Trimethylamine N-Oxide (TMAO) and Indoxyl Sulfate Concentrations in Patients with Alcohol Use Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. AUD Patients and Healthy Controls
2.2. Alcohol Screening Tool and Assessment of Alcohol Withdrawal Syndrome (AWS)
2.3. Neuropsychological Examination
2.4. Biological Analyzes
2.5. TMAO and IS Analysis
2.6. Statistical Analysis
3. Results
3.1. Serum TMAO Concentrations in AUD Patients and HC
3.2. Serum IS Concentrations in AUD Patients and HC
3.3. Evolution of Serum TMAO and IS Concentrations in AUD Patients during the Acute Alcohol Withdrawal Phase
3.4. Correlations of Serum TMAO or IS Concentrations with Biological and Clinical Markers in AUD Patients
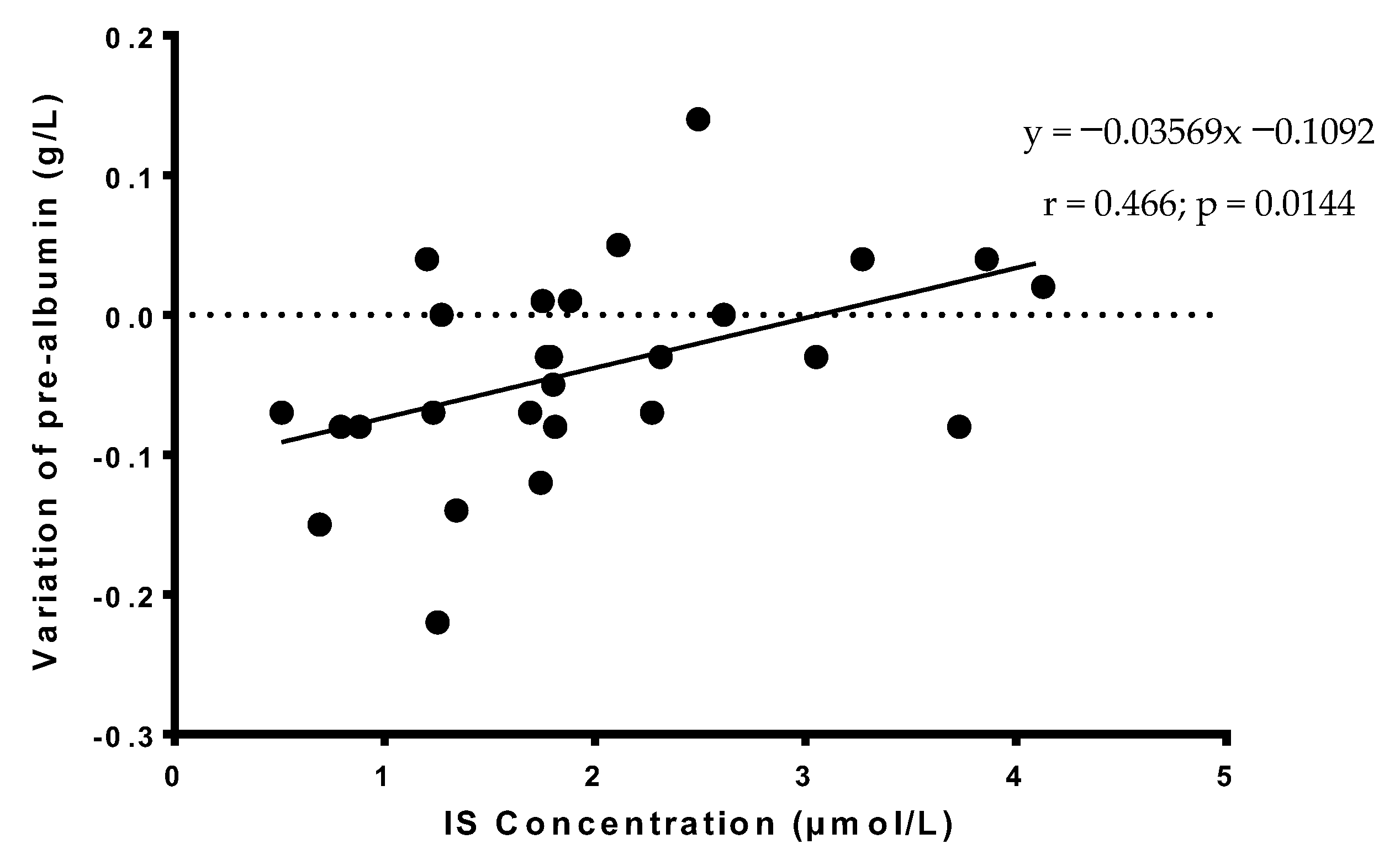
3.5. Correlations of Serum TMAO or IS Concentrations in AUD Patients with the Variation of Prealbumin or Albumin Concentrations during the Acute Phase of Alcohol Withdrawal
3.6. Predictors of TMAO and IS Concentrations in AUD Patients
3.7. Predictors of Clinical, Biological and Neuropsychological Parameters in AUD Patients
4. Discussion
5. Strengths and Limitations of This Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021, 13, 3173. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Souza-Smith, F.M.; Molina, P.E. Alcohol-Associated Tissue Injury: Current Views on Pathophysiological Mechanisms. Annu. Rev. Physiol. 2022, 84, 87–112. [Google Scholar] [CrossRef]
- Mendes, B.G.; Schnabl, B. From Intestinal Dysbiosis to Alcohol-Associated Liver Disease. Clin. Mol. Hepatol. 2020, 26, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Suk, K.T.; Kim, D.J. Gut Microbiota at the Intersection of Alcohol, Brain, and the Liver. J. Clin. Med. 2021, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Nowiński, A.; Ufnal, M. Trimethylamine N-Oxide: A Harmful, Protective or Diagnostic Marker in Lifestyle Diseases? Nutrition 2018, 46, 7–12. [Google Scholar] [CrossRef]
