Abstract
Herein we gathered updated knowledge regarding the alterations of gut microbiota (dysbiosis) and its correlation with human neurodegenerative and brain-related diseases, e.g., Alzheimer’s and Parkinson’s. This review underlines the importance of gut-derived metabolites and gut metabolic status as the main players in gut-brain crosstalk and their implications on the severity of neural conditions. Scientific evidence indicates that the administration of probiotic bacteria exerts beneficial and protective effects as reduced systemic inflammation, neuroinflammation, and inhibited neurodegeneration. The experimental results performed on animals, but also human clinical trials, show the importance of designing a novel microbiota-based probiotic dietary supplementation with the aim to prevent or ease the symptoms of Alzheimer’s and Parkinson’s diseases or other forms of dementia or neurodegeneration.
1. Introduction
The gut microbial community is the largest micro-ecosystem in the human body, formed by more than 1000 different species, with high density per mL and encoding approximately 4 × 106 genes, 150 times more unique than the human genome [1]. It is predicted that the total weight of our gut microbiota deposit circulates around 1–2 kg, approximately reaching the human brain weight [2]. Ninety-five percent of symbiotic microorganisms are located in the gut [3,4]. These multi-species ecological arrangements mostly comprise anaerobic bacteria, with at least 500–1000 different species and more than 7000 strains, mainly from the Firmicutes and Bacteroidetes families (accounting for approx. 90% of bacterial species). In addition, the gut microbiome is co-inhabited by bacteria belonging to Proteobacteria, Actinobacteria, and Verrucomicrobia phyla, as well as archaea, viruses, fungi, bacteriophages, and protozoa (especially during pathologic conditions), which reside in the gastrointestinal (GI) tract, maintaining symbiosis with the host [5,6,7].
The gut microbiome is crucially engaged in the regulation and maintenance of human health and homeostasis, i.e., proper intestine motion, fibre digestion, regulating the development of host immunity, and exerting a protective effect against pathogenic factors (such as Campylobacter jejuni, Helicobacter pylori, and Clostridium difficile) [8,9,10]. The Irish ELDERMET study (Elderly to benefit from Research Project) demonstrated the correlation between intestinal microbiota diversity and health outcomes such as overall health, frailty, and proper immune functions, underlying the gut microbiota composition as a possible indicator of healthy ageing [11]. Multiple studies showed the interplay between the intestinal microbiota and modulation of central nervous system (CNS) functions and neurodegeneration processes [11], pointing to the microbiome-gut–brain axis as one of the factors modulating host homeostasis in health and disease. In the past decade, the alterations of the gut microbiota, which are called dysbiosis, have been connected with the pathophysiology of numerous common diseases such as type 2 diabetes, obesity, gastrointestinal diseases, and cardiovascular events. Dysbiosis state is also implicated in neurological and neurodegenerative disorders such as autism, and Alzheimer’s (AD) or Parkinson’s (PD) diseases [9,12]. During dysbiosis, disrupted homeostasis allows the pathobionts to expand, which can further amend the bowel’s mucosal barrier, exerting neurogenic inflammatory processes [9,13,14].
However host–microbiota interplay is not exclusively restricted to cell–cell interactions. Intestinal microbes are capable of producing a plethora of different types of compounds, i.e., small molecules (amino acids, neurotransmitters, short-chain fatty acids (SCFAs) and other fatty acid derivatives, indoles, vitamins, and cofactors), polysaccharides, and proteins (i.e., bacteriocins), which have been reported as important factors in shaping the bacterial community and modulation of the immune system [15]. It has been demonstrated that intestinal bacterial strains such as Escherichia coli or Lactobacillus spp. interact directly with the host’s CNS (central nervous system), as they can release neurotransmitters such as dopamine, noradrenaline, histamine, acetylcholine, GABA (gamma-aminobutyric acid), or serotonin [16]. This metabolic status of the microbiome is also vulnerable to dysbiosis and compositional changes and often leads to metabolic disturbances. For example, dysbiosis can induce elevated reactive oxygen species (ROS) levels, intensifying oxidative stress and neuronal inflammatory processes [10,17]. It has been estimated that 40% of the metabolites detected in humans are the products of bacterial metabolism [18], which plays a fundamental role in the modulation of the host’s physiological processes, including immune-mediated neurodegeneration or affecting neural cell metabolism [19].
In this review, we summarized the influence of microbial metabolites on the severity and progression of neurodegenerative diseases and their correlation with microbial AD/PD-associated dysbiosis, pointing out particular genera/phyla responsible for increased/decreased production. Finally, the review concludes with a brief summary of actual knowledge regarding possible interventions to reverse the negative effects of a disbalanced microbiome in neurodegenerative diseases.
2. Microbiota–Brain–Gut Axis and Neurodegenerative Diseases/Brain Disorders
Some research reports underline the crucial role of the microbiome in the normal development, regulation of behavior, cognition, and brain functions, but also in the etiology, the pathogenesis, and the progression of debilitating neurological disorders, e.g., Alzheimer’s and Parkinson’s diseases, autism spectrum disorder, multiple sclerosis, or even bipolar disorder, depression, schizophrenia, Huntington’s, stroke, and brain ageing [18,20]. Animal studies have demonstrated the participation of microbiota in neural-related processes such as neurodevelopment, neuroinflammation, interactions with neurons and glia, and behavior [11]. It has been postulated that microbiome influences neurological homeostasis via the microbiome–gut–brain axis.
The gut microbiota and brain crosstalk is a complex process including the vagus nerve, the immune system, gut hormone signaling pathways, tryptophan amino acid metabolism, hypothalamic–pituitary–adrenal axis, and the products of bacterial metabolism such as short-chain fatty acids [21] or gut-derived neurotransmitters [22] (Figure 1). Experimental studies demonstrated that even minor modifications in gut microbiota composition can lead to serious modification of brain functions, subsequently affecting intestinal activity through the secretion of specific hormones, neuropeptides, and neurotransmitters [23]. For example, butyrate, acetate, or propionate can induce the release of leptin and glucagon-like peptide-1, which are the gut hormones interacting with the vagus nerve and brain receptors [24,25,26]. In a mice model, the reduction of the variety of microbiota significantly diminished the blood–brain barrier (BBB) expression of tight junction proteins, occludin and claudin-5, disrupting the barrier function of endothelial cells. Therefore, rodent neural cells become more susceptible to stimuli produced by bacteria, among them lipopolysaccharide (LPS) and factors such as oxidative stress mediators [27]. Moreover, defects in mucosal barrier tight junction proteins increase intestinal permeability, causing the “leaky gut” effect followed by systemic inflammation and pathogenic agents translocation into the bloodstream (e.g., LPS) with elicit pro-inflammatory cytokine release [28,29]. Aoyama et al. provided evidence of the spontaneous neutrophil apoptosis triggered by butyrate and propionate through a histone deacetylase (HDAC) inhibition, the expression of caspase-3, caspase-8, and caspase-9 pathways, and proteins belonging to the Bcl-2 family, affecting the intestines and different organs. Butyrate and propionate activated neutrophil apoptosis both in the absence and presence of LPS or tumor necrosis factor α (TNF-α), and therefore in normal and pro-inflammatory conditions. Moreover, the expression of the proapoptotic bax mRNA was significantly elevated and, just the opposite, the expression level of antiapoptotic mcl-1 and a1 mRNA was importantly decreased by the abovementioned SCFAs in non-activated neutrophils. LPS induction highly increased the expression of mcl-1 and a1 mRNA; interestingly, this effect was neutralized by SCFAs [30]. Moreover, clinical trials suggest an altered gut microbial composition of patients with neurodegenerative disorders, together with significant differences in microbial and serum metabolomic profiles [31]. These data indicate that both microbiome composition and microbiome metabolome affect neurological diseases. It is worth mentioning that gut peripheral tissues are a residue for 70% of the human immune system, which is a well-known mediator of changes in gut ecosystem, inflammatory response, and also regulates multiple processes in CNS [32]. A good example of the interplay between gut-located immune cells and brain cells is the influence of regulatory T cells, which exert a neuroprotective effect by stimulating oligodendrocyte differentiation and triggering neuronal remyelination by interleukin IL-10 [19]. At the molecular level, there is evidence that mammalian Toll-Like Receptors (TLRs) play an important role in neurodegeneration. TLRs receptors are one of the main receptor families engaged in transducing and shaping innate immune responses. They are expressed both in immune and non-immune cells, especially increasing after contact with microbial pathogens or bacterial elements, i.e., cell walls, peptidoglycans, or DNA [33]. TLR activation (mainly TLR2 and TLR4) induces an inflammatory response cascade, preceding the neuronal loss characteristic for Parkinson’s disease [34], which could be a result of a “cascade reaction”: disrupted microbial composition and metabolome, increased permeability of the gut cell wall, and increased exposure to TLR ligands. Moreover, the microbiome and its metabolites are associated with immune-mediated neuroinflammation processes, brain injury, and neurogenesis [35]. Microglia cells are brain glial-resident immune cells responsible for maintaining proper immune functions including phagocytosis, antigen presentation, the production of cytokines, and the subsequent inflammatory reactions [36,37]. Research reports indicate the influence of microbiota on microglial maturation and function; however, this mechanism is still unclear [38]. It has been confirmed that gut dysbiosis can augment intestinal permeability and bacterial translocation, causing an over-response of the immune system and the subsequent systemic/central nervous system inflammation [39,40]. The adaptive immune system also participates in the regulation of healthy microbiota. The crucial players in intestinal homeostasis maintenance are B cells, which produce secretory IgA antibodies targeted toward specific bacteria [41]. Additionally, gut microbiota can stably influence the host’s gene expression through epigenetic mechanisms, including histone modifications, methylation of DNA, and non-coding RNA expression [42]. Overall, the microbiome plays a big role in moderating the maturation of immune cells residing in the CNS tissues via an interplay and crosstalk with the peripheral immune system. Gut microbiota act as a multifunctional hub modulating the immune system via the production of immunomodulatory and anti-inflammatory signaling molecules, reaching immune cells. Commensal microbes fabricate various metabolites from digested food, SCFAs among them, which participate in the maintenance of intestinal homeostasis, exerting anti-inflammatory activity on the intestinal mucosa [43]. The gut microbiota participates in signal transduction pathways as it can communicate between the host’s innate immune system cells, which are located at the interface between the host and the microbiome [44].
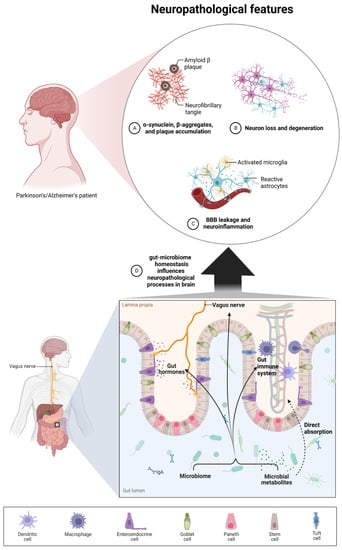
Figure 1.
The interplay between the microbiome, microbial metabolites, and neuropathological processes present during neurodegenerative diseases such as AD and PD. The microbiome and microbial metabolites could regulate brain homeostasis via four different ways: 1. direct absorption of specific metabolites which have the ability to cross the BBB; 2. interaction between peripheral immune systems, which interact with brain glia cells and astrocytes; 3. influence on gut hormones and the enteroendocrine system; 4. communication via the vagus nerve. These four routes may have an influence on pathological symptoms such as increased BBB leakage and neural inflammation, enhanced beta-amyloid and alpha-synuclein formation, and finally neuron loss and motor/cognitive deficits.
3. Microbiota Metabolites and the Severity of Neurodegenerative Diseases
Scientific evidence indicates that the metabolic status of microbiota exerts a more important role than the equilibrium between bacterial species. Microbial metabolites can act as positive modulators of the gut–brain-axis constituents, boosting the immune cells and exerting a positive/protective effect on neurodegenerative disease progression [45]. On the other hand, disrupted equilibrium between microbial species often leads to specific switches in the microbial metabolome, which may be harmful and associated with neurodegenerative disease progression. For example, dysregulation of Gram-negative bacteria in the gastrointestinal area leads to the production of harmful metabolites (i.e., endo- and exotoxins, saccharides, and amyloids) [46]. Exotoxins, such as LPS, which have the ability to activate the production of pro-inflammatory cytokines, exert negative effects. LPS belongs to glycolipid molecules, mostly produced by Gram-negative bacterial strains. These molecules participate in the integrity maintenance of the bacterial outer-membrane permeability barrier, playing an important role in host-pathogen interplay [47]. The overproduction of bacterial LPS can cause the activation of the macrophage–monocyte nod-like receptor P3 inflammasome, therefore stimulating the production of cytokines with pro-inflammatory activity, such as IL-18 or IL-1β. LPS can be translocated and intensify neuroinflammatory diseases such as AD [48]. Additionally, it was demonstrated that IL-1β can decrease the phagocytic function of microglial cells, stimulating the hyperphosphorylation of tau protein, and causing reduced synaptic plasticity and cognitive deterioration [48,49,50]. These cytokines can induce microbiota dysregulation, increasing gut permeability and influencing gut bacteria diversity [51,52].
On the other hand, the gut microbiome is able to produce a broad spectrum of metabolites, which have beneficial effects on human health (Figure 2).
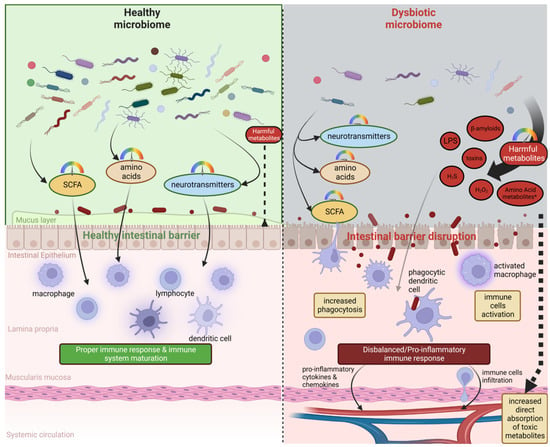
Figure 2.
