Melatonin Prevents Chondrocyte Matrix Degradation in Rats with Experimentally Induced Osteoarthritis by Inhibiting Nuclear Factor-κB via SIRT1
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Animal Ethics
2.3. Surgical Model of Anterior Cruciate Ligament Transection (ACLT)
2.4. Histological and Immunohistochemical (IHC) Analyses
2.5. Enzyme-Linked Immunosorbent Assay (ELISA) of Serum
2.6. Isolation and Culture of Chondrocytes
2.7. Cell Proliferation Assay
2.8. Western Blot (WB)
2.9. Real-Time PCR (qRT-PCR)
2.10. Immunofluorescence Staining (IF)
2.11. Statistical Analysis
3. Results
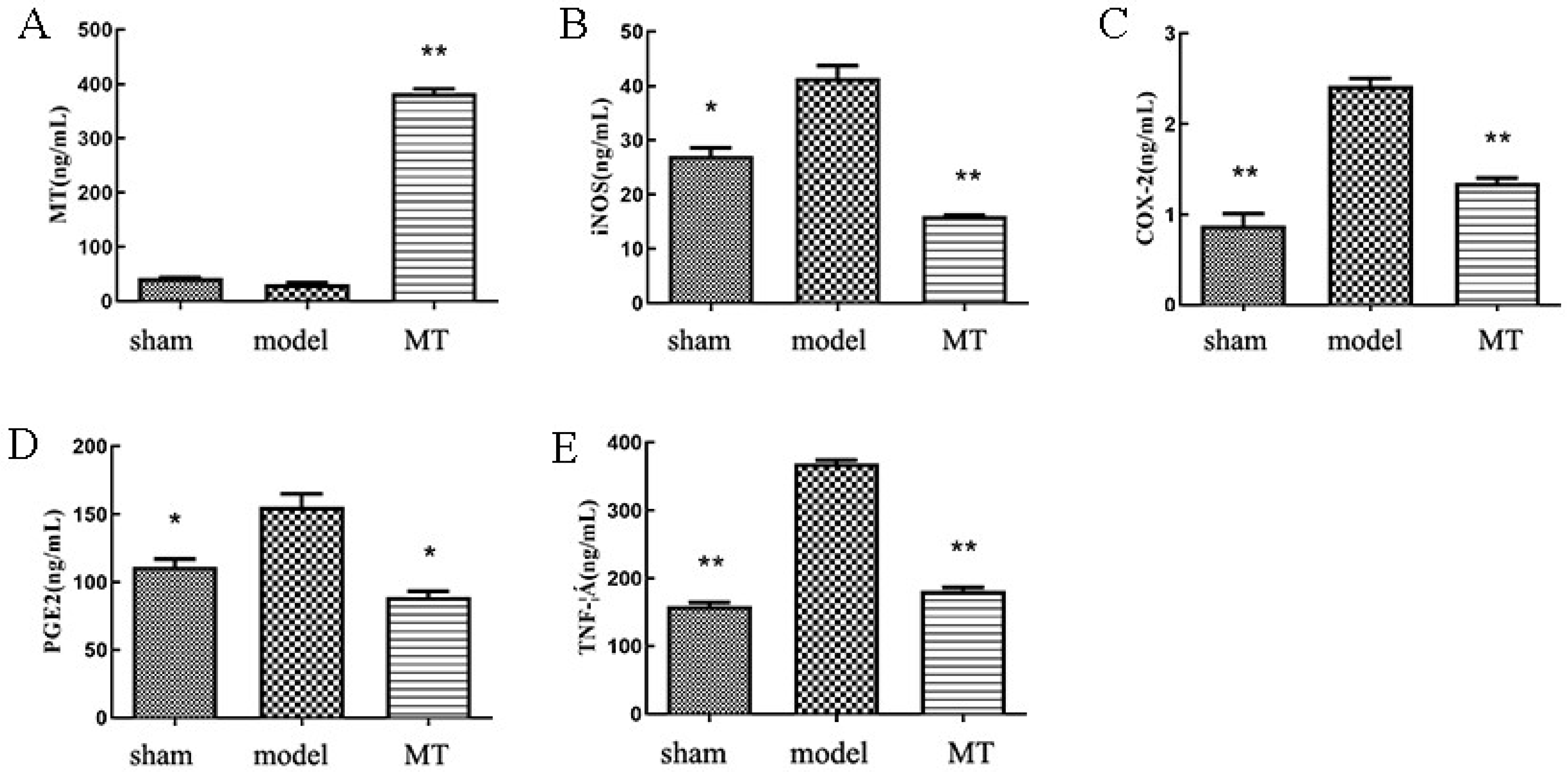
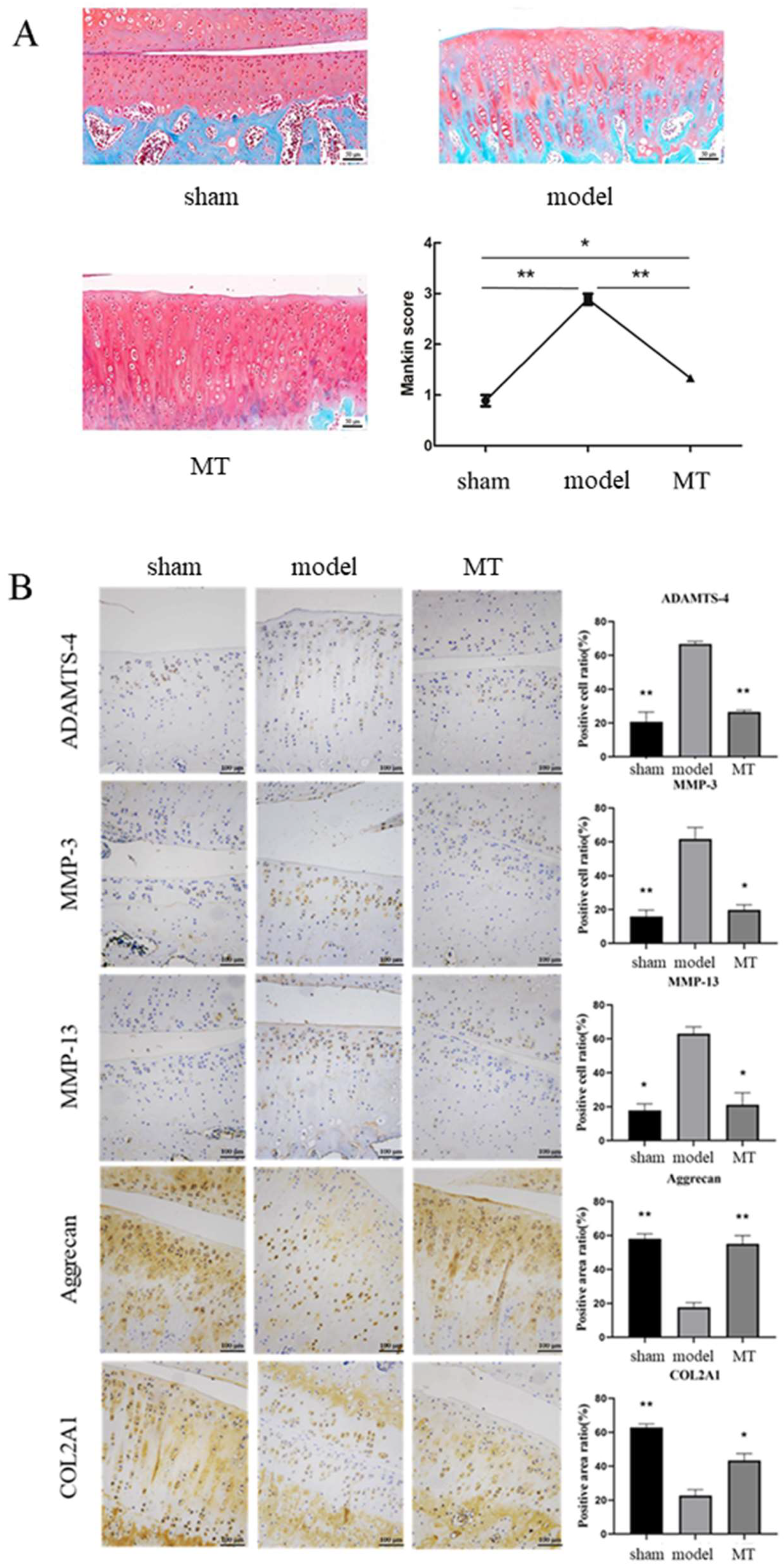
3.1. MT Attenuates Inflammation and Chondrocyte ECM Degradation in OA Rats
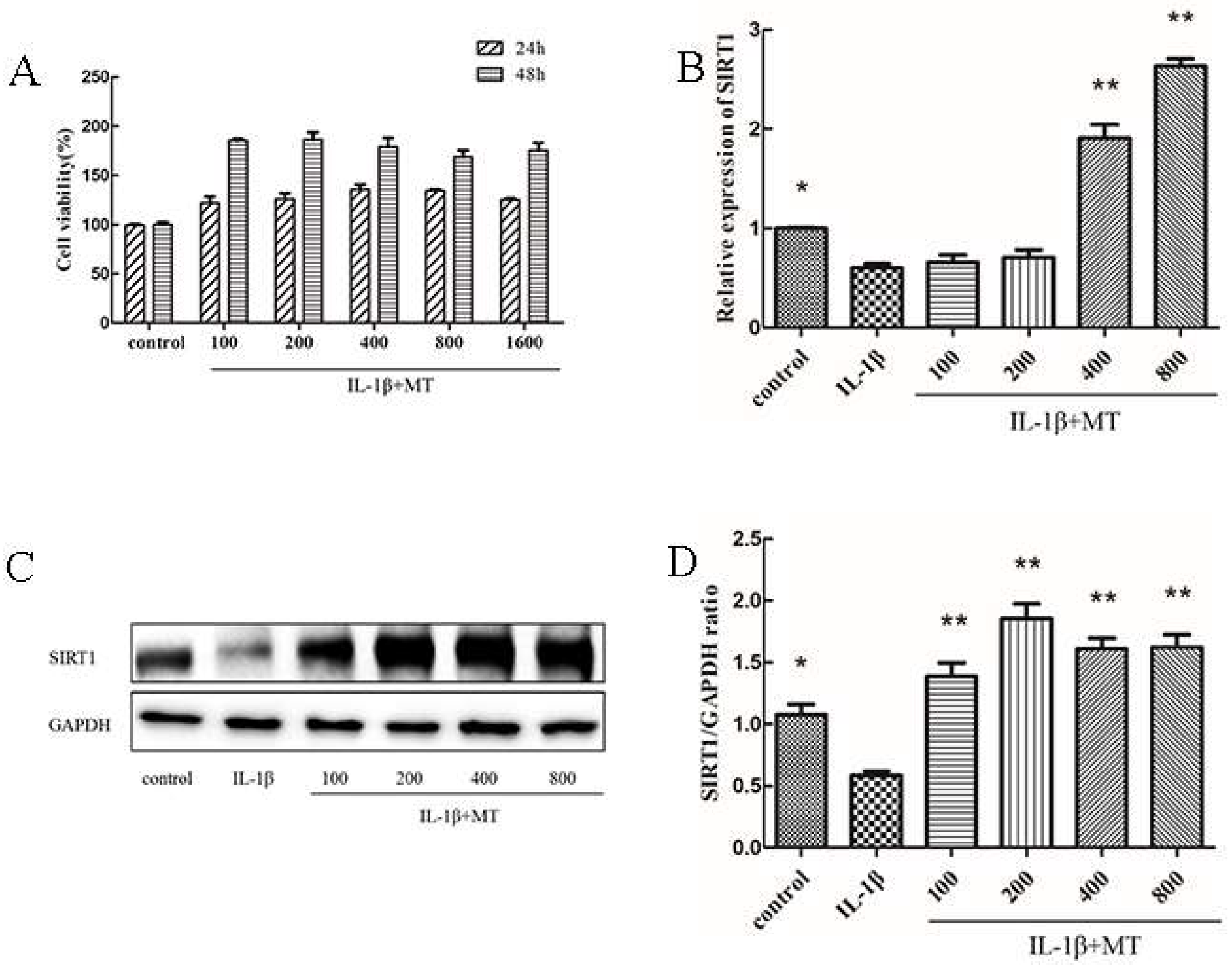
3.2. Co-Culture of MT with IL-1β Promotes Chondrocyte Proliferation, and MT Promotes SIRT1 Expression in IL-1β-Induced Chondrocytes
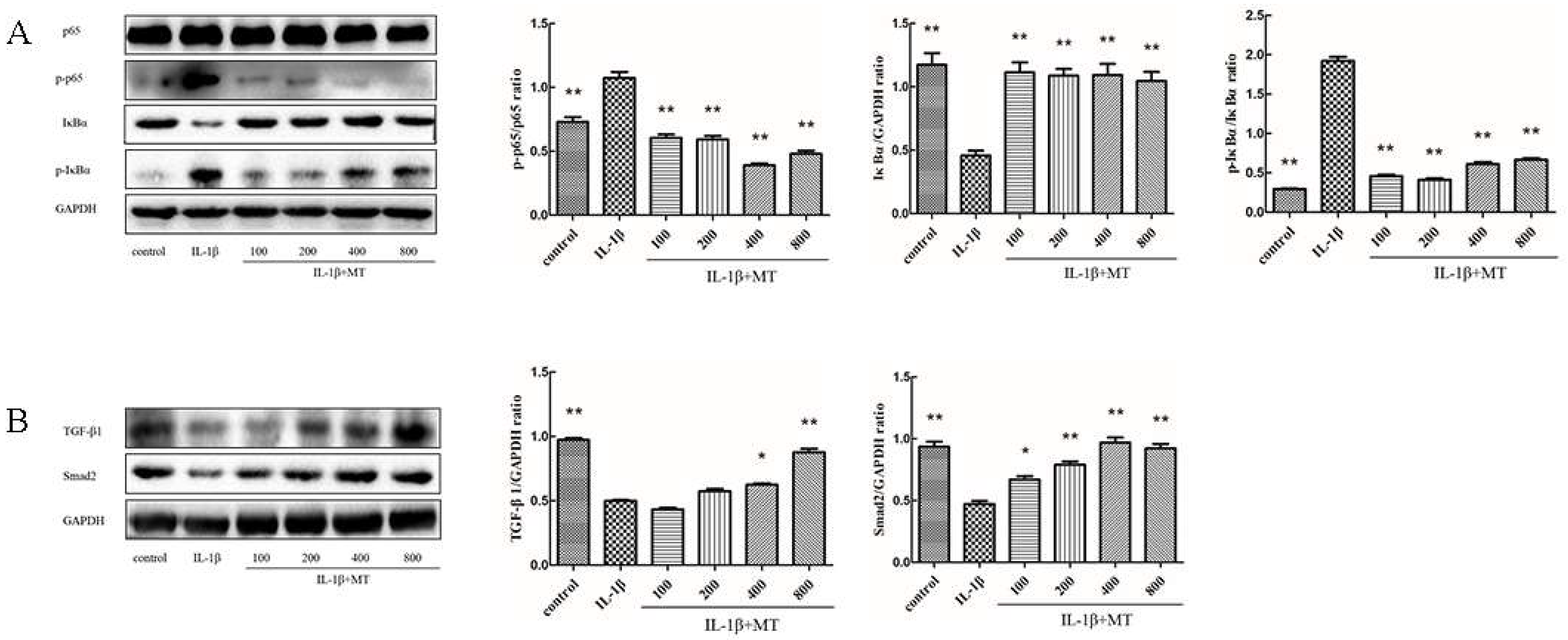
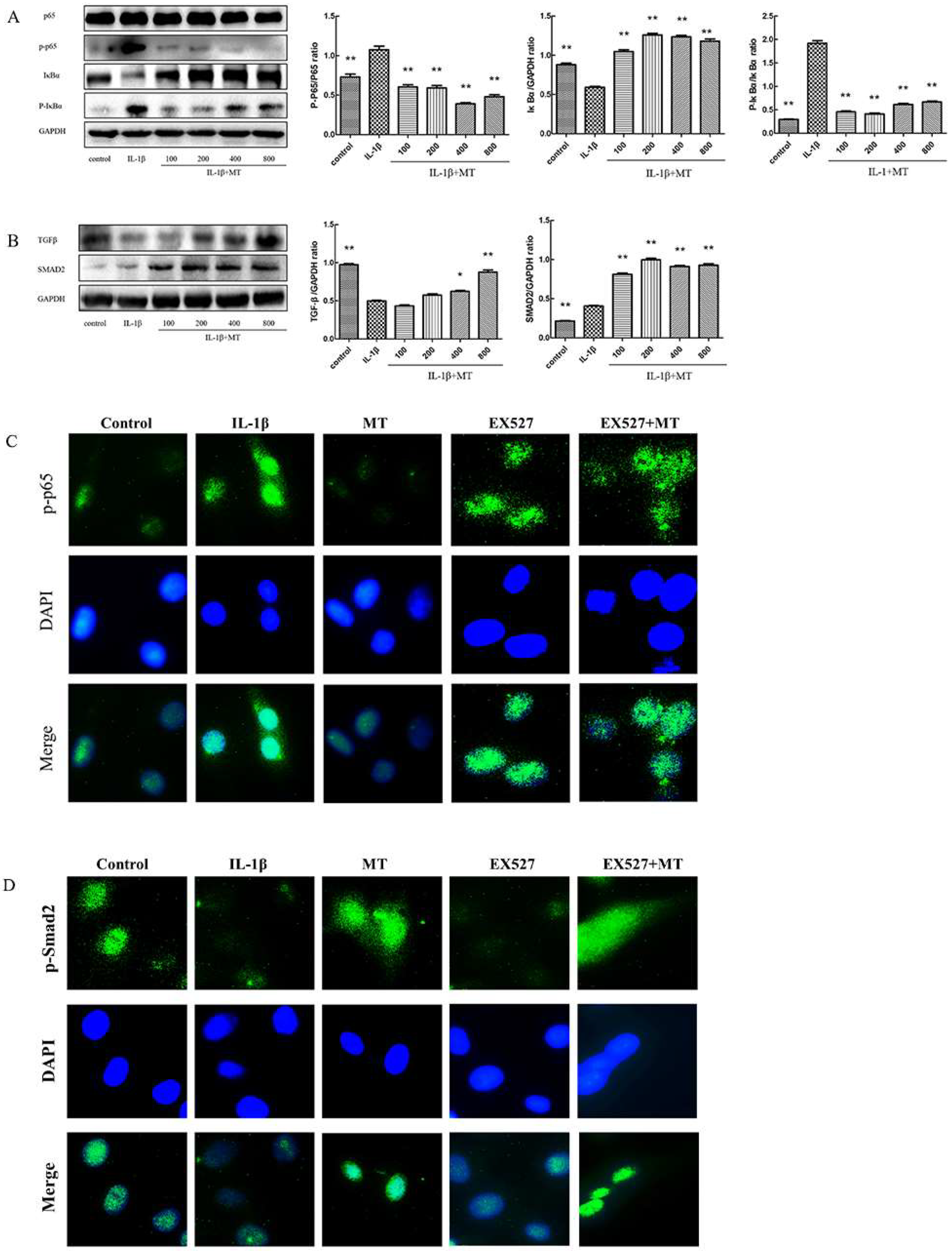
3.3. MT Inhibits NF-κB Signaling and Activates TGF-β1/Smad2 Pathway in IL-1β-Induced Chondrocytes
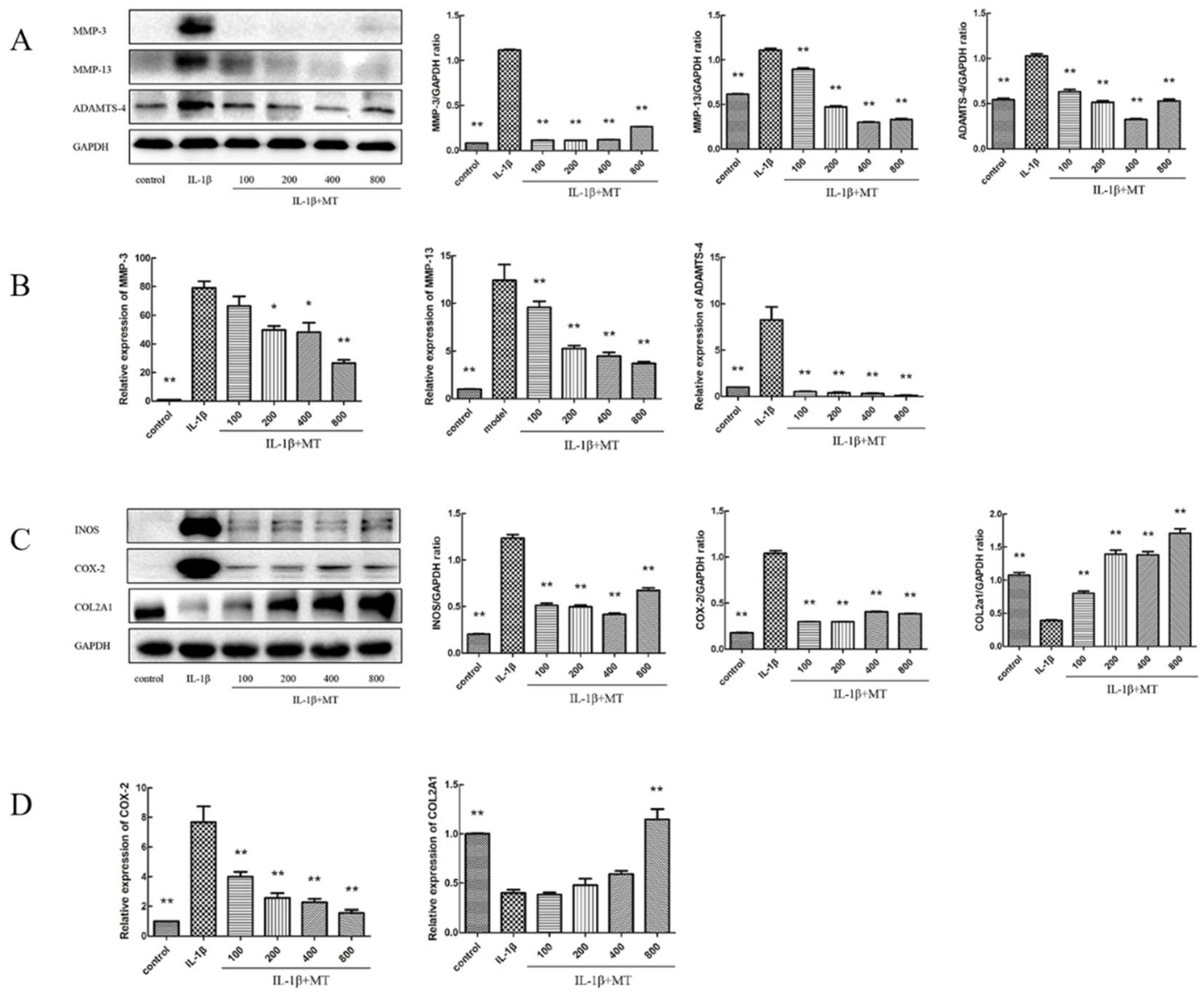
3.4. MT Inhibits the Expression of Matrix Degradation-Related Indicators in IL-1β-Induced Chondrocytes
3.5. EX527 Abrogates MT Activation of SIRT1 in IL-1β-Induced Chondrocytes
3.6. The Inhibitor EX527 Reverses the Inhibitory Effect of MT on the NF-κB Pathway but Does Not Affect the Activation of the TGF-β1/Smad2 Pathway
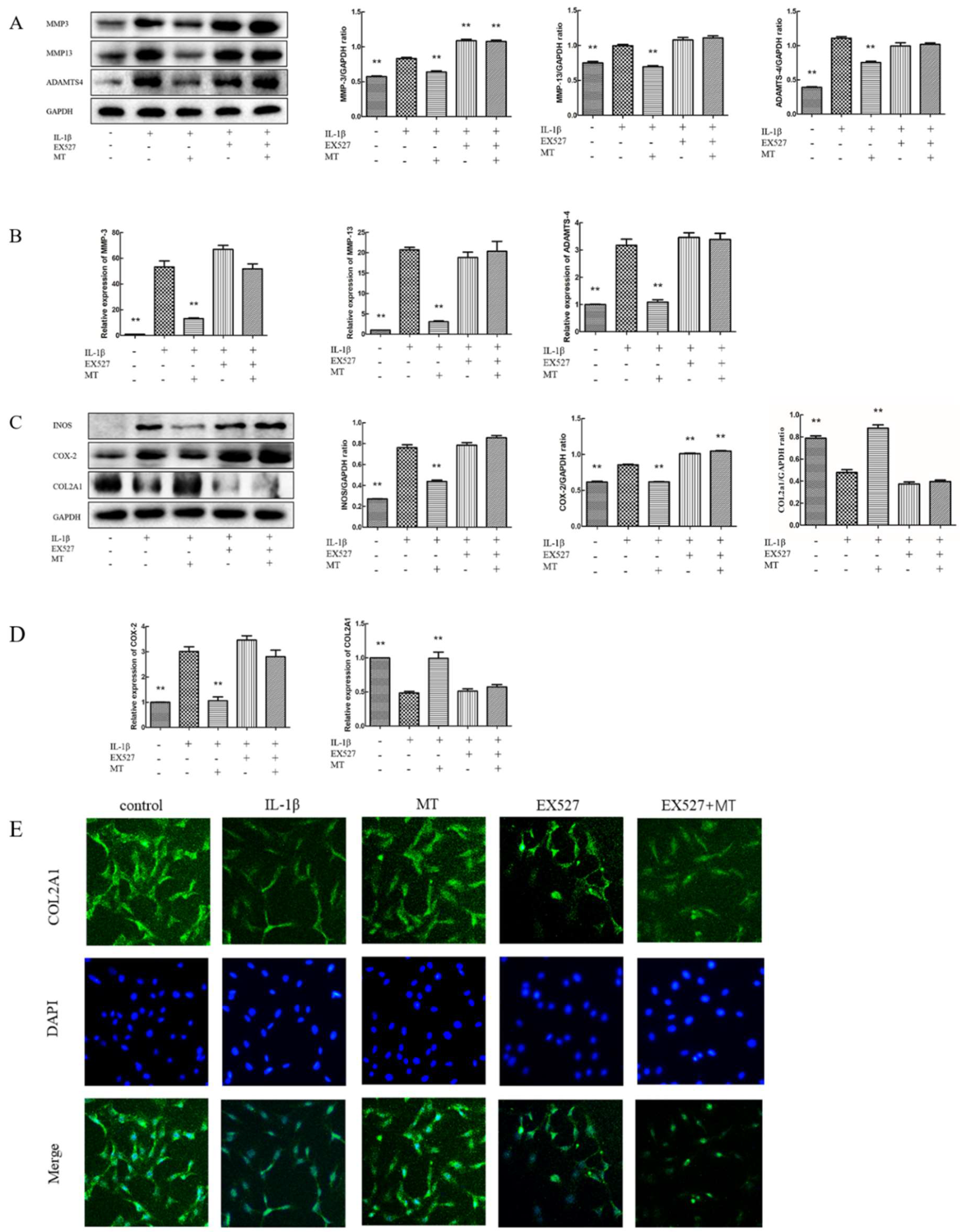
3.7. EX527 Reverses the Inhibitory Effect of MT on IL-1β-Induced Chondrocyte Matrix Degradation-Related Markers
4. Discussion
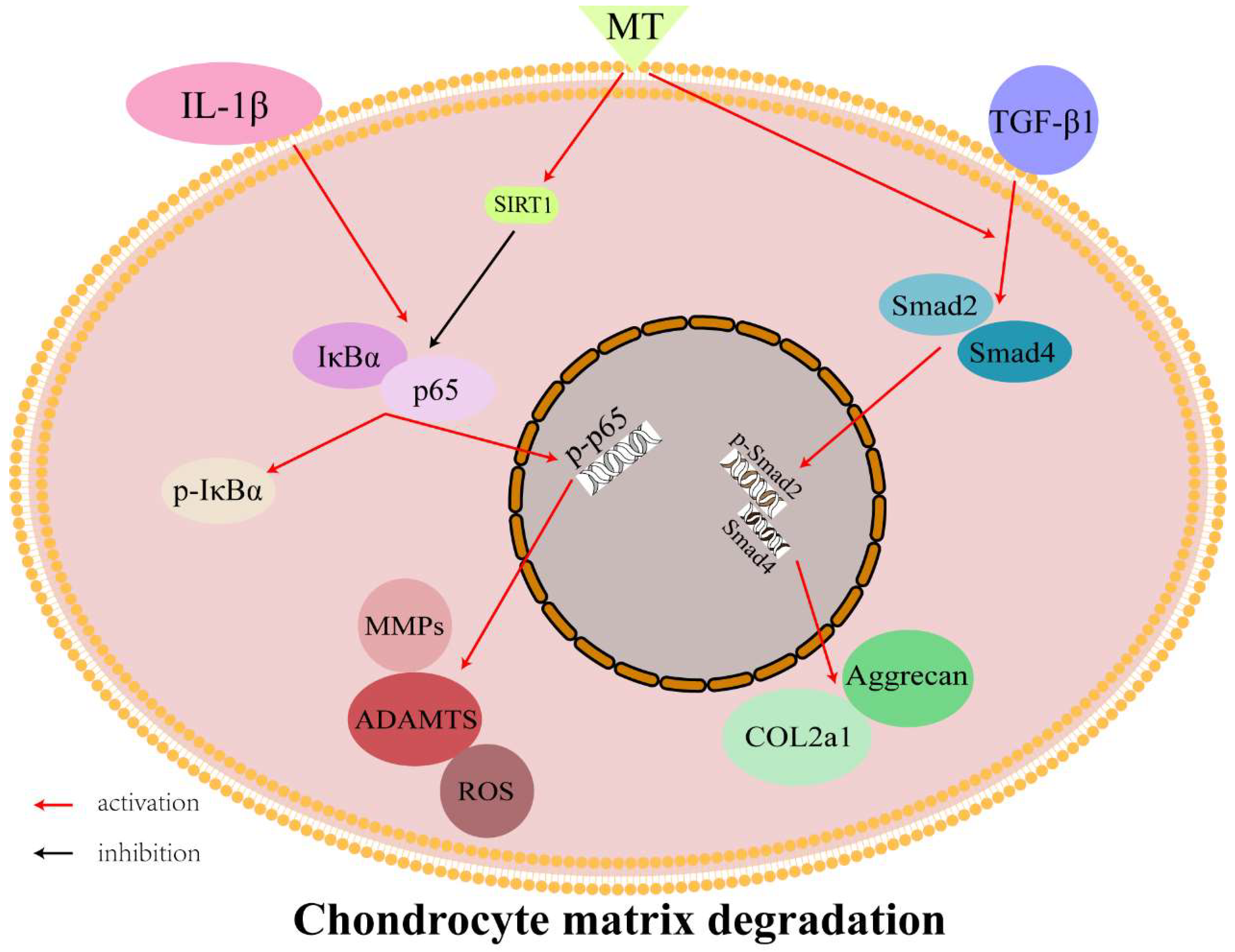
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y.; et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann. Intern. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Barbour, K.E.; Helmick, C.G.; Boring, M.; Brady, T.J. Vital Signs: Prevalence of Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation—United States, 2013–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef]
- Pereira, D.; Ramos, E.; Branco, J. Osteoarthritis. Acta Med. Port. 2015, 28, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Functional aspects of the pineal hormone melatonin in combating cell and tissue damage induced by free radicals. Eur. J. Endocrinol. 1996, 134, 412–420. [Google Scholar] [CrossRef]
