. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Lemieux, S.; Lafrenière, J.; Laramée, C.; Robitaille, J.; Morisset, A.S. Validation of a self-administered web-based 24-hour dietary recall among pregnant women. BMC Pregnancy Childbirth 2018, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Lemieux, S.; Weisnagel, S.J.; Fontaine-Bisson, B.; Gagnon, C.; Robitaille, J.; Morisset, A.S. Trimester-Specific Dietary Intakes in a Sample of French-Canadian Pregnant Women in Comparison with National Nutritional Guidelines. Nutrients 2018, 10, 768. [Google Scholar] [CrossRef]
- Henny, J.; Schiele, F.; Kruse-Jarres, J.D.; Kaiser, C.; Bonini, P.; Ceriotti, F.; Schlebusch, H.; Sorger, M.; Garcia-Beltran, L.; Jarausch, J.; et al. Detetermination of reference values for a colorimetric fructosamine assay. Klin. Lab. 1992, 38, 153–160. [Google Scholar]
- Lin, M.J.; Hoke, C.; Ettinger, B.; Coyne, R.V. Technical performance evaluation of BM/Hitachi 747-200 serum fructosamine assay. Clin. Chem. 1996, 42, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Berger, H.; Nisenbaum, R.; Lausman, A.Y.; MacGarvie, S.; Crerar, C.; Ray, J.G. Abdominal visceral adiposity in the first trimester predicts glucose intolerance in later pregnancy. Diabetes Care 2009, 32, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Cheng, A.Y. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Introduction. Can. J. Diabetes 2013, 37 (Suppl. 1), S1–S3. [Google Scholar] [CrossRef]
- McLorg, P.A. Body size and shape and glycemic control among Maya women in rural Yucatán. Am. J. Hum. Biol. 2003, 15, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Wu, O.; Leng, J.H.; Yang, F.F.; Yang, H.M.; Zhang, H.; Li, Z.F.; Zhang, X.Y.; Yuan, C.D.; Li, J.J.; Pan, Q.; et al. A comparative research on obesity hypertension by the comparisons and associations between waist circumference, body mass index with systolic and diastolic blood pressure, and the clinical laboratory data between four special Chinese adult groups. Clin. Exp. Hypertens. 2018, 40, 16–21. [Google Scholar] [CrossRef]
- Don, B.R.; Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef]
- Guo, J.; Lei, S.; Zhou, Y.; Pan, C. The ratio of estimated average glucose to fasting plasma glucose level as an indicator of insulin resistance in young adult diabetes: An observational study. Medicine 2020, 99, e22337. [Google Scholar] [CrossRef] [PubMed]
- Peer, N.; George, J.; Lombard, C.; Levitt, N.; Kengne, A.P. Associations of glycated albumin and fructosamine with glycaemic status in urban black South Africans. Clin. Chim. Acta 2021, 519, 291–297. [Google Scholar] [CrossRef]
- Hom, F.G.; Ettinger, B.; Lin, M.J. Comparison of serum fructosamine vs glycohemoglobin as measures of glycemic control in a large diabetic population. Acta Diabetol. 1998, 35, 48–51. [Google Scholar] [CrossRef]
- Pérez-López, L.; Boronat, M.; Melián, C.; Brito-Casillas, Y.; Wägner, A.M. Kidney function and glucose metabolism in overweight and obese cats. Vet. Q. 2020, 40, 132–139. [Google Scholar] [CrossRef]
- Burekovic, A.; Dizdarevic-Bostandzic, A.; Godinjak, A. Poorly Regulated Blood Glucose in Diabetic Patients-predictor of Acute Infections. Med. Arch. 2014, 68, 163–166. [Google Scholar] [CrossRef]