A healthy, abundant, and diverse microbiome (left side) is able to produce proper amounts of SCFAs, amino acids, and neurotransmitters. This type of microbiome and its metabolites positively influence gut barrier integrity and leads to the healthy maturation of the peripheral immune system, which is able to counteract external immune stimuli properly. A dysbiotic microbiome (right side) could be characterized by reduced diversity and an abundance of species able to produce positive metabolites and an increased abundance of species producing harmful metabolites such as LPS, beta-amyloids, small toxic metabolites, or other toxins. These switches may lead to disruption in the intestinal barrier, increased absorption of toxic metabolites, over-activation of the immune system, and, finally, pro-inflammatory responses which influence other organs, including the brain.
Recently, bacterial strains producing SCFAs have gained extreme popularity. SCFAs are small organic monocarboxylic acids, released after the fermentation of indigestible alimentary fiber (mainly galacto- or fructooligosaccharides), plant-derived polysaccharides, or the metabolism of amino acids, and are considered a key gut-derived metabolites with beneficial health effects and as essential in gut-brain crosstalk [53,54]. The type of formed SCFAs is correlated with the kind of fiber ingested and the overall gut microbiota population. For example, microbes belonging to Firmicutes phyla (e.g., Lachnospiraceae, Eubacterium, or Roseburia belonging to the Clostridia class) are responsible for the main production of butyrate, whereas Bifidobacteria spp. produce lactate and acetate [55]. SCFAs are significantly reduced during dysbiosis, where disrupted gut epithelial integrity, inflammation, and altered microbiome metabolic processes occur [56,57,58]. In a healthy colon, levels of SCFAs can vary depending on a diet; however, in multiple diseases, these levels are altered. SCFAs may also act in an indirect or direct way through G-protein-coupled receptors or as histone deacetylase epigenetic modulators [59]. They participate in various physiological processes, such as cell energetics and colonocyte metabolism and skeletal, adipose, and liver tissue modulation [60]. Their major functions comprise the activation of trophic factors, energy management, and the manufacturing of regulatory T-cells [61]. Short-chain fatty acids can be considered as key intermediaries in numerous neurological diseases such as Parkinson’s and Alzheimer’s diseases, stroke, and neuropsychiatric conditions.
3.1. Alzheimer’s Disease
Alzheimer’s disease (AD), commonly known as senile dementia, is a chronic, devastating, neurodegenerative, and neuroinflammatory disorder with rising incidence and is considered to be the most frequent form of dementia in aging individuals (caused by aberrant protein processing, trafficking, and aggregation in neural cells). The World Alzheimer Report indicates that 50 million people suffer from AD-related neurological problems and this number will increase to 152 million in 2050 [48]. It is predicted that in 2030 up to 66 million people will suffer from AD [9,35,62]. AD is a multifactorial disease characterized by synapse loss, extracellular cerebral accumulation of insoluble peptides belonging to amyloid-β (Aβ), the aggregation of hyperphosphorylated tau protein inside the cells, neurofibrillary mass formation, neuronal death (in the neocortex and hippocampus), progressive memory deterioration, and cognition decline leading to dementia. AD is associated with more than 700 genetic and environmental risk factors. From the genetic point of view, one of the most important risk factors related to AD development is the incidence of a “4 allele variant in the apolipoprotein” (APOE4) gene, which is associated with cholesterol transport in CNS, and also contributes to metabolomic and taxonomic changes in the microbiome [63,64].
3.1.1. Host and Microbiome Metabolomic Changes during AD: Short Story about Bad and Good Cop
Good Cop
As previously mentioned, the microbiome is able to produce SCFAs. Its indirect influence on Alzheimer’s disease progression has been reported in multiple studies [65]. The gut microbiota of AD patients represented a diminished amount and incidence of bacteria producing butyrate and proinflammatory-acting bacterial taxa [66]. Many studies revealed that the decreased levels of SCFAs can cause increased gut permeability together with altered gut pH levels, enabling the spread of opportunistic bacteria from Shigella and Escherichia species, elevated both in AD and PD [67]. Studies on the Japanese population underlined the negative correlation between SCFAs and the onset of AD and type 2 diabetes [68].
The main end product of bacterial fermentation, and therefore the most predominant, is the SCFA propionic acid (PA), which positively influences CNS regeneration processes such as remyelination (due to the former increase of the amount of intestinal T regulatory cells and protecting against the white matter and axonal loss) [69]. Hoyles et al. demonstrated the contribution of propionate to the BBB integrity and the modulation of gut-brain axis [70]. BBB homeostasis and integrity are necessary for proper CNS development and function. Recently, a positive correlation between microbial-induced BBB dysfunction (barrier integrity disturbances followed by leakage) and disorders such as AD has been established [3,71]. Another member of the SCFAs family, which has a documented influence on homeostasis and AD progression, is butyrate, an important driver of metabolic processes with the ability to influence intestinal macrophages, increasing immunomodulation and decreasing the histone deacetylase inhibition-mediated production of pro-inflammatory cytokines [72]. Butyrate, as an SCFA, influences the immune response crossing the BBB and drives the maturation and metabolism of microglia cells. It regulates the secretion of gut hormones, such as glucagon-like peptide-1, which can improve hippocampus neuroplasticity [38,56,73]. Additionally, in vitro tests conducted by Ho et al. revealed that bacterial SCFAs, such as valeric and butyric acid, exhibited a strong inhibitory effect on amyloid-β aggregation, which can be absorbed in the intestines and have a negative influence on disease progression [74].
Low concentration of SCFAs means a reduced degree of acetylation, therefore causing chromatin remodeling changes and low levels in plasma. An inadequate, low-fibre diet can seriously disturb the intestinal flora, as well as cause disproportions in the quantity and quality of its metabolites. The abovementioned metabolites include SCFAs, secondary bile acids (products of cholesterol metabolism and clearance), lipids, and vitamins [75,76].
Besides SCFAs, the human microbiome is able to produce other small molecules with bioactive potential. The species of the genera Bifidobacterium and Lactobacillus produce various metabolism products such as GABA, dopamine, histamine, serotonin, tryptophan catabolites, and noradrenaline, among others, with all of them belonging to neurotransmitters that regulate cognitive functions. Experimental studies have confirmed that bacterial strains such as Bifidobacterium and Lactobacillus can produce the GABA neurotransmitter through L-glutamate decarboxylation [77,78]. Neurotransmitters such as serotonin and dopamine can affect the immunological response, whereas GABA protects against bacterial translocation and is considered to be a major inhibitory neurotransmitter in the human CNS [79,80]. Moreover, they fulfill the role of modulators of neurodegenerative disease severity, mainly through immune-mediated neurodegeneration or microbial metabolites directly influencing neural cells. It was demonstrated that the aforementioned metabolites are the main signaling molecules for microbial effects on gut–brain communication and their disturbances are linked with gut dysbiosis and cognitive impairment [39]. For example, the results of metabolic profiling in AD patients indicated the disturbances in serotonin metabolic pathway metabolites, which were significantly decreased, especially 5-hydroxytryptophan and DL-5-methoxytryptophan, are mainly produced in the gut by enterochromaffin cells [48]. This process probably can be related to the disruptions in intestinal microbiota content, in particular to the reduction of Clostridium sporogenes and Ruminococcus gnavus abundance, which are considered the main tryptamine producers. Tryptamine impacts gut conditions through its action as a β-arylamine neurotransmitter capable of inducing serotonin (5-hydroxytryptamine) release by intestinal mucosal surface cells [81,82]. Several bacterial species, such as Escherichia coli, Clostridium spp., Bacteroides spp., Lactobacillus spp. Peptostreptococcus spp., or Vibrio cholerae are characterized by the ability to convert tryptophan into indole and indole derivatives through the tryptophanase enzyme, produced both by Gram-negative and Gram-positive bacteria [83,84]. Among them, 3-methylindole (skatole), indoleacetic acid, indoleacrylic acid, indolealdehyde, indolelactic acid, indolepropionic acid, or tryptamine can be found [85]. Interestingly, these metabolites are the ligands of the aryl hydrocarbon receptor (AHR), which can boost the innate and adaptive immune responses, being an important transcription factor expressed by immune cells [86]. Moreover, they can stimulate gut hormone secretion, and gastrointestinal motility, and improve the epithelial barrier of the intestine, regulating intestinal redox homeostasis [87]. Additionally, microbial tryptophan catabolites possess anti-oxidative and anti-inflammatory properties [85]. Previous scientific reports suggested that microbiota diversity impacts the tryptophan bioavailability and its downstream metabolites, which can participate in the host-microbiota crosstalk, being important intracellular signaling molecules [85]. This observation could be a result of disrupted homeostasis in the gut. In recent years, researchers have established a link between disturbed tryptophan metabolism (including indole derivatives and serotonin synthesis) and intestinal dysbiosis. Additionally, Huang et al. demonstrated that indole-3-propionic acid (tryptophan gut bacterial metabolite) could be used as a promising marker of AD, indicating progressive cognitive impairment correlated with disrupted gut microbiota composition and reduced indole metabolite concentration [88,89,90].
Bad Cop
When we talk about negative metabolites or microbiota by-products, which have a documented impact on AD progression, two molecules have been in the spotlight during the past decade. One is LPS and the second is gut-derived amyloid-β, both acting as pro-inflammatory agents.
Some of the studies performed show a connection between the pathological microbiota and Alzheimer’s disease. Screening tests identified the connection between Escherichia and Shigella bacterial species and increased systemic pro-inflammatory cytokine-related processes, reported in patients with Alzheimer’s. Increased inflammatory processes and oxidative stress conditions can trigger neurodegeneration observed in people with Alzheimer’s disease [11]. The previously mentioned species belong to Gram-negative bacteria, which are capable of producing huge amounts of pro-inflammatory lipopolysaccharides (LPSs), thereby causing intestinal inflammation and gut barrier disruption. Increased gut permeability permits the transport and the systemic and brain accumulation of LPS or other bacterial toxins and the propagation of an inflammatory state [91]. Moreover, this observation has been confirmed in in vivo studies, where the injection of bacterial lipopolysaccharide into the brain imitates inflammatory and pathological features such as cognitive impairment and fibrillogenesis observed in AD [92].
Besides the propagation of an inflammatory state, microbial endotoxins are probably involved in AD’s inflammatory amyloidosis. Metagenomics analysis showed that patients suffering from cognitive dysfunctions related to amyloidosis demonstrated elevated amounts of pro-inflammatory cytokines and neurotoxic compounds. In these patients, increased inflammation was probably related to Eubacterium deficiency, and an overabundance of gram-negative Escherichia and Shigella species [93], which are known amyloid producers [74]. They manufacture “curli” amyloid fibers, accumulated extracellularly through an elaborated system [94]. Further studies revealed that the overproduction of Aβ plaques is correlated with excessive growth of Escherichia coli, Bacillus subtilis, Klebsiella pneumoniae, Mycobacterium and Salmonella, Staphylococcus aureus, and Streptococcus spp. [95]. Galloway et al. found Aβ in small intestine epithelial cells, suggesting that gut-derived amyloids could be absorbed and finally reach systemic circulation, where they can act as pro-inflammatory factors and trigger fibrillogenesis [96,97]. Additionally, it was shown that amyloids generated by microbes can influence IL-17A and IL-22 proinflammatory cytokines production, considered as a key players in NFκB signaling, both associated with AD and capable of crossing the BBB and GI tract [9].
Another Gram-negative bacteria, and its metabolites, which have a confirmed association with AD, is Helicobacter pylori. H. pylori mainly colonizes the stomach, where it is able to produce H2O2, leading to increased homocysteine levels, and is a well-known metabolite connected with AD development. H2O2 alone harms BBB vascular endothelial cells, thereby disrupting cell homeostasis and creating dysfunctions, and consequently increasing the β-amyloid concentration in the brain [19,48,98].
At the end of this chapter, it is worth mentioning the fact that the interaction between the microbiome and the host is bidirectional, and some metabolic changes, which could be a pathological result of disease progression, also influence microbiome metabolism. A good example is a disturbance in cholesterol homeostasis, which correlates with an augmented probability of Alzheimer’s disease occurrence [63,64]. In addition, alteration of the intestinal microbiota composition with the subsequent fluctuations in the serum and brain levels of bile acids (BAs) is involved in the development of AD. BAs can act as detergents, affecting BBB permeability, and incrementing brain influx of peripheral cholesterol and BAs. Therefore, proper cholesterol elimination pathways play a crucial role in AD prevention and treatment [50]. The gut microbiome is a main “organ” metabolizing secondary bile acids. Peripheral cholesterol is eliminated mainly through its liver conversion to BAs. The gut microbiota performs BA transformation via deconjugation and dehydroxylation, leading to secondary BA formation [99]. Nho et al. investigated the correlation between neurodegeneration and BAs, B-amyloid, and tau protein concentration. The application of targeted metabolomics enabled the determination of the association between intestinal microbiota-derived secondary bile acids and sera BAs. Interestingly, the analysis indicated an imbalanced BA ratio, underlying an increased amount of secondary BAs over primary ones [91]. Another clinical study confirmed the correlation between bile acid metabolic profile and the prevalence of AD and mild cognitive impairment, showing similar BA ratio disruptions. Decreased cognitive functions were positively associated with increased concentrations of secondary bile acids such as deoxycholic acid, glycodeoxycholic acid, taurodeoxycholic acid, and glycolithocholic acid. The described results suggested the involvement of the pathological gut microbiota in AD development; however, still it requires more in-depth studies [100].
3.2. Parkinson’s Disease
Parkinson’s disease (PD) is one of the most widespread neurodegenerative diseases, second place in incidence among them, concerning around 1% of the population over the age of 60, reducing the quality of life and leading to progressive disability [101]. The death of dopaminergic neurons in the substantia nigra pars compacta is characteristic of PD. This disease causes symptoms such as rest tremors, bradykinesia, muscular rigidity, and postural stability problems. Parkinson’s disease is characterized not only by neurological disorders, but also by gastrointestinal disturbances, including nausea, constipation (affecting more than 70% of PD patients), vomiting, reduced colonic motility, and gut microbiota changes (small intestinal bacterial overgrowth among many others). Additionally, gastrointestinal comorbidities usually appear years prior to the onset of motor function symptoms [102,103].