- Mahabady, M.K.; Varzi, H.N.; Ranjbar, R.; Mehrzadi, S. Melatonin and Vitamin E Protects Against Sodium Arsenite-Induced Skeletal Malformations in Rats. Am. Eurasian J. Toxicol. Sci. 2011, 3, 184–189. [Google Scholar]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Kawamoto, M.; Sato, S.; Tamura, I.; Maekawa, R.; Taketani, T.; Aasada, H.; Takaki, E.; Nakai, A.; Reiter, R.J.; et al. Long-term melatonin treatment delays ovarian aging. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; He, F.; Wei, L.; Rawson, A. Melatonin enhances cartilage matrix synthesis by porcine articular chondrocytes. J. Pineal Res. 2009, 46, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Lin, J.; Zhou, X.F.; Chen, X.; Chen, A.C.; Pi, B.; Pan, G.Q.; Pei, M.; Yang, H.L.; Liu, T.; et al. Melatonin Prevents Osteoarthritis-Induced Cartilage Degradation via Targeting MicroRNA-140. Oxidat. Med. Cell. Longev. 2019, 2019, 16. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, C.; Liu, P.; Huang, H.; Zhang, S.; Wang, X. Anti-Apoptosis and Autophagy Effects of Melatonin Protect Rat Chondrocytes against Oxidative Stress via Regulation of AMPK/Foxo3 Pathways. Cartilage 2021, 13, 1041S–1053S. [Google Scholar] [CrossRef]
- Paulino Silva, K.M.; De Sousa, F.L.; Alves, A.C.B.; Rocha, P.A.; Da Costa, H.; Ferreira, W.R.; Reis, T.S.; De Oliveira, T.K.B.; Cabral Batista, S.R.; Lapa Neto, C.J.C.; et al. Chondroprotective effect of melatonin and strontium ranelate in animal model of osteoarthritis. Heliyon 2021, 7, e06760. [Google Scholar] [CrossRef]
- Shah, S.A.; Khan, M.; Jo, M.H.; Jo, M.G.; Amin, F.U.; Kim, M.O. Melatonin Stimulates the SIRT1/Nrf2 Signaling Pathway Counteracting Lipopolysaccharide (LPS)-Induced Oxidative Stress to Rescue Postnatal Rat Brain. CNS Neurosci. Ther. 2017, 23, 33–44. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Lim, H.D.; Kim, Y.S.; Ko, S.H.; Yoon, I.J.; Cho, S.G.; Chun, Y.H.; Choi, B.J.; Kim, E.C. Cytoprotective and anti-inflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J. Pineal Res. 2012, 53, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Li, F.; Wen, Y.B.; Cui, H.X.; Guo, M.L.; Zhang, L.; Zhang, Y.F.; Guo, Y.J.; Guo, Y.X. Melatonin inhibits Sirt1-dependent NAMPT and NFAT5 signaling in chondrocytes to attenuate osteoarthritis. Oncotarget 2017, 8, 55967–55983. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, K.A.; Papavassiliou, A.G. Redox and NF-κB signaling in osteoarthritis. Free Radic. Biol. Med. 2019, 132, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, X.; Liu, M.; Fan, H.; Zheng, H.; Zhang, S.; Rahman, N.; Wołczyński, S.; Kretowski, A.; Li, X. Melatonin inhibits inflammasome-associated activation of endothelium and macrophages attenuating pulmonary arterial hypertension. Cardiovasc. Res. 2020, 116, 2156–2169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gan, L.; Xu, Y.; Luo, D.; Ren, Q.; Wu, S.; Sun, C. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J. Pineal Res. 2017, 63, e12414. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chiou, C.H.; Liu, S.C.; Hu, S.L.; Su, C.M.; Tsai, C.H.; Tang, C.H. Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. J. Pineal Res. 2019, 66, e12560. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Chen, S.; Tan, Z.; Xiong, K.; Li, Y.; Ye, Y.; Luo, Z.P.; He, F.; Gong, Y. Rescue of proinflammatory cytokine-inhibited chondrogenesis by the antiarthritic effect of melatonin in synovium mesenchymal stem cells via suppression of reactive oxygen species and matrix metalloproteinases. Free Radic. Biol. Med. 2014, 68, 234–246. [Google Scholar] [CrossRef]
- Assoian, R.K.; Komoriya, A.; Meyers, C.A.; Miller, D.M.; Sporn, M.B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem. 1983, 258, 7155–7160. [Google Scholar] [CrossRef]
- Shi, Y.; Massagué, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Wu, M.; Zhang, S. Transforming growth factor beta signaling and cartilage initiation, development, and maintenance. Chin. J. Bone Jt. Surg. 2018, 11, 556–560. [Google Scholar]
- Tian, F.; Cui, X.; Zhang, S.; Shi, Y.; Jin, Q. Effects of transforming growth factor β1 and interleukin-1β on endplate chondrocytes. Chongqing Med. 2012, 41, 4. [Google Scholar]
- Huang, C.; Liu, J.; Hu, Y. Effects of melatonin on the expression of TGF-β1 and IL-1β in articular cartilage of osteoarthritis rats. Chin. J. Repair Reconstr. Surg. 2010, 24, 1082–1087. [Google Scholar]