- Kravchenko, I.V.; Furalyov, V.A.; Popov, V.O. Glycated albumin stimulates expression of inflammatory cytokines in muscle cells. Cytokine 2020, 128, 154991. [Google Scholar] [CrossRef]
- Chang, D.C.; Xu, X.; Ferrante, A.W., Jr.; Krakoff, J. Reduced plasma albumin predicts type 2 diabetes and is associated with greater adipose tissue macrophage content and activation. Diabetol. Metab. Syndr. 2019, 11, 14. [Google Scholar] [CrossRef] [PubMed]
| Variables | Mean ± SD or n (%) |
|---|---|
| Age, years | 32.2 ± 3.5 |
| Parity a | |
| 0 | 37 (36.6) |
| ≥1 | 64 (63.4) |
| Pre-pregnancy body mass index, kg/m2 | 25.5 ± 5.5 |
| Underweight | 3 (2.9) |
| Normal weight | 55 (54.5) |
| Overweight | 25 (24.8) |
| Obesity | 18 (17.8) |
| Gestational diabetes mellitus | 11 (10.9) |
| Ethnicity | |
| European descent | 98 (97.0) |
| African | 1 (1.0) |
| Latin American | 2 (2.0) |
| Education | |
| High school | 5 (5.0) |
| College b | 18 (17.8) |
| University | 78 (77.2) |
| Annual household income | |
| <60,000 CAD | 20 (19.8) |
| 60,000–79,999 CAD | 14 (13.9) |
| 80,000–99,999 CAD | 22 (21.8) |
| ≥100,000 CAD | 44 (43.6) |
| Income not disclosed | 1 (0.9) |
| Fructosamine (μmol/L) | |
| First trimester | 215.5 ± 14.7 |
| Second trimester | 197.6 ± 13.0 |
| Third trimester | 193.1 ± 12.0 |
| Albumin (g/L) | |
| First trimester | 33.5 ± 2.3 |
| Second trimester | 28.9 ± 2.1 |
| Third trimester | 27.9 ± 1.9 |
| Albumin-corrected fructosamine (μmol/g) | |
| First trimester | 193.4 ± 13.7 |
| Second trimester | 205.8 ± 16.8 |
| Third trimester | 207.9 ± 14.0 |
| Ultrasound-estimated SAT thickness (mm) c | 17.4 ± 7.7 |
| Ultrasound-estimated VAT thickness (mm) c | 27.0 ± 14.7 |
| Leptin (ng/mL) d | |
| First trimester | 33.6 ± 20.2 |
| Second trimester | 38.9 ± 27.6 |
| Third trimester | 41.2 ± 26.8 |
| Fasting glucose (mmol/L) | |
| First trimester e | 4.5 ± 0.3 |
| Second trimester f | 4.4 ± 0.4 |
| Third trimester f | 4.6 ± 0.5 |
| Fasting insulin (pmol/L) | |
| First trimester g | 63.1 ± 34.0 |
| Second trimester h | 74.8 ± 41.4 |
| Third trimester c | 97.5 ± 47.9 |
| HOMA-IR | |
| First trimester g | 1.8 ± 1.1 |
| Second trimester h | 2.2 ± 1.4 |
| Third trimester c | 2.9 ± 1.6 |
| Adiponectin (mg/mL) d | |
| First trimester | 10.2 ± 3.2 |
| Second trimester | 9.2 ± 3.2 |
| Third trimester | 7.7 ± 2.7 |
| IL-6 (pg/mL) i | |
| First trimester | 1.0 ± 0.6 |
| Second trimester | 1.2 ± 0.9 |
| Third trimester | 1.5 ± 0.9 |
| CRP(mg/L) j | |
| First trimester | 7.0 ± 7.4 |
| Second trimester | 6.5 ± 5.6 |
| Third trimester | 6.5 ± 6.0 |
| First Trimester | Second Trimester | Third Trimester | |||||
|---|---|---|---|---|---|---|---|
| n | r | radj | r | radj | r | radj | |
| Adiposity markers | |||||||
| ppBMI | 101 | −0.55 *** | - | - | - | - | - |
| SAT thickness | 84 | −0.61 *** | −0.16 | - | - | - | - |
| VAT thickness | 84 | −0.48 *** | −0.03 | - | - | - | - |
| Leptin | 74 | −0.47 *** | −0.003 | −0.26 * | −0.08 | −0.25 * | −0.01 |
| Glucose homeostasis markers | |||||||
| Fasting glucose | 88 | −0.04 | 0.16 | 0.16 | 0.24 * | 0.10 | 0.24 * |
| Fasting insulin | 88 | −0.46 *** | 0.01 | −0.16 | −0.002 | −0.27 * | 0.01 |
| HOMA-IR | 88 | −0.46 *** | 0.05 | −0.10 | 0.06 | −0.22 * | 0.05 |
| Inflammatory markers | |||||||
| Adiponectin | 72 | 0.33 ** | 0.16 | 0.23 † | 0.13 | 0.13 | 0.08 |
| IL-6 | 70 | −0.38 ** | −0.07 | −0.05 | 0.05 | 0.06 | 0.09 |
| CRP | 70 | −0.19 | 0.08 | −0.07 | 0.09 | 0.10 | 0.27 * |