The origins of Parkinson’s disease are the result of many factors such as increased age, polygenic factors (disease-segregating mutations in α-synuclein protein occurring in 15% of PD patients), toxins, and infectious agents. In 2006, Braak et al. assumed the gut-related origins of Parkinson’s disease [104]. One of the key pathological hallmarks of PD is Lewy bodies, being eosinophilic inclusion forms of misfolded α-synuclein aggregates. Importantly, α-synuclein protein has a tendency to misfold and form aggregates. Its accumulation has been detected during bacterial and viral infections in the gut. Moreover, it can be recognized as a characteristic indicator of PD neural degeneration [105]. This protein aggregate can boost the local immune response [106]. Interestingly, α-synuclein deposition has been confirmed in the neurons of the intestinal submucosa, together with a possibility of the α-synuclein transport to the brain via the vagus nerve (translocation of prion-like proteins) [103,104,107,108]. Misfolded α-synuclein triggers neuronal dysfunction and progressive degeneration, thereby enabling disease progression [109]. Additionally, α-synuclein deposition in CNS is associated with intestinal hyperpermeability, TLRs, and pro-inflammatory cytokine overexpression. Animal models showed that α-synuclein aggregation in PD is associated with disorders of lipid metabolism, especially concerning phospholipids and sphingolipids [110,111].
3.2.1. Host and Microbiome Metabolomic Changes during PD: Short Story about Bad and Good Cop
Good Cop
Patients suffering from PD have different bacterial flora compared with healthy controls [112]. Microbiota have an impact on disease progression, which has been established in the fecal-transplantation experiments. Administration of the microbiota of patients with Parkinson’s disease to mice showed the development of neuroinflammatory processes and motor deficits [113].
The PD microbiome has a diminished variety of bacterial taxonomic groups, especially the ones associated with anti-inflammatory and neuroprotective effects and the increase of phyla, which has toxic effects [114,115]. Predominantly, the reduction was observed in the Lachnospiraceae family, including Butyrivibrio, Pseudobutyrivibrio, Coprococcus, Blautia and SCFAs-producing Prevotellaceae [115]. The increased amount of Enterobacteriaceae is linked to the severity of postural instability and walking difficulties [116]. Moreover, the PD microbiome is characterised by increased Akkermansia and Bifidobacterium species and decreased amount of Faecalibacterium and Lachnospiraceae. The overgrowth of Akkermansia spp. in PD patients may be strain-specific and can be related to immune-response alterations, decreased mucus thicknesses, and increased constipation [117]. Faecalibacterium family is a crucial player producing SCFAs and anti-inflammatory metabolites, maintaining gut health [118]. Faecalibacterium balance disturbances can lead to impaired gut-barrier function, increasing the risk of infection with enteric pathogens and boosting α-synuclein formation [119]. Similarly, the Lachnospiraceae family can produce butyrate, important for the proper function of the gut epithelium [120]. Decreased Lachnospiraceae quantity might aggravate gut inflammation, stimulate toxin production, and damage the integrity of the gut epithelial barrier [121,122]. PD patients’ intestinal microflora were characterised by a diminished abundance of Lachnospiraceae, which was positively correlated with PD progress, cognitive decline, and impaired motor functions [122,123]. These microbiome’s compositional changes correlate well with some metabolic switches. PD microbiota show reduced SCFAs production, decreased carbohydrate fermentation, and augmented proteolytic fermentation [102]. Chen et al. demonstrated that the SCFA level was relevant to microbiota composition changes and the clinical severity of PD. The levels of SCFAs acetate, butyrate, and propionate in fecal samples of PD patients were significantly reduced, contrary to their elevated concentration in plasma [57]. Rodent models of PD disease have shown a positive effect of SCFAs on behavior and PD symptoms [11,124]. Butyrate, as a histone deacetylase inhibitor, exerts neuroprotective activity, reducing dopaminergic cell death, and motor impairment, and improving cognitive functions [125,126]. Taken together, SCFAs play an important role in protection from PD disease and could be proposed as representative gut-oriented indicators for diversifying individuals with PD and placing disease progression [127].
During PD development, microbiota changes can be accompanied by reduced concentrations of branched-chain amino acids (BCAAs) and aromatic amino-acids in comparison with the healthy control group [128]. It has been demonstrated that the alteration of the metabolites occurred, especially during disease progression [129]. Experimental results indicate the correlation between PD and mitochondrial dysfunction. Additionally, the lower levels of BCAAs and aromatic amino acids have been observed in stool samples during this period in comparison with the healthy control group, indicating a dysbiosis state in the gut microbiota [128]. Moreover, the PD-disrupted gut microbiome may negatively influence the level of methionine in human plasma [130,131].
Similar to the AD case, the host metabolome is affected during disease, which is not without its influence on microbiome composition/metabolome. Bile acid synthesis is a common part of the metabolic system which is affected both in AD and PD [132,133,134]. Regarding this, there were increased levels of bile acids conjugated with taurine (taurodeoxycholic, taurolithocholic, and taurochenodeoxycholic acid) observed, which correlates with motor activity in PD [135]. It is worth underlining the role of taurine as an inhibitory neurotransmitter. Moreover, it is fabricated and secreted by neurons during stress conditions, e.g., mitochondrial dysfunctions, where taurine seems to increase the neuronal survival rate due to calcium influx regulation and the intensified antioxidant gene expression. In summary, taurine demonstrates an important role in proper brain function and development. Additionally, it contributes to the volume regulation of brain cells, and interferes with synaptic amino acid receptors [136,137].
Bad Cop
Functional analysis of the samples obtained from PD patients exposes an augmented microbial ability to conduct the degradation of mucin and glycans [138], influence on folate deficiency and hyperhomocysteinemia [138], and finally increased proteolytic fermentation with the creation of toxic amino acid metabolites such as p-cresol or phenylacetylglutamine [102]. Preclinical tests on genetically modified mice with PD demonstrated the correlation between Akkermansia muciniphila and Bilophila wadsworthia abundance and decreased the mice motor activity. Furthermore, Akkermansia muciniphila produces high amounts of pro-inflammatory hydrogen sulphide, correlated with PD pathology and being a neurotoxin affecting brain mitochondrial energy homeostasis through the inhibition of brain glutathione concentration [135,139]. The second bacterial species, Bilophila wadsworthia, is considered to be the main producer of sulphite in the human gut microbiome, leading to intestinal inflammatory states and the reduction of blood glutathione levels [135].
3.3. Other Neurodegenerative and Psychiatric Diseases
The term “neurodegenerative diseases” is much wider than only PD and AD [140]. However, according to the state of knowledge, microbiome, and microbiome–metabolome influence on disease progression in these two diseases are the best described so far (Table 1). Interestingly, microbiome and microbiome–metabolome have gained a lot of attention recently [141,142], and new studies shed light on their engagement in multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD) [143].

Table 1.
Changes in microbiome composition, its metabolic status, and influence on host homeostasis and metabolome in AD and PD.
3.4. Microbiota, Multiple Sclerosis (MS), and Amyotrophic Lateral Sclerosis (ALS)
Multiple sclerosis is a chronic neurodegenerative autoimmune disorder, where the human immune system attacks the myelin sheath surrounding axon terminals, thereby triggering long-lasting inflammation within the CNS, abrasion formation, and demyelination of axons. Besides, afterward, it can cause autonomic and cognitive problems, motor, sensory, and visual flaws, and finally paralysis and disruption of the BBB [144,145,146]. Various animal and human studies suggested the participation of gut microbiota in the development of MS [147,148,149]. Animal studies on an experimental autoimmune encephalomyelitis mice model revealed a significant reduction in Lactobacillus phyla, contributing to an impairment of the mice’s immune systems [150]. In patients diagnosed with MS, a reduction in the variety and quantity of gut bacteria was detected, confirming a moderate dysbiosis state [151]. Fecal samples obtained from MS patients indicated the reduced amount of bacteria belonging to 19 species, Bacteroidetes, Firmicutes, Faecalibacterium, Prevotella, and Anaerostipes species among them, and the increased quantity of Actinobacteria, Bifidobacterium, and Streptococcus genera [146].
Experimental research confirms the hypothesis regarding SCFA reduction in autoimmune diseases (MS among others) and altered gut microbiota. A low level of SCFAs dysregulates Treg cell functions, leading to insufficient immune tolerance towards endogenous components and enteric microbiota. However, this process can be reversed by SCFAs supplementation (e.g., propionic acid) [147,152]. Furthermore, research results indicated that MS is also connected with intestinal permeability disruption and the altered metabolism of bile acids, causing the dysregulation of the immune and nervous systems. Bile acids can act as CNS inflammatory signalling modulators, e.g., ursodeoxycholic acid can exert microglia inflammatory-inhibiting activity. Moreover, tauroursodeoxycholic acid (TUDCA) can direct microglia phenotypes towards an anti-inflammatory state, reducing NFκB activation and exerting a neuroprotective effect both in the mouse model of acute neuroinflammation and on microglial cells in vitro. TUDCA binds to the G protein-coupled bile acid receptor 1/Takeda G protein-coupled receptor 5 (GPBAR1/TGR5) expressed in microglial cells [153]. This binding follows an increased level of microglial intracellular cyclic adenosine monophosphate (cAMP), inducing the release of the anti-inflammatory cytokines and chemokines (e.g., IL-4Rα, IL-10, IL-1 receptor-associated kinase-M IRAK-M, programmed cell death ligand 1 PD-L1, and sphingosine kinase 1 Sphk1) and reducing the ones with pro-inflammatory activity (ionised calcium-binding adapter molecule 1 Iba-1, inducible nitric oxide synthase iNOS). Experimental results demonstrated that TUDCA acted as a double-edged sword, triggering microglia enhancement in anti-inflammatory and alternatively activated markers and on the other hand reducing the infiltration of macrophages and microglia with expressed pro-inflammatory markers [153]. Interestingly, during neuroinflammation, microglial cells change their morphology, converting themselves into reactive macrophage-like forms. However, microglial cells are flexible, as they can sometimes polarize towards alternatively activated phenotypes, promoting CNS pathologies (proinflammatory or M1 microglia), or exert anti-inflammatory effects, boosting cell repair and remodeling. Bile acids were identified as nuclear hormone receptor farnesoid X receptor agonists, leading to experimental autoimmune encephalomyelitis (EAE) depletion (in a mouse model) and neuroinflammatory process adjustment [153,154]. The administration of the complex probiotic positively influenced the intestinal flora balance in patients diagnosed with MS. The probiotic prevented the onset of dysbiosis, significantly increasing the amount of Methanobrevibacter and Akkermansia, Prevotella, and Sutterella in MS patients, influencing gene expression and the production of beneficial bacterial metabolites [155].
Amyotrophic Lateral Sclerosis (ALS) is a fatal health condition caused by the progressive neural death of the spinal cord, brain stem, and motor cortex neurons, causing advanced weakness, paralysis, eating difficulties, and respiratory failure [156]. One of the main signs of ALS is the accumulation of phosphorylated protein, which binds to DNA in the form of protein aggregates deposited in glia and motor neurons [157,158]. Next-Generation Sequencing (NGS) methods, such as 16S rRNA gene sequencing, have demonstrated the alteration of numerous bacteria, including Parabacteroides distasonis, Lactobacillus gasseri, Prevotella melaninogenica, Ruminococcus torques, and Akkermansia muciniphila [159]. Changes in bacterial abundance and diversity correlate with ASL origins (as one of the environmental factors) together with impaired metabolism, immunological functions, and toxin accumulation, leading to brain damage. It has been demonstrated that Akkermansia muciniphila alleviates ALS symptoms, although Ruminococcus torques and Parabacteroides distasonis aggravate them [157]. The main switches have been observed in SCFA-producing bacteria, leading to reduced levels of SCFAs, e.g., butyrate and propionate [157,158].
3.5. Huntington’s Disease (HD)
Recent studies underlined the important role of the gut microbiota in bidirectional crosstalk in Huntington’s disease (HD) [160]. Huntington’s disease has genetic origins related to trinucleotide expansion (CAG) in the huntingtin coding gene. Moreover, experiments performed in mice models revealed that the altered microbiota observed in the HD-suffering individuals at the pre-motor symptomatic stage highly influence health status. HD microbiota composition indicated dysbiosis, disturbances in cytokine levels, and an increase in sulphur metabolism characterized by hydrogen sulphide overproduction and negatively influencing gut health [160,161]. Ultimately, the gut dysbiosis state was also described in a transgenic mice model representing Huntington’s disease [162,163]. Kong et al. found increased Bacteroidetes and decreased Firmicutes levels in a R6/1 transgenic mice model. In this study, dysbiosis was followed by body weight deterioration, gastrointestinal motility problems, and the loss of motor ability [162]. Subsequent studies performed by the same group revealed the negative correlation between Blautia producta and butyrate production. Moreover, Prevotella scopos was negatively associated with ATP levels [161]. Clinical trials performed on patients suffering from Huntington’s disease revealed the prevalence of bacterial phyla belonging to Firmicutes (83%), Actinobacteria (9%), Bacteroidetes (4%), and Verrucomicrobia (1.1%) detected in the collected fecal samples [164]. The results of performed human randomized clinical trials positively correlate with the data obtained in mouse models in which gut microbiota dysbiosis was observed. A Huntington’s mouse model revealed endocrine hormonal abnormalities, improper intestinal morphology (decreased mucosal thickness, and villus length), and damaged neuropeptide production [165,166,167].