- Teeple, E.; Jay, G.D.; Elsaid, K.A.; Fleming, B.C. Animal models of osteoarthritis: Challenges of model selection and analysis. AAPS J. 2013, 15, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluijs, J.A.; Geesink, R.G.; van der Linden, A.J.; Bulstra, S.K.; Kuyer, R.; Drukker, J. The reliability of the Mankin score for osteoarthritis. J. Orthop. Res. 1992, 10, 58–61. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Qiu, J.; Huang, X. 8-Methoxypsoralen has Anti-inflammatory and Antioxidant Roles in Osteoarthritis Through SIRT1/NF-κB Pathway. Front. Pharmacol. 2021, 12, 692424. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiol. (Bethesda) 2014, 29, 325–333. [Google Scholar] [CrossRef]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Sumsuzzman, D.M.; Choi, J.; Khan, Z.A.; Kamenos, G.; Hong, Y. Melatonin Maintains Anabolic-Catabolic Equilibrium and Regulates Circadian Rhythm During Osteoarthritis Development in Animal Models: A Systematic Review and Meta-analysis. Front. Pharmacol. 2021, 12, 714974. [Google Scholar] [CrossRef]
- Kouri, V.P.; Olkkonen, J.; Kaivosoja, E.; Ainola, M.; Juhila, J.; Hovatta, I.; Konttinen, Y.T.; Mandelin, J. Circadian timekeeping is disturbed in rheumatoid arthritis at molecular level. PLoS ONE 2013, 8, e54049. [Google Scholar] [CrossRef]
- Satyanarayanan, S.K.; Su, H.; Lin, Y.W.; Su, K.P. Circadian Rhythm and Melatonin in the Treatment of Depression. Curr. Pharm. Des. 2018, 24, 2549–2555. [Google Scholar] [CrossRef]
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. BioMed Res. Int. 2013, 2013, 284873. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, C.; Jouzeau, J.Y.; Gegout-Pottie, P.; Presle, N.; Bordji, K.; Netter, P.; Terlain, B. Modulation of IL-1-induced cartilage injury by NO synthase inhibitors: A comparative study with rat chondrocytes and cartilage entities. Br. J. Pharmacol. 1998, 124, 1719–1727. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clynes, M.A.; Jameson, K.A.; Edwards, M.H.; Cooper, C.; Dennison, E.M. Impact of osteoarthritis on activities of daily living: Does joint site matter? Aging Clin. Exp. Res. 2019, 31, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liu, M.; Zhao, W.; Peng, H.T. Study on the pain-inducing effect of NF-α and IL-1β in lumbar disc herniation. J. Neck Low Back Pain 2010, 31, 174–176. [Google Scholar]
- Liang, Y. Relationship between TNF-α and Apoptosis of Nucleus Pulposus in Lumbar Disc Herniation; Huazhong University of Science and Technology: Wuhan, China, 2006. [Google Scholar]