| First Trimester | Second Trimester | Third Trimester | |||||
|---|---|---|---|---|---|---|---|
| n | r | radj | r | radj | r | radj | |
| Adiposity markers | |||||||
| ppBMI | 101 | −0.20 * | - | - | - | - | - |
| SAT thickness | 84 | −0.30 ** | −0.15 | - | - | - | - |
| VAT thickness | 84 | −0.30 ** | −0.17 | - | - | - | - |
| Leptin | 74 | −0.15 | −0.02 | −0.06 | 0.19 | 0.02 | 0.09 |
| Glucose homeostasis markers | |||||||
| Fasting glucose | 88 | −0.09 | −0.04 | 0.02 | −0.04 | 0.12 | 0.15 |
| Fasting insulin | 88 | −0.23 * | −0.09 | −0.02 | 0.10 | −0.003 | 0.05 |
| HOMA-IR | 88 | −0.22 * | −0.09 | −0.001 | 0.10 | −0.004 | 0.05 |
| Inflammatory markers | |||||||
| Adiponectin | 72 | 0.22 † | 0.16 | −0.02 | −0.05 | −0.03 | −0.04 |
| IL-6 | 70 | −0.17 | −0.10 | 0.29 * | 0.31 ** | 0.06 | 0.06 |
| CRP | 70 | 0.20 † | 0.27 * | −0.05 | −0.03 | 0.06 | 0.09 |
| First Trimester | Second Trimester | Third Trimester | |||||
|---|---|---|---|---|---|---|---|
| n | r | radj | r | radj | r | radj | |
| Adiposity markers | |||||||
| ppBMI | 101 | −0.40 *** | - | - | - | - | - |
| SAT thickness | 84 | −0.28 * | 0.04 | - | - | - | - |
| VAT thickness | 84 | −0.10 | 0.14 | - | - | - | - |
| Leptin | 74 | −0.34 ** | 0.02 | −0.34 ** | −0.20 † | −0.27 * | −0.10 |
| Glucose homeostasis markers | |||||||
| Fasting glucose | 88 | −0.09 | 0.20 † | 0.10 | 0.18 † | −0.03 | 0.06 |
| Fasting insulin | 88 | −0.21 * | 0.14 | −0.13 | −0.02 | −0.22 * | −0.09 |
| HOMA-IR | 88 | −0.21 † | 0.17 | −0.09 | 0.01 | −0.18 † | −0.05 |
| Inflammatory markers | |||||||
| Adiponectin | 72 | 0.17 | 0.01 | 0.25 * | 0.16 | 0.17 | 0.10 |
| IL-6 | 70 | −0.26 * | 0.01 | −0.28 * | −0.26 * | 0.002 | 0.06 |
| CRP | 70 | −0.29 * | −0.28 * | −0.07 | 0.08 | 0.03 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernier, E.; Lachance, A.; Plante, A.-S.; Lemieux, P.; Mourabit Amari, K.; Weisnagel, S.J.; Gagnon, C.; Michaud, A.; Tchernof, A.; Morisset, A.-S. Trimester-Specific Serum Fructosamine in Association with Abdominal Adiposity, Insulin Resistance, and Inflammation in Healthy Pregnant Individuals. Nutrients 2022, 14, 3999. https://doi.org/10.3390/nu14193999
Bernier E, Lachance A, Plante A-S, Lemieux P, Mourabit Amari K, Weisnagel SJ, Gagnon C, Michaud A, Tchernof A, Morisset A-S. Trimester-Specific Serum Fructosamine in Association with Abdominal Adiposity, Insulin Resistance, and Inflammation in Healthy Pregnant Individuals. Nutrients. 2022; 14(19):3999. https://doi.org/10.3390/nu14193999
Chicago/Turabian StyleBernier, Emilie, Amélie Lachance, Anne-Sophie Plante, Patricia Lemieux, Karim Mourabit Amari, S. John Weisnagel, Claudia Gagnon, Andréanne Michaud, André Tchernof, and Anne-Sophie Morisset. 2022. "Trimester-Specific Serum Fructosamine in Association with Abdominal Adiposity, Insulin Resistance, and Inflammation in Healthy Pregnant Individuals" Nutrients 14, no. 19: 3999. https://doi.org/10.3390/nu14193999
APA StyleBernier, E., Lachance, A., Plante, A.-S., Lemieux, P., Mourabit Amari, K., Weisnagel, S. J., Gagnon, C., Michaud, A., Tchernof, A., & Morisset, A.-S. (2022). Trimester-Specific Serum Fructosamine in Association with Abdominal Adiposity, Insulin Resistance, and Inflammation in Healthy Pregnant Individuals. Nutrients, 14(19), 3999. https://doi.org/10.3390/nu14193999