3.6. Autism, Schizophrenia, and ADHD
Autism spectrum disorder (ASD) can be classified as a neurodevelopmental problem represented by repetitive behaviors, social communication, and cognitive function impairment beginning in early childhood [168]. It is predicted that more than 50% of the neurobiology in autism disorders may be determined by non-genetic factors, such as environmental factors, parental age, and preterm birth, among many others. Autism is not only associated with disorders of a neurological nature, but also with disturbances in the qualitative and quantitative arrangement of the gut microbiota, intestinal immune inflammation, and dysbiosis, as confirmed in many studies, and correlated with disease severity [169,170]. For example, gut microbiota transplantation from patients suffering from autistic disorders into antibiotic-treated mice induced pathological autistic social and repetitive behaviors in rodents [11,171].
In accordance with the microbiome–metabolome, research has shown that reductions in microbiome diversity were mainly expressed by the decrease of the SCFAs-producing Prevotella, Coprococcus, Faecalibacterium, and unclassified Veillonellaceae species [172,173]. However, it has been found that ameliorating and modulating these behaviors by administering probiotics containing Bacteroides fragilis or Lactobacillus reuteri, or by performing fecal transplantations from healthy individuals, is possible. In this way, the composition of the beneficial gut microbiota can be restored, providing adequate levels of SCFAs and intestinal homeostasis [11,171].
Schizophrenia is a severe neuropsychological disorder affecting approximately 1% of the world’s population [174]. 16S rRNA sequencing and diversity analyses of the fecal-derived microbiota obtained from patients suffering from schizophrenia indicated lower microbiome diversity. Performed analyses revealed the abundance of Proteobacteria and decreased amount of SCFAs synthesizing bacteria from Blautia, Coprococcus, and Roseburia species. It has been reported that butyrate and other SCFAs can cross the gastrointestinal endothelium and can pass through the BBB (exploiting active membrane transporters) and inhibit histone deacetylase 1 (HDAC1) [6]. Abnormal microbiota can lead to reduced levels of butyrate and can cause the elevation of HDAC1 levels in the prefrontal cortex and hippocampus of patients with diagnosed schizophrenia, causing altered DNA methylation, impaired synaptic plasticity and short-term memory, and the development of impaired cognitive function and social abilities [175].
The analysis of the intestinal microbiome of adolescent and adult ADHD patients (Attention Deficit Hyperactivity Disorder) indicated changes and imbalances in the gut microbiota followed by elevated levels of Bifidobacterium and Eggerthella genera related to boosted dopamine precursor formation and connected with ADHD development [176]. Some reports indicated the implication of the microbiome in the regulation of brain dopamine levels [177]. The Bifidobacterium genus plays an important role in dopamine precursor production (cyclohexadienyl dehydratase) in ADHD patients [178]. Jiang et al. reported a reduction of Faecalibacterium spp. in paediatric patients diagnosed with ADHD and they suggested Faecalibacterium as a new marker of ADHD [179]. Faecalibacterium depletion was followed by increased cytokine levels and inflammation [180,181]. Interestingly, three bacterial species (Bacteroides uniformis, Bacteroides ovatus, and Sutterella stercoricanis) were identified as potential biomarkers of ADHD [182]. Tengeler et al. reported increased anxiety in a GF mice model with human microbiota transplanted from ADHD-suffering individuals [183]. Another clinical trial performed on infants indicated the beneficial and protective effect of Lactobacillus rhamnosus GG administration in the prevention of ADHD development. Moreover, L. rhamnosus probiotic administration reduced the quantity of the following proinflammatory factors: IL-12 p70, IL-10, TNF-α, and IL-6 [184].
3.7. Brain Injury and Stroke
It has been proven that risk factors for civilization diseases such as stroke and cardiovascular diseases are also associated with the diversity of the intestinal flora. The abovementioned factors comprise atherosclerosis and hypertension. Moreover, the dysregulation of microbiota was observed in patients with stroke together with increased plasma and urinary concentrations of the proatherosclerotic gut bacterial metabolite trimethylamine N-oxide (TMAO), also co-responsible for the development of cardiovascular diseases (atherosclerotic coronary artery disease among others) [185], AD, and gestational diabetes [186]. TMAO is produced from dietary phosphatidylcholine by the intestinal microbiota [11]. Preclinical models of cerebral ischemia indicate the coexistence of pathological microflora which negatively affect intestinal permeability and motility. Some reported experimental results indicate the relationship of intestinal microbiota with neuroinflammatory processes by modulating intestinal T-cell trafficking to the CNS. Animal models of cerebral ischemia-reperfusion injury clearly pointed out the beneficial, neuroprotective effect of the administration of a probiotic containing the Clostridium butyricum bacterial strain on the prognosis of animals after a stroke [187].
4. Nutritional Intervention as a Promising Solution to Prevent the Progression of Neurodegenerative Diseases
Currently, we have defined multiple factors which have an influence on the microbiome’s composition and its metabolome, i.e., environmental (such as exposure to pesticides or heavy metals), biological (infections), and sociodemographic factors such as inadequate diet, stress, mode of delivery at birth, lack of breastfeeding during the neonatal period, or antibiotic therapy [161,188,189]. All of these factors could negatively modulate the microbiome and finally lead to dysbiosis. However, if we try to indicate the most powerful factor, then diet is one of the strongest lifetime modulators of microbiome composition [190,191].
For example, the Western diet is a well-known negative modifier of microbiome composition and a promoting factor of multiple common diseases [192]. It is characterized by high fat and sugar ingestion and low fiber content, which are positively linked to Bacteroidetes and Actinobacteria abundance, but negatively correlated with fiber-rich nourishment. A contradictory connotation could be observed for Proteobacteria and Firmicutes [193].
Contrarily, as a positive example, balanced diets (i.e., Mediterranean diet and Dietary Approaches to Stop Hypertension) can be mentioned. Diets abundant in natural compounds with anti-inflammatory activity, antioxidants, polyunsaturated fatty acids, and plant-origin nutrients (proteins, polyphenols, vitamins, and fibers), together with reduced caloric intake, such as the Mediterranean diet, are correlated with a lower risk of dementia, decrease age-related cognitive deterioration, and the risk of neurodegeneration occurrence [194,195,196]. Moreover, the Mediterranean diet increases the amount of gut Bifidobacterium and Lactobacillus and reduces malignant bacteria belonging to Clostridium and Bacteroides, thereby improving memory and cognitive processes in healthy females after menopause and disorder patients. Additionally, following such a diet significantly reduces amyloid aggregation and therefore the frequency of amyloid-related diseases in the aging population [14,197,198]. There are scientific reports that he gut dysfunction is related to the inflammation of the CNS, which can especially be observed in Parkinson’s disease patients, where dysregulation of the gut–brain axis was observed in 80% of patients [199].
In the past decade, single dietary factors which can positively manipulate the gut microbiota–brain axis through microbiome modulation have gained significant attention [200,201]. One of these factors is probiotic bacteria and/or prebiotics. Probiotics are defined as microbial organisms with a beneficial role in the host’s health and homeostasis, which help to preserve digestive, metabolic, immune, and neuroendocrine functions [202]. Naturally probiotic bacteria can be found in fermented food [203,204]. Experimental results revealed that the administration of probiotics alone or in combination with prebiotics (fructooligosaccharides or galactooligosaccharides) improved the gastrointestinal barrier [205,206], which is the first line of “defence” from harmful factors occurring in food, and the microbiome. Probiotics and prebiotics are well-documented “problem-solution” for gastrointestinal diseases [207,208]. Moreover, probiotics can reduce CNS inflammatory processes and activation of microglia, demonstrating promising potential for use in neurodegenerative diseases as ingestible “psychobiotics”. Many studies indicate that the ingestion of probiotics can serve as a microbiological strategy against neurodegenerative diseases such as AD [209,210,211]; however, the exact mechanism is under investigation [212]. Experiments performed on mice underline the hypothesis that mechanisms related to neuroprotective effects of Bifidobacterium breve administration may be connected with neuroinflammation and cognition pathways, probably through the production of the brain-derived neurotrophic factor (BDNF) neurotransmitter and the regulation of the gut microbial composition [213]. Clinical results of a randomized double-blind controlled trial revealed that 3-month exposure to Lactobacillus spp. And a Bifidobacterium bifidum “cocktail” enhanced cognitive functions and memory, as examined in a mini-mental state test [214]. It was demonstrated that Bifidobacterium breve exerts positive and unique activity in AD patients, improving cognitive functions and reducing neuroinflammation. Controlled, double-blind, randomized clinical trials with a 12-week multispecies probiotic treatment (comprised of Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum) caused a significant decrease in malondialdehyde in the probiotic-treated group [214].
4.1. Clinical Trials Related to Probiotic Administration and Brain-Related/Stress Disorders
Actually, some clinical trials regarding probiotic administration and brain-related disorders have been carried out. Herein, we gathered information regarding this issue. An intense clinical trial search was performed between 16 September and 19 September 2022 on the National Library of Medicine (NIH) ClinicalTrials website (clinicaltrials.gov) using the term “probiotics” in the “condition” field, yielding 853 clinical studies. Only completed studies were taken into consideration. After the initial selection, we collected only those trials related to brain related/stress disorders (Table 2). Interestingly, the majority of the presented clinical trials demonstrated a positive correlation between probiotic administration and the amelioration of disease/condition symptoms, encouraging the daily administration of pro/symbiotics or the introduction of fermented-food additives into the daily diet.

Table 2.
Clinical trials developed regarding probiotics administration and brain-related disorders.
4.2. Another Factors including Gender Aspects Related to Gut Microbiome and Neurodegenerative Diseases
The health of the gut microbiome can vary depending on various factors including diet (e.g., ingestion of saturated/unsaturated dietary fats, vitamins, minerals, dairy products, alcoholic beverages, polyphenols containing food such as cocoa-rich chocolate, tea, or coffee), dietary patterns, geographical area, and diet-related habits (sometimes varying between males and females) and sex (the influence of sex hormones). A positive correlation between polyunsaturated fatty acids (PUFA), omega 3 polyunsaturated fatty acids (N-3 PUFA), and α-linolenic acid (aLNA) ingestion and protection against PD development in dose-response trends was demonstrated [253]. American studies demonstrated that PUFA replacement with increased animal fat intake (Western Diet) significantly increased the risk of PD in males only [254]. Excessive animal fat intake can promote dysbiosis and the production of harmful microbial metabolites, which can increase the prevalence of the development of neurodegenerative diseases. Moreover, sex differences are implicated in the mental health and prevalence of psychiatric, neurodevelopmental, and neurodegenerative disorders [255]. It has been demonstrated that PD patients usually have altered microbiome compositions compared with controls [120]. Similar to AD, many sex differences in PD are correlated with the protective nature of estrogens, as they are impacted by the gut-brain axis and protect against oxidative damage, thereby supporting dopaminergic function [256]. It has been demonstrated that at age 45, the estimated overall lifetime risk for AD is about 20% for females and 10% for males, which is followed by a disruption in the patient’s gut microbiota composition [257]. In summary, it is worth underlining the correlation between the gut microbiome, immune system and brain, mental health, and behavior [258].
5. Conclusions
Overall, gut microbiota and their implication in the gut-brain axis can be recognized as a crucial factor in neurodegenerative and neurological disorders. Moreover, human studies indicated the correlation between gut dysbiosis and major neurological and psychiatric disorders. The experimental results showed the importance of designing a novel microbiota-based probiotic dietary supplementation with the aim to prevent or ease the symptoms of AD, PD, or other forms of dementia. Promisingly, neuropsychiatric and neurodegenerative problems can be ameliorated through targeting the microbiota by microbiota transplantation, antibiotic treatment, or the administration of properly selected probiotics. The results of numerous studies indicate the beneficial effect of probiotic administration, including intestinal epithelial integrity enhancement and a protective role against neuroinflammation, neurodegeneration, and barrier disruption. Such solutions in the future may open new therapeutic paths and provide hope for improving the living condition of patients suffering from neurodegenerative diseases.