- León, J.; Escames, G.; Rodríguez, M.I.; López, L.C.; Tapias, V.; Entrena, A.; Camacho, E.; Carrión, M.D.; Gallo, M.A.; EspINOSa, A.; et al. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem. 2006, 98, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Tan, D.X.; Hardeland, R.; Leon, J.; Rodriguez, C.; Reiter, R.J. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 2005, 165, 139–149. [Google Scholar] [CrossRef]
- Lv, K.; Lei, W.; Ma, J.; Liu, G.; Sun, D.; Jiang, M. Effects of arthroscopic debridement combined with intra-articular injection of triamcinolone acetonide and sodium hyaluronate on serum bone metabolism-related indexes in knee osteoarthritis. J. Guangxi Med. Univ. 2018, 35, 1392–1396. [Google Scholar] [CrossRef]
- Rodriguez, M.I.; Escames, G.; Lopez, L.C.; Lopez, A.; Garcia, J.A.; Ortiz, F.; Acuna-Castroviejo, D. Chronic melatonin treatment reduces the age-dependent inflammatory process in senescence-accelerated mice. J. Pineal Res. 2007, 42, 272–279. [Google Scholar] [CrossRef]
- Zhang, Y.; He, F.; Chen, Z.; Su, Q.; Yan, M.; Zhang, Q.; Tan, J.; Qian, L.; Han, Y. Melatonin modulates IL-1β-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging 2019, 11, 10499–10512. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Hou, M.; Liu, H.; Yang, H.; Chen, X.; Liu, T.; He, F.; Zhu, X. Melatonin Prevents Cartilage Degradation in Early-Stage Osteoarthritis Through Activation of miR-146a/NRF2/HO-1 Axis. J. Bone Miner. Res. 2022, 37, 1056–1072. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kim, H.; Lee, Y.; Lee, S.; Kim, K.; Jin, Y.; Lee, S.R.; Chang, K.T.; Hong, Y. Salutary effects of melatonin combined with treadmill exercise on cartilage damage. J. Pineal Res. 2014, 57, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Sharan, K.; Lewis, K.; Furukawa, T.; Yadav, V.K. Regulation of bone mass through pineal-derived melatonin-MT2 receptor pathway. J. Pineal Res. 2017, 63, e12423. [Google Scholar] [CrossRef]