Author Contributions
Conceptualisation, R.J. and M.A.M.; formal analysis, M.A.M.; investigation, M.A.M.; data curation, M.A.M.; writing—original draft preparation, M.A.M.; writing—review and editing, J.M., R.J.; visualisation (figures and tables), R.J., M.A.M.; supervision, J.M.; project administration, J.M., M.A.M. and R.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Centre for Research and Development (NCBiR, Poland), under the grant number POIR.01.01.01-00-0985/17.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial Genes, Brain & Behaviour—Epigenetic Regulation of the Gut-Brain Axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The Progress of Gut Microbiome Research Related to Brain Disorders. J. Neuroinflamm. 2020, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Blum, H.E. The Human Microbiome. Adv. Med. Sci. 2017, 62, 414–420. [Google Scholar] [CrossRef]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as Modifiers of Gut Microbial Communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Hooper, L.V.; Gordon, J.I. Commensal Host-Bacterial Relationships in the Gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Sun, M.-F.; Shen, Y.-Q. Dysbiosis of Gut Microbiota and Microbial Metabolites in Parkinson’s Disease. Ageing Res. Rev. 2018, 45, 53–61. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, Probiotics and Neurodegenerative Diseases: Deciphering the Gut Brain Axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Antonioli, L.; Colucci, R.; Blandizzi, C.; Fornai, M. Interplay among Gut Microbiota, Intestinal Mucosal Barrier and Enteric Neuro-Immune System: A Common Path to Neurodegenerative Diseases? Acta Neuropathol. 2018, 136, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Thaiss, C.A.; Elinav, E. Metabolites: Messengers between the Microbiota and the Immune System. Genes Dev. 2016, 30, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, E.; Musich, P.R.; Lin, F. Mitochondrial Dysfunction in Neurodegenerative Diseases and the Potential Countermeasure. CNS Neurosci. Ther. 2019, 25, 816–824. [Google Scholar] [CrossRef]
- Konjevod, M.; Nikolac Perkovic, M.; Sáiz, J.; Svob Strac, D.; Barbas, C.; Rojo, D. Metabolomics Analysis of Microbiota-Gut-Brain Axis in Neurodegenerative and Psychiatric Diseases. J. Pharm. Biomed. Anal. 2021, 194, 113681. [Google Scholar] [CrossRef]
- Hirschberg, S.; Gisevius, B.; Duscha, A.; Haghikia, A. Implications of Diet and The Gut Microbiome in Neuroinflammatory and Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3109. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Gut Instincts: Microbiota as a Key Regulator of Brain Development, Ageing and Neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef]
- Wang, S.; Cui, W.; Zeng, M.; Ren, Y.; Han, S.; Li, J. The Increased Release of Amino Acid Neurotransmitters of the Primary Somatosensory Cortical Area in Rats Contributes to Remifentanil-Induced Hyperalgesia and Its Inhibition by Lidocaine. J. Pain Res. 2018, 11, 1521–1529. [Google Scholar] [CrossRef]
- Santoro, A.; Ostan, R.; Candela, M.; Biagi, E.; Brigidi, P.; Capri, M.; Franceschi, C. Gut Microbiota Changes in the Extreme Decades of Human Life: A Focus on Centenarians. Cell. Mol. Life Sci. 2018, 75, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein–Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome of Prebiotic-Treated Mice Reveals Novel Targets Involved in Host Response during Obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Arrona Cardoza, P.; Spillane, M.B.; Morales Marroquin, E. Alzheimer’s Disease and Gut Microbiota: Does Trimethylamine N-Oxide (TMAO) Play a Role? Nutr. Rev. 2022, 80, 271–281. [Google Scholar] [CrossRef]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Unhealthy Gut, Unhealthy Brain: The Role of the Intestinal Microbiota in Neurodegenerative Diseases. Neurochem. Int. 2018, 120, 149–163. [Google Scholar] [CrossRef]
- Aoyama, M.; Kotani, J.; Usami, M. Butyrate and Propionate Induced Activated or Non-Activated Neutrophil Apoptosis via HDAC Inhibitor Activity but without Activating GPR-41/GPR-43 Pathways. Nutrition 2010, 26, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Padhi, P.; Worth, C.; Zenitsky, G.; Jin, H.; Sambamurti, K.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Mechanistic Insights Into Gut Microbiome Dysbiosis-Mediated Neuroimmune Dysregulation and Protein Misfolding and Clearance in the Pathogenesis of Chronic Neurodegenerative Disorders. Front. Neurosci. 2022, 16, 836605. [Google Scholar] [CrossRef] [PubMed]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of Gut-Brain Axis, Gut Microbial Composition, and Probiotic Intervention in Alzheimer’s Disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Drouin-Ouellet, J.; Cicchetti, F. Inflammation and Neurodegeneration: The Story “Retolled”. Trends Pharmacol. Sci. 2012, 33, 542–551. [Google Scholar] [CrossRef]
- Juźwik, C.A.; Drake, S.S.; Zhang, Y.; Paradis-Isler, N.; Sylvester, A.; Amar-Zifkin, A.; Douglas, C.; Morquette, B.; Moore, C.S.; Fournier, A.E. microRNA Dysregulation in Neurodegenerative Diseases: A Systematic Review. Prog. Neurobiol. 2019, 182, 101664. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia Development and Function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef]
- Nayak, D.; Zinselmeyer, B.H.; Corps, K.N.; McGavern, D.B. In Vivo Dynamics of Innate Immune Sentinels in the CNS. Intravital 2012, 1, 95–106. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Caputi, V.; Giron, M.C. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 1689. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.A.; McNulty, N.P.; Guruge, J.L.; Gordon, J.I. IgA Response to Symbiotic Bacteria as a Mediator of Gut Homeostasis. Cell Host Microbe 2007, 2, 328–339. [Google Scholar] [CrossRef]
- Nichols, R.G.; Davenport, E.R. The Relationship between the Gut Microbiome and Host Gene Expression: A Review. Hum. Genet. 2021, 140, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.M.; Vieira, A.T.; Vinolo, M.A.R.; Oliveira, F.A.; Curi, R.; Martins, F. dos S. The Central Role of the Gut Microbiota in Chronic Inflammatory Diseases. J. Immunol. Res. 2014, 2014, 689492. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The Microbiome and Innate Immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Doifode, T.; Giridharan, V.V.; Generoso, J.S.; Bhatti, G.; Collodel, A.; Schulz, P.E.; Forlenza, O.V.; Barichello, T. The Impact of the Microbiota-Gut-Brain Axis on Alzheimer’s Disease Pathophysiology. Pharmacol. Res. 2021, 164, 105314. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer’s Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front. Cell. Infect. Microbiol. 2017, 7, 318. [Google Scholar] [CrossRef]
- Whitfield, C.; Trent, M.S. Biosynthesis and Export of Bacterial Lipopolysaccharides. Annu. Rev. Biochem. 2014, 83, 99–128. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host-Microbe Interplay. Nutrients 2021, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and Functions of Inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Jia, W.; Rajani, C.; Kaddurah-Daouk, R.; Li, H. Expert Insights: The Potential Role of the Gut Microbiome-Bile Acid-Brain Axis in the Development and Progression of Alzheimer’s Disease and Hepatic Encephalopathy. Med. Res. Rev. 2020, 40, 1496–1507. [Google Scholar] [CrossRef]
- Samsel, A.; Seneff, S. Glyphosate’s Suppression of Cytochrome P450 Enzymes and Amino Acid Biosynthesis by the Gut Microbiome: Pathways to Modern Diseases. Entropy 2013, 15, 1416–1463. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Venegas, D.P.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, A.; Elli, M. The Role of Gut Microbiota in Human Obesity: Recent Findings and Future Perspectives. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–gut–brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Chen, S.-J.; Chen, C.-C.; Liao, H.-Y.; Lin, Y.-T.; Wu, Y.-W.; Liou, J.-M.; Wu, M.-S.; Kuo, C.-H.; Lin, C.-H. Association of Fecal and Plasma Levels of Short-Chain Fatty Acids with Gut Microbiota and Clinical Severity in Patients With Parkinson Disease. Neurology 2022, 98, e848–e858. [Google Scholar] [CrossRef]
- Verbeke, K.A.; Boobis, A.R.; Chiodini, A.; Edwards, C.A.; Franck, A.; Kleerebezem, M.; Nauta, A.; Raes, J.; van Tol, E.A.F.; Tuohy, K.M. Towards Microbial Fermentation Metabolites as Markers for Health Benefits of Prebiotics. Nutr. Res. Rev. 2015, 28, 42–66. [Google Scholar] [CrossRef]
- Galland, L. The Gut Microbiome and the Brain. J. Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Ali, S.A.; Singh, R.K. Emerging Role of Gut Microbiota in Modulation of Neuroinflammation and Neurodegeneration with Emphasis on Alzheimer’s Disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110112. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, C.; Filadi, R.; Rossi, A.; Mammucari, C.; Pizzo, P. PSEN2 (presenilin 2) Mutants Linked to Familial Alzheimer Disease Impair Autophagy by Altering Ca2+ Homeostasis. Autophagy 2019, 15, 2044–2062. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; Kim, T.-W. Linking Lipids to Alzheimer’s Disease: Cholesterol and beyond. Nat. Rev. Neurosci. 2011, 12, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Mayeux, R. Alzheimer Disease: Epidemiology, Diagnostic Criteria, Risk Factors and Biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Narengaowa, K.W.; Lan, F.; Awan, U.F.; Qing, H.; Ni, J. The Oral-Gut-Brain AXIS: The Influence of Microbes in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 633735. [Google Scholar] [CrossRef]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. MBio 2019, 10, e00632-19. [Google Scholar] [CrossRef]
- Solch, R.J.; Aigbogun, J.O.; Voyiadjis, A.G.; Talkington, G.M.; Darensbourg, R.M.; O’Connell, S.; Pickett, K.M.; Perez, S.R.; Maraganore, D.M. Mediterranean Diet Adherence, Gut Microbiota, and Alzheimer’s or Parkinson’s Disease Risk: A Systematic Review. J. Neurol. Sci. 2022, 434, 120166. [Google Scholar] [CrossRef]
- Fujii, Y.; Khasnobish, A.; Morita, H. Morita Relationship between Alzheimer’s Disease and the Human Microbiome. Exon Publ. 2019, 9, 147–158. [Google Scholar]
- Pasinetti, G.M. Handbook of Microbiome and Gut-Brain-Axis in Alzheimer’s Disease; IOS Press: Amsterdam, The Netherlands, 2022; ISBN 9781643682891. [Google Scholar]
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome-Host Systems Interactions: Protective Effects of Propionate upon the Blood-Brain Barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, Maintenance and Disruption of the Blood-Brain Barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Szentirmai, É.; Millican, N.S.; Massie, A.R.; Kapás, L. Butyrate, a Metabolite of Intestinal Bacteria, Enhances Sleep. Sci. Rep. 2019, 9, 7035. [Google Scholar] [CrossRef]
- Tan, A.H.; Chong, C.W.; Lim, S.-Y.; Yap, I.K.S.; Teh, C.S.J.; Loke, M.F.; Song, S.-L.; Tan, J.Y.; Ang, B.H.; Tan, Y.Q.; et al. Gut Microbial Ecosystem in Parkinson Disease: New Clinicobiological Insights from Multi-Omics. Ann. Neurol. 2021, 89, 546–559. [Google Scholar] [CrossRef]
- Ho, L.; Ono, K.; Tsuji, M.; Mazzola, P.; Singh, R.; Pasinetti, G.M. Protective Roles of Intestinal Microbiota Derived Short Chain Fatty Acids in Alzheimer’s Disease-Type Beta-Amyloid Neuropathological Mechanisms. Expert Rev. Neurother. 2018, 18, 83–90. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary Gut Microbial Metabolites, Short-Chain Fatty Acids, and Host Metabolic Regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell 2016, 64, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric Acid Production by Culturable Bacteria from the Human Intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Luan, H.; Wang, X.; Cai, Z. Mass Spectrometry-Based Metabolomics: Targeting the Crosstalk between Gut Microbiota and Brain in Neurodegenerative Disorders. Mass Spectrom. Rev. 2019, 38, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Baganz, N.L.; Blakely, R.D. A Dialogue between the Immune System and Brain, Spoken in the Language of Serotonin. ACS Chem. Neurosci. 2013, 4, 48–63. [Google Scholar] [CrossRef]
- Besser, M.J.; Ganor, Y.; Levite, M. Dopamine by Itself Activates Either D2, D3 or D1/D5 Dopaminergic Receptors in Normal Human T-Cells and Triggers the Selective Secretion of Either IL-10, TNFalpha or Both. J. Neuroimmunol. 2005, 169, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Takaki, M.; Mawe, G.M.; Barasch, J.M.; Gershon, M.D.; Gershon, M.D. Physiological Responses of Guinea-Pig Myenteric Neurons Secondary to the Release of Endogenous Serotonin by Tryptamine. Neuroscience 1985, 16, 223–240. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin Signalling in the Gut--Functions, Dysfunctions and Therapeutic Targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, J. Indole as an Intercellular Signal in Microbial Communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Devlin, A.S.; Marcobal, A.; Dodd, D.; Nayfach, S.; Plummer, N.; Meyer, T.; Pollard, K.S.; Sonnenburg, J.L.; Fischbach, M.A. Modulation of a Circulating Uremic Solute via Rational Genetic Manipulation of the Gut Microbiota. Cell Host Microbe 2016, 20, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial Tryptophan Catabolites in Health and Disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The Aryl Hydrocarbon Receptor: Multitasking in the Immune System. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.B. Redox Reactions in Food Fermentations. Curr. Opin. Food Sci. 2018, 19, 98–103. [Google Scholar] [CrossRef]
- Moya, A.; Ferrer, M. Functional Redundancy-Induced Stability of Gut Microbiota Subjected to Disturbance. Trends Microbiol. 2016, 24, 402–413. [Google Scholar] [CrossRef]
- De-Paula, D.J.R.V.; Forlenza, A.S.; Forlenza, O.V. Relevance of Gutmicrobiota in Cognition, Behaviour and Alzheimer’s Disease. Pharmacol. Res. 2018, 136, 29–34. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Lin, C.-H.; Tsai, T.-H.; Huang, C.-H.; Li, J.-L.; Chen, L.-K.; Li, C.-H.; Tsai, T.-F.; Wang, P.-N. Discovery of a Metabolic Signature Predisposing High Risk Patients with Mild Cognitive Impairment to Converting to Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 10903. [Google Scholar] [CrossRef]
- Nho, K.; Kueider-Paisley, A.; MahmoudianDehkordi, S.; Arnold, M.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; Kastenmüller, G.; et al. Altered Bile Acid Profile in Mild Cognitive Impairment and Alzheimer’s Disease: Relationship to Neuroimaging and CSF Biomarkers. Alzheimers. Dement. 2019, 15, 232–244. [Google Scholar] [CrossRef]
- Hauss-Wegrzyniak, B.; Vraniak, P.D.; Wenk, G.L. LPS-Induced Neuroinflammatory Effects Do Not Recover with Time. Neuroreport 2000, 11, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of Brain Amyloidosis with pro-Inflammatory Gut Bacterial Taxa and Peripheral Inflammation Markers in Cognitively Impaired Elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.D.; Matthews, S.J. New Insight into the Molecular Control of Bacterial Functional Amyloids. Front. Cell. Infect. Microbiol. 2015, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Friedland, R.P. Mechanisms of Molecular Mimicry Involving the Microbiota in Neurodegeneration. J. Alzheimers. Dis. 2015, 45, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S.; Jian, L.; Johnsen, R.; Chew, S.; Mamo, J.C.L. Beta-Amyloid or Its Precursor Protein Is Found in Epithelial Cells of the Small Intestine and Is Stimulated by High-Fat Feeding. J. Nutr. Biochem. 2007, 18, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S.; Takechi, R.; Pallebage-Gamarallage, M.M.S.; Dhaliwal, S.S.; Mamo, J.C.L. Amyloid-Beta Colocalizes with Apolipoprotein B in Absorptive Cells of the Small Intestine. Lipids Health Dis. 2009, 8, 46. [Google Scholar] [CrossRef]
- Park, A.-M.; Omura, S.; Fujita, M.; Sato, F.; Tsunoda, I. Helicobacter Pylori and Gut Microbiota in Multiple Sclerosis versus Alzheimer’s Disease: 10 Pitfalls of Microbiome Studies. Clin. Exp. Neuroimmunol. 2017, 8, 215–232. [Google Scholar] [CrossRef]
- Garcia, C.J.; Kosek, V.; Beltrán, D.; Tomás-Barberán, F.A.; Hajslova, J. Production of New Microbially Conjugated Bile Acids by Human Gut Microbiota. Biomolecules 2022, 12, 687. [Google Scholar] [CrossRef]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered Bile Acid Profile Associates with Cognitive Impairment in Alzheimer’s Disease-An Emerging Role for Gut Microbiome. Alzheimers. Dement. 2019, 15, 76–92. [Google Scholar] [CrossRef]
- de Lau, L.M.L.; Breteler, M.M.B. Epidemiology of Parkinson’s Disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Cirstea, M.S.; Yu, A.C.; Golz, E.; Sundvick, K.; Kliger, D.; Radisavljevic, N.; Foulger, L.H.; Mackenzie, M.; Huan, T.; Finlay, B.B.; et al. Microbiota Composition and Metabolism Are Associated with Gut Function in Parkinson’s Disease. Mov. Disord. 2020, 35, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Lew, M. Overview of Parkinson’s Disease. Pharmacotherapy 2007, 27, 155S–160S. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; de Vos, R.A.I.; Bohl, J.; Del Tredici, K. Gastric Alpha-Synuclein Immunoreactive Inclusions in Meissner’s and Auerbach’s Plexuses in Cases Staged for Parkinson’s Disease-Related Brain Pathology. Neurosci. Lett. 2006, 396, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s Disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Ghaisas, S.; Maher, J.; Kanthasamy, A. Gut Microbiome in Health and Disease: Linking the Microbiome-Gut-Brain Axis and Environmental Factors in the Pathogenesis of Systemic and Neurodegenerative Diseases. Pharmacol. Ther. 2016, 158, 52–62. [Google Scholar] [CrossRef]
- Hilton, D.; Stephens, M.; Kirk, L.; Edwards, P.; Potter, R.; Zajicek, J.; Broughton, E.; Hagan, H.; Carroll, C. Accumulation of α-Synuclein in the Bowel of Patients in the Pre-Clinical Phase of Parkinson’s Disease. Acta Neuropathol. 2014, 127, 235–241. [Google Scholar] [CrossRef]
- Pouclet, H.; Lebouvier, T.; Coron, E.; Des Varannes, S.B.; Neunlist, M.; Derkinderen, P. A Comparison between Colonic Submucosa and Mucosa to Detect Lewy Pathology in Parkinson’s Disease. Neurogastroenterol. Motil. 2012, 24, e202–e205. [Google Scholar] [CrossRef]
- Chung, C.G.; Lee, H.; Lee, S.B. Mechanisms of Protein Toxicity in Neurodegenerative Diseases. Cell. Mol. Life Sci. 2018, 75, 3159–3180. [Google Scholar] [CrossRef]
- Perez-Pardo, P.; Dodiya, H.B.; Engen, P.A.; Forsyth, C.B.; Huschens, A.M.; Shaikh, M.; Voigt, R.M.; Naqib, A.; Green, S.J.; Kordower, J.H.; et al. Role of TLR4 in the Gut-Brain Axis in Parkinson’s Disease: A Translational Study from Men to Mice. Gut 2019, 68, 829–843. [Google Scholar] [CrossRef]
- Galvagnion, C. The Role of Lipids Interacting with α-Synuclein in the Pathogenesis of Parkinson’s Disease. J. Parkinsons. Dis. 2017, 7, 433–450. [Google Scholar] [CrossRef]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-Analysis of the Parkinson’s Disease Gut Microbiome Suggests Alterations Linked to Intestinal Inflammation. NPJ Parkinsons Dis. 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [PubMed]
- Wallen, Z.D. Comparison Study of Differential Abundance Testing Methods Using Two Large Parkinson Disease Gut Microbiome Datasets Derived from 16S Amplicon Sequencing. BMC Bioinform. 2021, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, S.; Palmas, V.; Melis, M.; Pisanu, S.; Cusano, R.; Uva, P.; Perra, D.; Madau, V.; Sarchioto, M.; Oppo, V.; et al. Gut Microbiota and Metabolome Alterations Associated with Parkinson’s Disease. mSystems 2020, 5, e00561-20. [Google Scholar] [CrossRef] [PubMed]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut Microbiota Are Related to Parkinson’s Disease and Clinical Phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Wu, F.; Zhang, M.; Yi, M.; Peng, Y. Different Subtype Strains of Akkermansia Muciniphila Abundantly Colonize in Southern China. J. Appl. Microbiol. 2016, 120, 452–459. [Google Scholar] [CrossRef]
- Ferreira-Halder, C.V.; de Sousa Faria, A.V.; Andrade, S.S. Action and Function of Faecalibacterium Prausnitzii in Health and Disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.-U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.-H. Short Chain Fatty Acids and Gut Microbiota Differ between Patients with Parkinson’s Disease and Age-Matched Controls. Parkinsonism Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s Disease and Parkinson’s Disease Medications Have Distinct Signatures of the Gut Microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef]
- Lin, A.; Zheng, W.; He, Y.; Tang, W.; Wei, X.; He, R.; Huang, W.; Su, Y.; Huang, Y.; Zhou, H.; et al. Gut Microbiota in Patients with Parkinson’s Disease in Southern China. Parkinsonism Relat. Disord. 2018, 53, 82–88. [Google Scholar] [CrossRef]
- Barichella, M.; Severgnini, M.; Cilia, R.; Cassani, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cancello, R.; Ceccarani, C.; Faierman, S.; et al. Unraveling Gut Microbiota in Parkinson’s Disease and Atypical Parkinsonism. Mov. Disord. 2019, 34, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic Bacterial Composition in Parkinson’s Disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-Chain Fatty Acids: Microbial Metabolites That Alleviate Stress-Induced Brain-Gut Axis Alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- Rane, P.; Shields, J.; Heffernan, M.; Guo, Y.; Akbarian, S.; King, J.A. The Histone Deacetylase Inhibitor, Sodium Butyrate, Alleviates Cognitive Deficits in Pre-Motor Stage PD. Neuropharmacology 2012, 62, 2409–2412. [Google Scholar] [CrossRef]
- Srivastav, S.; Neupane, S.; Bhurtel, S.; Katila, N.; Maharjan, S.; Choi, H.; Hong, J.T.; Choi, D.-Y. Probiotics Mixture Increases Butyrate, and Subsequently Rescues the Nigral Dopaminergic Neurons from MPTP and Rotenone-Induced Neurotoxicity. J. Nutr. Biochem. 2019, 69, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.M.-S.; Mohajeri, M.H. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients 2021, 13, 732. [Google Scholar] [CrossRef] [PubMed]
- Minato, T.; Maeda, T.; Fujisawa, Y.; Tsuji, H.; Nomoto, K.; Ohno, K.; Hirayama, M. Progression of Parkinson’s Disease Is Associated with Gut Dysbiosis: Two-Year Follow-up Study. PLoS ONE 2017, 12, e0187307. [Google Scholar] [CrossRef]
- Romo-Vaquero, M.; Fernández-Villalba, E.; Gil-Martinez, A.-L.; Cuenca-Bermejo, L.; Espín, J.C.; Herrero, M.T.; Selma, M.V. Urolithins: Potential Biomarkers of Gut Dysbiosis and Disease Stage in Parkinson’s Patients. Food Funct. 2022, 13, 6306–6316. [Google Scholar] [CrossRef]
- Ye, J.; Lv, L.; Wu, W.; Li, Y.; Shi, D.; Fang, D.; Guo, F.; Jiang, H.; Yan, R.; Ye, W.; et al. Butyrate Protects Mice Against Methionine-Choline-Deficient Diet-Induced Non-Alcoholic Steatohepatitis by Improving Gut Barrier Function, Attenuating Inflammation and Reducing Endotoxin Levels. Front. Microbiol. 2018, 9, 1967. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Li, P.; Killinger, B.A.; Ensink, E.; Beddows, I.; Yilmaz, A.; Lubben, N.; Lamp, J.; Schilthuis, M.; Vega, I.E.; Woltjer, R.; et al. Gut Microbiota Dysbiosis Is Associated with Elevated Bile Acids in Parkinson’s Disease. Metabolites 2021, 11, 29. [Google Scholar] [CrossRef]
- Mulak, A. Bile Acids as Key Modulators of the Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Alzheimers. Dis. 2021, 84, 461–477. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Nochi, H. The Biosynthesis, Signaling, and Neurological Functions of Bile Acids. Biomolecules 2019, 9, 232. [Google Scholar] [CrossRef]
- Hertel, J.; Harms, A.C.; Heinken, A.; Baldini, F.; Thinnes, C.C.; Glaab, E.; Vasco, D.A.; Pietzner, M.; Stewart, I.D.; Wareham, N.J.; et al. Integrated Analyses of Microbiome and Longitudinal Metabolome Data Reveal Microbial-Host Interactions on Sulfur Metabolism in Parkinson’s Disease. Cell Rep. 2019, 29, 1767–1777.e8. [Google Scholar] [CrossRef] [PubMed]
- Saransaari, P.; Oja, S.S. Taurine Release in Mouse Brain Stem Slices under Cell-Damaging Conditions. Amino Acids 2007, 32, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Wu, H.; Jin, Y.; Wei, J.; Sha, D.; Prentice, H.; Lee, H.-H.; Lin, C.-H.; Lee, Y.-H.; Yang, L.-L. Mechanism of Neuroprotective Function of Taurine. Adv. Exp. Med. Biol. 2009, 643, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Rosario, D.; Bidkhori, G.; Lee, S.; Bedarf, J.; Hildebrand, F.; Le Chatelier, E.; Uhlen, M.; Ehrlich, S.D.; Proctor, G.; Wüllner, U.; et al. Systematic Analysis of Gut Microbiome Reveals the Role of Bacterial Folate and Homocysteine Metabolism in Parkinson’s Disease. Cell Rep. 2021, 34, 108807. [Google Scholar] [CrossRef]
- Amorim Neto, D.P.; Bosque, B.P.; Pereira de Godoy, J.V.; Rodrigues, P.V.; Meneses, D.D.; Tostes, K.; Costa Tonoli, C.C.; Faustino de Carvalho, H.; González-Billault, C.; de Castro Fonseca, M. Akkermansia Muciniphila Induces Mitochondrial Calcium Overload and α -Synuclein Aggregation in an Enteroendocrine Cell Line. iScience 2022, 25, 103908. [Google Scholar] [CrossRef]
- Wolfe, M.S. The Molecular and Cellular Basis of Neurodegenerative Diseases: Underlying Mechanisms; Academic Press: New York, NY, USA, 2018; ISBN 9780128113059. [Google Scholar]
- Faintuch, J.; Faintuch, S. Microbiome and Metabolome in Diagnosis, Therapy, and Other Strategic Applications; Academic Press: New York, NY, USA, 2019; ISBN 9780128152508. [Google Scholar]
- Xia, Y.; Sun, J. An Integrated Analysis of Microbiomes and Metabolomics; American Chemical Society: Washington, DC, USA, 2022; ISBN 9780841299542. [Google Scholar]
- Ghezzi, L.; Cantoni, C.; Rotondo, E.; Galimberti, D. The Gut Microbiome-Brain Crosstalk in Neurodegenerative Diseases. Biomedicines 2022, 10, 1486. [Google Scholar] [CrossRef]