- Bang, J.; Chang, H.W.; Jung, H.R.; Cho, C.H.; Hur, J.A.; Lee, S.I.; Choi, T.H.; Kim, S.H.; Ha, E. Melatonin attenuates clock gene cryptochrome1, which may aggravate mouse anti-type II collagen antibody-induced arthritis. Rheumatol. Int. 2012, 32, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Kayani, B.; Konan, S.; Ahmed, S.S.; Chang, J.S.; Ayuob, A.; Haddad, F.S. The effect of anterior cruciate ligament resection on knee biomechanics. Bone Jt. J. 2020, 102, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. The Mechanism of Melatonin Delaying the Pathogenesis of Osteoarthritis through miR-140-5p; Suzhou University: Suzhou, China, 2020. [Google Scholar]
- Abramson, S.B. Osteoarthritis and nitric oxide. Osteoarthr. Cartil. 2008, 16 (Suppl. 2), S15-20. [Google Scholar] [CrossRef]
- Xiao, C.; Li, R.; Zeng, L.; Mu, F. Effects of Fuyuan Capsule on iNOS and serum PGE_2 and NO in cartilage of knee osteoarthritis in rats. Chin. J. Gerontol. 2011, 31, 1580–1582. [Google Scholar]
- Mabey, T.; Honsawek, S.; Tanavalee, A.; Yuktanandana, P.; Wilairatana, V.; Poovorawan, Y. Plasma and synovial fluid inflammatory cytokine profiles in primary knee osteoarthritis. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2016, 21, 639–644. [Google Scholar] [CrossRef]
- Feng, K.; Chen, Z.; Pengcheng, L.; Zhang, S.; Wang, X. Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell. Physiol. 2019, 234, 18192–18205. [Google Scholar] [CrossRef]
- Fujita, N.; Matsushita, T.; Ishida, K.; Kubo, S.; Matsumoto, T.; Takayama, K.; Kurosaka, M.; Kuroda, R. Potential involvement of SIRT1 in the pathogenesis of osteoarthritis through the modulation of chondrocyte gene expressions. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2011, 29, 511–515. [Google Scholar] [CrossRef]
- Xu, M.; Feng, M.; Peng, H.; Qian, Z.; Zhao, L.; Wu, S. Epigenetic regulation of chondrocyte hypertrophy and apoptosis through Sirt1/P53/P21 pathway in surgery-induced osteoarthritis. Biochem. Biophys. Res. Commun. 2020, 528, 179–185. [Google Scholar] [CrossRef]
- Dvir-Ginzberg, M.; Gagarina, V.; Lee, E.J.; Hall, D.J. Regulation of cartilage-specific gene expression in human chondrocytes by SIRT1 and nicotinamide phosphoribosyltransferase. J. Biol. Chem. 2008, 283, 36300–36310. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cai, L.; Jiang, P.; Wang, J.; Gao, C.; Feng, H.; Wang, C.; Pan, H.; Yang, Y. SIRT1 inhibition by melatonin exerts antitumor activity in human osteosarcoma cells. Eur. J. Pharmacol. 2013, 715, 219–229. [Google Scholar] [CrossRef]