- Dopkins, N.; Nagarkatti, P.S.; Nagarkatti, M. The Role of Gut Microbiome and Associated Metabolome in the Regulation of Neuroinflammation in Multiple Sclerosis and Its Implications in Attenuating Chronic Inflammation in Other Inflammatory and Autoimmune Disorders. Immunology 2018, 154, 178–185. [Google Scholar] [CrossRef]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of Microbiota on Central Nervous System and Neurological Diseases: The Gut-Brain Axis. J. Neuroinflamm. 2019, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Miyake, S.; Kim, S.; Suda, W.; Oshima, K.; Nakamura, M.; Matsuoka, T.; Chihara, N.; Tomita, A.; Sato, W.; Kim, S.-W.; et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS ONE 2015, 10, e0137429. [Google Scholar] [CrossRef] [PubMed]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; Stangl, G.I.; Eilers, E.; Bader, V.; Haase, S.; Kaisler, J.; David, C.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067–1080.e16. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Repáraz, J.; Kirby, T.O.; Kasper, L.H. The Gut Microbiome and Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a029017. [Google Scholar] [CrossRef]
- Ghezzi, L.; Cantoni, C.; Pinget, G.V.; Zhou, Y.; Piccio, L. Targeting the Gut to Treat Multiple Sclerosis. J. Clin. Investig. 2021, 131, e143774. [Google Scholar] [CrossRef]
- He, B.; Hoang, T.K.; Tian, X.; Taylor, C.M.; Blanchard, E.; Luo, M.; Bhattacharjee, M.B.; Freeborn, J.; Park, S.; Couturier, J.; et al. Lactobacillus Reuteri Reduces the Severity of Experimental Autoimmune Encephalomyelitis in Mice by Modulating Gut Microbiota. Front. Immunol. 2019, 10, 385. [Google Scholar] [CrossRef]
- Navarro-López, V.; Méndez-Miralles, M.Á.; Vela-Yebra, R.; Fríes-Ramos, A.; Sánchez-Pellicer, P.; Ruzafa-Costas, B.; Núñez-Delegido, E.; Gómez-Gómez, H.; Chumillas-Lidón, S.; Picó-Monllor, J.A.; et al. Gut Microbiota as a Potential Predictive Biomarker in Relapsing-Remitting Multiple Sclerosis. Genes 2022, 13, 930. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Yanguas-Casás, N.; Asunción Barreda-Manso, M.; Nieto-Sampedro, M.; Romero-Ramírez, L. TUDCA: An Agonist of the Bile Acid Receptor GPBAR1/TGR5 With Anti-Inflammatory Effects in Microglial Cells. J. Cell. Physiol. 2017, 232, 2231–2245. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Metz, L.M.; Yong, V.W. Focus on the Gut-Brain Axis: Multiple Sclerosis, the Intestinal Barrier and the Microbiome. World J. Gastroenterol. 2018, 24, 4217–4223. [Google Scholar] [CrossRef]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the Human Gut Microbiome in Multiple Sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Greco, V.; Longone, P.; Spalloni, A.; Pieroni, L.; Urbani, A. Crosstalk Between Oxidative Stress and Mitochondrial Damage: Focus on Amyotrophic Lateral Sclerosis. Adv. Exp. Med. Biol. 2019, 1158, 71–82. [Google Scholar] [CrossRef]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential Roles of Gut Microbiome and Metabolites in Modulating ALS in Mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Romero, C.; Guo, K.; Murdock, B.J.; Paez-Colasante, X.; Bassis, C.M.; Mikhail, K.A.; Raue, K.D.; Evans, M.C.; Taubman, G.F.; McDermott, A.J.; et al. Temporal Evolution of the Microbiome, Immune System and Epigenome with Disease Progression in ALS Mice. Dis. Model. Mech. 2019, 13, dmm041947. [Google Scholar] [CrossRef]
- Tilocca, B.; Pieroni, L.; Soggiu, A.; Britti, D.; Bonizzi, L.; Roncada, P.; Greco, V. Gut-Brain Axis and Neurodegeneration: State-of-the-Art of Meta-Omics Sciences for Microbiota Characterization. Int. J. Mol. Sci. 2020, 21, 4045. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Dong, W.; Yang, Q.; Yu, X.; Ma, J.; Gu, W.; Huang, Y. Altered Gut Microbiota Related to Inflammatory Responses in Patients with Huntington’s Disease. Front. Immunol. 2020, 11, 603594. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Ellul, S.; Narayana, V.K.; Kanojia, K.; Ha, H.T.T.; Li, S.; Renoir, T.; Cao, K.-A.L.; Hannan, A.J. An Integrated Metagenomics and Metabolomics Approach Implicates the Microbiota-Gut-Brain Axis in the Pathogenesis of Huntington’s Disease. Neurobiol. Dis. 2021, 148, 105199. [Google Scholar] [CrossRef]
- Kong, G.; Cao, K.-A.L.; Judd, L.M.; Li, S.; Renoir, T.; Hannan, A.J. Microbiome Profiling Reveals Gut Dysbiosis in a Transgenic Mouse Model of Huntington’s Disease. Neurobiol. Dis. 2020, 135, 104268. [Google Scholar] [CrossRef]
- Radulescu, C.I.; Garcia-Miralles, M.; Sidik, H.; Bardile, C.F.; Yusof, N.A.B.; Lee, H.U.; Ho, E.X.P.; Chu, C.W.; Layton, E.; Low, D.; et al. Manipulation of Microbiota Reveals Altered Callosal Myelination and White Matter Plasticity in a Model of Huntington Disease. Neurobiol. Dis. 2019, 127, 65–75. [Google Scholar] [CrossRef]
- Wasser, C.I.; Mercieca, E.-C.; Kong, G.; Hannan, A.J.; McKeown, S.J.; Glikmann-Johnston, Y.; Stout, J.C. Gut Dysbiosis in Huntington’s Disease: Associations among Gut Microbiota, Cognitive Performance and Clinical Outcomes. Brain Commun. 2020, 2, fcaa110. [Google Scholar] [CrossRef]
- Ferrante, R.J.; Andreassen, O.A.; Jenkins, B.G.; Dedeoglu, A.; Kuemmerle, S.; Kubilus, J.K.; Kaddurah-Daouk, R.; Hersch, S.M.; Beal, M.F. Neuroprotective Effects of Creatine in a Transgenic Mouse Model of Huntington’s Disease. J. Neurosci. 2000, 20, 4389–4397. [Google Scholar] [CrossRef] [PubMed]
- van der Burg, J.M.M.; Winqvist, A.; Aziz, N.A.; Maat-Schieman, M.L.C.; Roos, R.A.C.; Bates, G.P.; Brundin, P.; Björkqvist, M.; Wierup, N. Gastrointestinal Dysfunction Contributes to Weight Loss in Huntington’s Disease Mice. Neurobiol. Dis. 2011, 44, 1–8. [Google Scholar] [CrossRef]
- McCourt, A.C.; O’Donovan, K.L.; Ekblad, E.; Sand, E.; Craufurd, D.; Rosser, A.; Sanders, D.; Stoy, N.; Rickards, H.; Wierup, N.; et al. Characterization of Gastric Mucosa Biopsies Reveals Alterations in Huntington’s Disease. PLoS Curr. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism Spectrum Disorder. Nat. Rev. Dis Primers 2020, 6, 5. [Google Scholar] [CrossRef]
- Mayer, E.A.; Padua, D.; Tillisch, K. Altered Brain-Gut Axis in Autism: Comorbidity or Causative Mechanisms? Bioessays 2014, 36, 933–939. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal Flora and Gastrointestinal Status in Children with Autism--Comparisons to Typical Children and Correlation with Autism Severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef]
- Kang, D.-W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; Labaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef]
- Kessler, R.C.; Chiu, W.T.; Demler, O.; Merikangas, K.R.; Walters, E.E. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Bahari-Javan, S.; Varbanov, H.; Halder, R.; Benito, E.; Kaurani, L.; Burkhardt, S.; Anderson-Schmidt, H.; Anghelescu, I.; Budde, M.; Stilling, R.M.; et al. HDAC1 Links Early Life Stress to Schizophrenia-like Phenotypes. Proc. Natl. Acad. Sci. USA 2017, 114, E4686–E4694. [Google Scholar] [CrossRef] [PubMed]
- Song, J.G.; Yu, M.-S.; Lee, B.; Lee, J.; Hwang, S.-H.; Na, D.; Kim, H.W. Analysis Methods for the Gut Microbiome in Neuropsychiatric and Neurodegenerative Disorders. Comput. Struct. Biotechnol. J. 2022, 20, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef]
- Aarts, E.; Ederveen, T.H.A.; Naaijen, J.; Zwiers, M.P.; Boekhorst, J.; Timmerman, H.M.; Smeekens, S.P.; Netea, M.G.; Buitelaar, J.K.; Franke, B.; et al. Gut Microbiome in ADHD and Its Relation to Neural Reward Anticipation. PLoS ONE 2017, 12, e0183509. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Zhou, Y.-Y.; Zhou, G.-L.; Li, Y.-C.; Yuan, J.; Li, X.-H.; Ruan, B. Gut Microbiota Profiles in Treatment-Naïve Children with Attention Deficit Hyperactivity Disorder. Behav. Brain Res. 2018, 347, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.H.B.; Goldstein, B.I. Inflammation in Children and Adolescents with Neuropsychiatric Disorders: A Systematic Review. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 274–296. [Google Scholar] [CrossRef]
- Martin, C.R.; Preedy, V.R.; Rajendram, R. Diagnosis, Management and Modeling of Neurodevelopmental Disorders: The Neuroscience of Development; Academic Press: New York, NY, USA, 2021; ISBN 9780128179895. [Google Scholar]
- Prehn-Kristensen, A.; Zimmermann, A.; Tittmann, L.; Lieb, W.; Schreiber, S.; Baving, L.; Fischer, A. Reduced Microbiome Alpha Diversity in Young Patients with ADHD. PLoS ONE 2018, 13, e0200728. [Google Scholar] [CrossRef]
- Tengeler, A.C.; Dam, S.A.; Wiesmann, M.; Naaijen, J.; van Bodegom, M.; Belzer, C.; Dederen, P.J.; Verweij, V.; Franke, B.; Kozicz, T.; et al. Gut Microbiota from Persons with Attention-Deficit/hyperactivity Disorder Affects the Brain in Mice. Microbiome 2020, 8, 44. [Google Scholar] [CrossRef]
- Kumperscak, H.G.; Gricar, A.; Ülen, I.; Micetic-Turk, D. A Pilot Randomized Control Trial with the Probiotic Strain Lactobacillus Rhamnosus GG (LGG) in ADHD: Children and Adolescents Report Better Health-Related Quality of Life. Front. Psychiatry 2020, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Li, P.; Zhong, C.; Li, S.; Sun, T.; Huang, H.; Chen, X.; Zhu, Y.; Hu, X.; Peng, X.; Zhang, X.; et al. Plasma Concentration of Trimethylamine-N-Oxide and Risk of Gestational Diabetes Mellitus. Am. J. Clin. Nutr. 2018, 108, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, F.; Ling, Z.; Yu, X.; Chen, W.; Li, H.; Jin, J.; Pang, M.; Zhang, H.; Yu, J.; et al. Clostridium Butyricum Attenuates Cerebral Ischemia/reperfusion Injury in Diabetic Mice via Modulation of Gut Microbiota. Brain Res. 2016, 1642, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Howarth, G.S.; Wang, H. Role of Endogenous Microbiota, Probiotics and Their Biological Products in Human Health. Nutrients 2013, 5, 58–81. [Google Scholar] [CrossRef]
- Ajslev, T.A.; Andersen, C.S.; Gamborg, M.; Sørensen, T.I.A.; Jess, T. Childhood Overweight after Establishment of the Gut Microbiota: The Role of Delivery Mode, Pre-Pregnancy Weight and Early Administration of Antibiotics. Int. J. Obes. 2011, 35, 522–529. [Google Scholar] [CrossRef]
- Davis, E.C.; Dinsmoor, A.M.; Wang, M.; Donovan, S.M. Microbiome Composition in Pediatric Populations from Birth to Adolescence: Impact of Diet and Prebiotic and Probiotic Interventions. Dig. Dis. Sci. 2020, 65, 706–722. [Google Scholar] [CrossRef]
- Kashtanova, D.A.; Popenko, A.S.; Tkacheva, O.N.; Tyakht, A.B.; Alexeev, D.G.; Boytsov, S.A. Association between the Gut Microbiota and Diet: Fetal Life, Early Childhood, and Further Life. Nutrition 2016, 32, 620–627. [Google Scholar] [CrossRef]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Casini, A. Mediterranean Diet and Minimizing Neurodegeneration. Curr. Nutr. Rep. 2013, 2, 75–80. [Google Scholar] [CrossRef]
- Féart, C.; Samieri, C.; Rondeau, V.; Amieva, H.; Portet, F.; Dartigues, J.-F.; Scarmeas, N.; Barberger-Gateau, P. Adherence to a Mediterranean Diet, Cognitive Decline, and Risk of Dementia. JAMA 2009, 302, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Morris, M.C.; Dhana, K.; Ventrelle, J.; Johnson, K.; Bishop, L.; Hollings, C.S.; Boulin, A.; Laranjo, N.; Stubbs, B.J.; et al. Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Study: Rationale, Design and Baseline Characteristics of a Randomized Control Trial of the MIND Diet on Cognitive Decline. Contemp. Clin. Trials 2021, 102, 106270. [Google Scholar] [CrossRef]
- Joseph, J.; Cole, G.; Head, E.; Ingram, D. Nutrition, Brain Aging, and Neurodegeneration. J. Neurosci. 2009, 29, 12795–12801. [Google Scholar] [CrossRef]
- van de Rest, O.; Berendsen, A.A.; Haveman-Nies, A.; de Groot, L.C. Dietary Patterns, Cognitive Decline, and Dementia: A Systematic Review. Adv. Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Mulak, A.; Bonaz, B. Brain-Gut-Microbiota Axis in Parkinson’s Disease. World J. Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Gut-Brain Axis in 2016: Brain-Gut-Microbiota Axis—Mood, Metabolism and Behaviour. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Matsuda, K.; Oishi, K. Probiotics: A Dietary Factor to Modulate the Gut Microbiome, Host Immune System, and Gut–Brain Interaction. Microorganisms 2020, 8, 1401. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Ravishankar, R.V.; Bai, J.A. Beneficial Microbes in Fermented and Functional Foods; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781482206623. [Google Scholar]
- Fuloria, S.; Mehta, J.; Talukdar, M.P.; Sekar, M.; Gan, S.H.; Subramaniyan, V.; Rani, N.N.I.M.; Begum, M.Y.; Chidambaram, K.; Nordin, R.; et al. Synbiotic Effects of Fermented Rice on Human Health and Wellness: A Natural Beverage That Boosts Immunity. Front. Microbiol. 2022, 13, 950913. [Google Scholar] [CrossRef]
- Gill, H.S.; Rutherfurd, K.J.; Cross, M.L.; Gopal, P.K. Enhancement of Immunity in the Elderly by Dietary Supplementation with the Probiotic Bifidobacterium Lactis HN019. Am. J. Clin. Nutr. 2001, 74, 833–839. [Google Scholar] [CrossRef]
- Vulevic, J.; Drakoularakou, A.; Yaqoob, P.; Tzortzis, G.; Gibson, G.R. Modulation of the Fecal Microflora Profile and Immune Function by a Novel Trans-Galactooligosaccharide Mixture (B-GOS) in Healthy Elderly Volunteers. Am. J. Clin. Nutr. 2008, 88, 1438–1446. [Google Scholar] [CrossRef]
- Floch, M.H.; Ringel, Y.; Allen Walker, W. The Microbiota in Gastrointestinal Pathophysiology: Implications for Human Health, Prebiotics, Probiotics, and Dysbiosis; Academic Press: New York, NY, USA, 2016; ISBN 9780128040621. [Google Scholar]
- Hojsak, I.; Kolaček, S.; Mihatsch, W.; Mosca, A.; Shamir, R.; Szajewska, H.; Vandenplas, Y. Working Group on Probiotics and Prebiotics of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition* Synbiotics in The Management of Pediatric Gastrointestinal Disorders: Position Paper of the Espghan Special Interest Group on Gut Microbiota and Modifications. J. Pediatr. Gastroenterol. Nutr. 2022. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Heidari-Soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and Selenium Co-Supplementation, and the Effects on Clinical, Metabolic and Genetic Status in Alzheimer’s Disease: A Randomized, Double-Blind, Controlled Trial. Clin. Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kinoshita, T.; Matsumoto, A.; Yoshino, K.; Saito, I.; Xiao, J.-Z. Bifidobacterium Breve A1 Supplementation Improved Cognitive Decline in Older Adults with Mild Cognitive Impairment: An Open-Label, Single-Arm Study. J. Prev. Alzheimer’s Dis. 2019, 6, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Pourjafar, H.; Tabrizi, A.; Homayouni, A. The Effects of Probiotics and Prebiotics on Mental Disorders: A Review on Depression, Anxiety, Alzheimer, and Autism Spectrum Disorders. Curr. Pharm. Biotechnol. 2020, 21, 555–565. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Administration of Bifidobacterium Breve Improves the Brain Function of Aβ1-42-Treated Mice via the Modulation of the Gut Microbiome. Nutrients 2021, 13, 1602. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- Wu, S.-I.; Wu, C.-C.; Cheng, L.-H.; Noble, S.W.; Liu, C.-J.; Lee, Y.-H.; Lin, C.-J.; Hsu, C.-C.; Chen, W.-L.; Tsai, P.-J.; et al. Psychobiotic Supplementation of HK-PS23 Improves Anxiety in Highly Stressed Clinical Nurses: A Double-Blind Randomized Placebo-Controlled Study. Food Funct. 2022, 13, 8907–8919. [Google Scholar] [CrossRef]
- Wu, S.-I.; Wu, C.-C.; Tsai, P.-J.; Cheng, L.-H.; Hsu, C.-C.; Shan, I.-K.; Chan, P.-Y.; Lin, T.-W.; Ko, C.-J.; Chen, W.-L.; et al. Psychobiotic Supplementation of PS128TM Improves Stress, Anxiety, and Insomnia in Highly Stressed Information Technology Specialists: A Pilot Study. Front. Nutr. 2021, 8, 614105. [Google Scholar] [CrossRef]
- Rode, J.; Edebol Carlman, H.M.T.; König, J.; Repsilber, D.; Hutchinson, A.N.; Thunberg, P.; Andersson, P.; Persson, J.; Kiselev, A.; Lathrop Stern, L.; et al. Probiotic Mixture Containing Lactobacillus Helveticus, Bifidobacterium Longum and Lactiplantibacillus Plantarum Affects Brain Responses Toward an Emotional Task in Healthy Subjects: A Randomized Clinical Trial. Front. Nutr. 2022, 9, 827182. [Google Scholar] [CrossRef]
- Sanborn, V.; Aljumaah, M.; Azcarate-Peril, M.A.; Gunstad, J. Examining the Cognitive Benefits of Probiotic Supplementation in Physically Active Older Adults: A Randomized Clinical Trial. Appl. Physiol. Nutr. Metab. 2022, 47, 871–882. [Google Scholar] [CrossRef]
- Sherman, H.T.; Liu, K.; Kwong, K.; Chan, S.-T.; Li, A.C.; Kong, X.-J. Carbon Monoxide (CO) Correlates with Symptom Severity, Autoimmunity, and Responses to Probiotics Treatment in a Cohort of Children with Autism Spectrum Disorder (ASD): A Post-Hoc Analysis of a Randomized Controlled Trial. BMC Psychiatry 2022, 22, 536. [Google Scholar] [CrossRef]
- Kong, X.-J.; Liu, J.; Li, J.; Kwong, K.; Koh, M.; Sukijthamapan, P.; Guo, J.J.; Sun, Z.J.; Song, Y. Probiotics and Oxytocin Nasal Spray as Neuro-Social-Behavioral Interventions for Patients with Autism Spectrum Disorders: A Pilot Randomized Controlled Trial Protocol. Pilot Feasibility Stud. 2020, 6, 20. [Google Scholar] [CrossRef]
- Vanuytsel, T.; van Wanrooy, S.; Vanheel, H.; Vanormelingen, C.; Verschueren, S.; Houben, E.; Salim Rasoel, S.; Tόth, J.; Holvoet, L.; Farré, R.; et al. Psychological Stress and Corticotropin-Releasing Hormone Increase Intestinal Permeability in Humans by a Mast Cell-Dependent Mechanism. Gut 2014, 63, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Van Oudenhove, L.; Accarie, A.; Geboers, K.; Geysen, H.; Toth, J.; Luypaerts, A.; Verbeke, K.; Smokvina, T.; Raes, J.; et al. Lactobacillus Rhamnosus CNCM I-3690 Decreases Subjective Academic Stress in Healthy Adults: A Randomized Placebo-Controlled Trial. Gut Microbes 2022, 14, 2031695. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Tojo, R.; Suárez, A.; Clemente, M.G.; de los Reyes-Gavilán, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal Microbiota in Health and Disease: Role of Bifidobacteria in Gut Homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef] [PubMed]
- Brenner, L.A.; Forster, J.E.; Stearns-Yoder, K.A.; Stamper, C.E.; Hoisington, A.J.; Brostow, D.P.; Mealer, M.; Wortzel, H.S.; Postolache, T.T.; Lowry, C.A. Evaluation of an Immunomodulatory Probiotic Intervention for Veterans with Co-Occurring Mild Traumatic Brain Injury and Posttraumatic Stress Disorder: A Pilot Study. Front. Neurol. 2020, 11, 1015. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef]
- El Aidy, S.; Dinan, T.G.; Cryan, J.F. Immune Modulation of the Brain-Gut-Microbe Axis. Front. Microbiol. 2014, 5, 146. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.-A. Gut-Brain Axis: How the Microbiome Influences Anxiety and Depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Wang, F.; Yu, X.; Ling, Z.; Li, H.; Zhang, H.; Jin, J.; Chen, W.; Pang, M.; et al. Neuroprotective Effects of Clostridium Butyricum against Vascular Dementia in Mice via Metabolic Butyrate. Biomed. Res. Int. 2015, 2015, 412946. [Google Scholar] [CrossRef]
- Liu, W.-H.; Chuang, H.-L.; Huang, Y.-T.; Wu, C.-C.; Chou, G.-T.; Wang, S.; Tsai, Y.-C. Alteration of Behavior and Monoamine Levels Attributable to Lactobacillus Plantarum PS128 in Germ-Free Mice. Behav. Brain Res. 2016, 298, 202–209. [Google Scholar] [CrossRef]
- Möhle, L.; Mattei, D.; Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Alutis, M.; French, T.; Hambardzumyan, D.; Matzinger, P.; Dunay, I.R.; et al. Ly6C(hi) Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Rep. 2016, 15, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.J.K.; Milev, R. The Effects of Probiotics on Depressive Symptoms in Humans: A Systematic Review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef]
- Wang, H.; Lee, I.-S.; Braun, C.; Enck, P. Effect of Probiotics on Central Nervous System Functions in Animals and Humans: A Systematic Review. J. Neurogastroenterol. Motil. 2016, 22, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorff, F.; Akkermans, L.M.A.; Söderholm, J.D. The Role of Microbiota and Probiotics in Stress-Induced Gastro-Intestinal Damage. Curr. Mol. Med. 2008, 8, 282–298. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic Gut Effect Prevents the Chronic Psychological Stress-Induced Brain Activity Abnormality in Mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Schaub, A.-C.; Schneider, E.; Vazquez-Castellanos, J.F.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Clinical, Gut Microbial and Neural Effects of a Probiotic Add-on Therapy in Depressed Patients: A Randomized Controlled Trial. Transl. Psychiatry 2022, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.; Adamos, M.; Katsafanas, E.; Khushalani, S.; Origoni, A.; Savage, C.; Schweinfurth, L.; Stallings, C.; Sweeney, K.; Goga, J.; et al. Adjunctive Probiotic Microorganisms to Prevent Rehospitalization in Patients with Acute Mania: A Randomized Controlled Trial. Bipolar Disord. 2018, 20, 614–621. [Google Scholar] [CrossRef]
- Arteaga-Henríquez, G.; Rosales-Ortiz, S.K.; Arias-Vásquez, A.; Bitter, I.; Ginsberg, Y.; Ibañez-Jimenez, P.; Kilencz, T.; Lavebratt, C.; Matura, S.; Reif, A.; et al. Treating Impulsivity with Probiotics in Adults (PROBIA): Study Protocol of a Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. Trials 2020, 21, 161. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible Association of Bifidobacterium and Lactobacillus in the Gut Microbiota of Patients with Major Depressive Disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of Probiotic and Prebiotic vs Placebo on Psychological Outcomes in Patients with Major Depressive Disorder: A Randomized Clinical Trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and Metabolic Response to Probiotic Administration in Patients with Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Dickerson, F.B.; Stallings, C.; Origoni, A.; Katsafanas, E.; Savage, C.L.G.; Schweinfurth, L.A.B.; Goga, J.; Khushalani, S.; Yolken, R.H. Effect of Probiotic Supplementation on Schizophrenia Symptoms and Association with Gastrointestinal Functioning: A Randomized, Placebo-Controlled Trial. Prim. Care Companion CNS Disord. 2014, 16, 26294. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A Randomized Controlled Trial to Test the Effect of Multispecies Probiotics on Cognitive Reactivity to Sad Mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Marteau, T.M.; Bekker, H. The Development of a Six-Item Short-Form of the State Scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br. J. Clin. Psychol. 1992, 31, 301–306. [Google Scholar] [CrossRef]
- Wang, H.; Braun, C.; Enck, P. Effects of Rifaximin on Central Responses to Social Stress-a Pilot Experiment. Neurotherapeutics 2018, 15, 807–818. [Google Scholar] [CrossRef]
- Cannavale, C.N.; Mysonhimer, A.R.; Bailey, M.A.; Cohen, N.J.; Holscher, H.D.; Khan, N.A. Consumption of a Fermented Dairy Beverage Improves Hippocampal-Dependent Relational Memory in a Randomized, Controlled Cross-over Trial. Nutr. Neurosci. 2022, 1–10. [Google Scholar] [CrossRef]
- Tan, A.H.; Lim, S.-Y.; Chong, K.K.; Manap, A.M.A.A.; Hor, J.W.; Lim, J.L.; Low, S.C.; Chong, C.W.; Mahadeva, S.; Lang, A.E. Probiotics for Constipation in Parkinson Disease: A Randomized Placebo-Controlled Study. Neurology 2021, 96, e772–e782. [Google Scholar] [CrossRef]
- Fulceri, F.; Morelli, M.; Santocchi, E.; Cena, H.; Del Bianco, T.; Narzisi, A.; Calderoni, S.; Muratori, F. Gastrointestinal Symptoms and Behavioral Problems in Preschoolers with Autism Spectrum Disorder. Dig. Liver Dis. 2016, 48, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Santocchi, E.; Guiducci, L.; Fulceri, F.; Billeci, L.; Buzzigoli, E.; Apicella, F.; Calderoni, S.; Grossi, E.; Morales, M.A.; Muratori, F. Gut to Brain Interaction in Autism Spectrum Disorders: A Randomized Controlled Trial on the Role of Probiotics on Clinical, Biochemical and Neurophysiological Parameters. BMC Psychiatry 2016, 16, 183. [Google Scholar] [CrossRef] [PubMed]
- Santocchi, E.; Guiducci, L.; Prosperi, M.; Calderoni, S.; Gaggini, M.; Apicella, F.; Tancredi, R.; Billeci, L.; Mastromarino, P.; Grossi, E.; et al. Effects of Probiotic Supplementation on Gastrointestinal, Sensory and Core Symptoms in Autism Spectrum Disorders: A Randomized Controlled Trial. Front. Psychiatry 2020, 11, 550593. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Griffin, S.M.; Ibarra, A.; Ellsiepen, E.; Hellhammer, J. Lacticaseibacillus Paracasei Lpc-37® Improves Psychological and Physiological Markers of Stress and Anxiety in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled and Parallel Clinical Trial (the Sisu Study). Neurobiol. Stress 2020, 13, 100277. [Google Scholar] [CrossRef]
- Saccarello, A.; Montarsolo, P.; Massardo, I.; Picciotto, R.; Pedemonte, A.; Castagnaro, R.; Brasesco, P.C.; Guida, V.; Picco, P.; Fioravanti, P.; et al. Oral Administration of S-Adenosylmethionine (SAMe) and Lactobacillus Plantarum HEAL9 Improves the Mild-To-Moderate Symptoms of Depression: A Randomized, Double-Blind, Placebo-Controlled Study. Prim. Care Companion CNS Disord. 2020, 22, 23164. [Google Scholar] [CrossRef]
- Boulos, C.; Yaghi, N.; El Hayeck, R.; Heraoui, G.N.; Fakhoury-Sayegh, N. Nutritional Risk Factors, Microbiota and Parkinson’s Disease: What Is the Current Evidence? Nutrients 2019, 11, 1896. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.M.; Hernán, M.A.; Willett, W.C.; Ascherio, A. Dietary Intakes of Fat and Risk of Parkinson’s Disease. Am. J. Epidemiol. 2003, 157, 1007–1014. [Google Scholar] [CrossRef]
- Tierney, M.C.; Curtis, A.F.; Chertkow, H.; Rylett, R.J. Integrating Sex and Gender into Neurodegeneration Research: A Six-Component Strategy. Alzheimers. Dement. 2017, 3, 660–667. [Google Scholar] [CrossRef]
- Cox, L.M.; Abou-El-Hassan, H.; Maghzi, A.H.; Vincentini, J.; Weiner, H.L. The Sex-Specific Interaction of the Microbiome in Neurodegenerative Diseases. Brain Res. 2019, 1724, 146385. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Christian, L.M.; Galley, J.D.; Hade, E.M.; Schoppe-Sullivan, S.; Kamp Dush, C.; Bailey, M.T. Gut Microbiome Composition Is Associated with Temperament during Early Childhood. Brain Behav. Immun. 2015, 45, 118–127. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).