- Sidiropoulos, P.I.; Goulielmos, G.; Voloudakis, G.K.; Petraki, E.; Boumpas, D.T. Inflammasomes and rheumatic diseases: Evolving concepts. Ann. Rheum. Dis. 2008, 67, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, T.; Yang, H.; He, F.; Zhu, X. Melatonin: A novel candidate for the treatment of osteoarthritis. Ageing Res. Rev. 2022, 78, 101635. [Google Scholar] [CrossRef]
- Su, P. Study on the Effect and Mechanism of Melatonin in Promoting Cartilage Repair; The First Affiliated Hospital of Sun Yat-Sen University: Guangzhou, China, 2020. [Google Scholar]
- Wang, J.; Ma, S.; Yu, J.; Zuo, D.; He, X.; Peng, H.; Shi, X.; Huang, W.; Li, Q. MiR-9-5p promotes M1 cell polarization in osteoarthritis progression by regulating NF-κB and AMPK signaling pathways by targeting SIRT1. Int. Immunopharmacol. 2021, 101 (Pt A), 108207. [Google Scholar] [CrossRef]
- Zhou, L. Melatonin Regulates Oxidative Stress-Induced Premature Aging in Mesenchymal Stem Cells and the Molecular Mechanism Mediated by SIRT1; Suzhou University: Suzhou, China, 2016. [Google Scholar]
- Li, J.; Huang, J.; Dai, L.; Yu, D.; Chen, Q.; Zhang, X.; Dai, K. miR-146a, an IL-1β responsive miRNA, induces vascular endothelial growth factor and chondrocyte apoptosis by targeting Smad4. Arthritis Res. Ther. 2012, 14, R75. [Google Scholar] [CrossRef]
- Chinzei, N.; Brophy, R.H.; Duan, X.; Cai, L.; Nunley, R.M.; Sandell, L.J.; Rai, M.F. Molecular influence of anterior cruciate ligament tear remnants on chondrocytes: A biologic connection between injury and osteoarthritis. Osteoarthr. Cartil. 2018, 26, 588–599. [Google Scholar] [CrossRef]
- Wang, Y.J.; Shen, M.; Wang, S.; Wen, X.; Han, X.R.; Zhang, Z.F.; Li, H.; Wang, F.; Wu, D.M.; Lu, J.; et al. Inhibition of the TGF-β1/Smad signaling pathway protects against cartilage injury and osteoarthritis in a rat model. Life Sci. 2017, 189, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Chen, T.Y.; Zhao, X.J.; Xu, Q.; Jiao, R.Q.; Li, J.M.; Kong, L.D. Pterostilbene prevents hepatocyte epithelial-mesenchymal transition in fructose-induced liver fibrosis through suppressing miR-34a/Sirt1/p53 and TGF-β1/Smads signalling. Br. J. Pharmacol. 2019, 176, 1619–1634. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ma, Y.; Chen, F.; Wang, S.; Chen, B.; Shi, J. miR-133b and miR-199b knockdown attenuate TGF-β1-induced epithelial to mesenchymal transition and renal fibrosis by targeting SIRT1 in diabetic nephropathy. Eur. J. Pharmacol. 2018, 837, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, Z.; Karkhaneh, A.; Nokhbatolfoghahaei, H.; Farzad-Mohajeri, S.; Rezai-Rad, M.; Dehghan, M.M.; Aminishakib, P.; Khojasteh, A. Cartilage regeneration with dual-drug-releasing injectable hydrogel/microparticle system: In vitro and in vivo study. J. Cell. Physiol. 2021, 236, 2194–2204. [Google Scholar] [CrossRef] [PubMed]

| Forward Primer | Reverse Primer | |
|---|---|---|
| SIRT1 MMP-3 | 5′-TCAGTGTCATGGTTCCTTTGC-3′ 5′-TTTGGCCGTCTTCTCATCC-3′ | 5′-AATCTGCTCCTTTGCCACTCT-3′ 5′-GCATCGATCTTCTGGACGGT-3′ |
| MMP-13 | 5′-TTCTGGTCTTCTGCCACACG-3 | 5′-TGGAGCTGCTTGTCCAGGT-3′ |
| ADMTS-4 | 5′-AGGAGGCGCCCTTAACTCTG-3′ | 5′-CTACTCAGCGAAGCGAAGCG-3′ |
| COX-2 | 5′-AGAAGCGAGGACCTGGGTTCAC-3′ | 5′-ACACCTCTCCACCGATGACCTG-3′ |
| COL2A1 | 5-′ACGAAGCGGCTGGCAACCTCA-3′ | 5′-CCCTGTGAATGGGCGGAAAG-3′ |
| GAPDH | 5-’GATGCCCCCATGTTTGTGAT-3′ | 5-′GGCATGGACTGTGGTCATGAG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Song, X.; Chen, H.; Ma, T.; Tang, J.; Wang, X.; Yu, Y.; Lv, L.; Jia, L.; Gao, L. Melatonin Prevents Chondrocyte Matrix Degradation in Rats with Experimentally Induced Osteoarthritis by Inhibiting Nuclear Factor-κB via SIRT1. Nutrients 2022, 14, 3966. https://doi.org/10.3390/nu14193966
Zhao M, Song X, Chen H, Ma T, Tang J, Wang X, Yu Y, Lv L, Jia L, Gao L. Melatonin Prevents Chondrocyte Matrix Degradation in Rats with Experimentally Induced Osteoarthritis by Inhibiting Nuclear Factor-κB via SIRT1. Nutrients. 2022; 14(19):3966. https://doi.org/10.3390/nu14193966
Chicago/Turabian StyleZhao, Mingchao, Xiaopeng Song, Hong Chen, Tianwen Ma, Jilang Tang, Xinyu Wang, Yue Yu, Liangyu Lv, Lina Jia, and Li Gao. 2022. "Melatonin Prevents Chondrocyte Matrix Degradation in Rats with Experimentally Induced Osteoarthritis by Inhibiting Nuclear Factor-κB via SIRT1" Nutrients 14, no. 19: 3966. https://doi.org/10.3390/nu14193966
APA StyleZhao, M., Song, X., Chen, H., Ma, T., Tang, J., Wang, X., Yu, Y., Lv, L., Jia, L., & Gao, L. (2022). Melatonin Prevents Chondrocyte Matrix Degradation in Rats with Experimentally Induced Osteoarthritis by Inhibiting Nuclear Factor-κB via SIRT1. Nutrients, 14(19), 3966. https://doi.org/10.3390/nu14193966