- Kalagi, N.A.; Abbott, K.A.; Alburikan, K.A.; Alkofide, H.A.; Stojanovski, E.; Garg, M.L. Modulation of Circulating Trimethylamine N-Oxide Concentrations by Dietary Supplements and Pharmacological Agents: A Systematic Review. Adv. Nutr. 2019, 10, 876–887. [Google Scholar] [CrossRef]
- Wu, W.-K.; Chen, C.-C.; Liu, P.-Y.; Panyod, S.; Liao, B.-Y.; Chen, P.-C.; Kao, H.-L.; Kuo, H.-C.; Kuo, C.-H.; Chiu, T.H.T.; et al. Identification of TMAO-Producer Phenotype and Host-Diet-Gut Dysbiosis by Carnitine Challenge Test in Human and Germ-Free Mice. Gut 2019, 68, 1439–1449. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; von Eckardstein, A.; Müller, D. Plasma Concentrations of Trimethylamine-N-Oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J. Nutr. 2016, 146, 283–289. [Google Scholar] [CrossRef]
- Lombardo, M.; Aulisa, G.; Marcon, D.; Rizzo, G.; Tarsisano, M.G.; Di Renzo, L.; Federici, M.; Caprio, M.; De Lorenzo, A. Association of Urinary and Plasma Levels of Trimethylamine N-Oxide (TMAO) with Foods. Nutrients 2021, 13, 1426. [Google Scholar] [CrossRef]
- Coutinho-Wolino, K.S.; de F Cardozo, L.F.M.; de Oliveira Leal, V.; Mafra, D.; Stockler-Pinto, M.B. Can Diet Modulate Trimethylamine N-Oxide (TMAO) Production? What Do We Know so Far? Eur. J. Nutr. 2021, 60, 3567–3584. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.; Skye, S.M.; Graham, B.; Zabell, A.; Li, X.S.; Li, L.; Shelkay, S.; Fu, X.; Neale, S.; O’Laughlin, C.; et al. Dietary Choline Supplements, but Not Eggs, Raise Fasting TMAO Levels in Participants with Normal Renal Function: A Randomized Clinical Trial. Am. J. Med. 2021, 134, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Hung, W.-L.; Ford, A.L.; Suh, J.H.; Auger, J.; Nagulesapillai, V.; Wang, Y. In Older Women, a High-Protein Diet Including Animal-Sourced Foods Did Not Impact Serum Levels and Urinary Excretion of Trimethylamine-N-Oxide. Nutr. Res. 2020, 78, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef]
- Azuar, J.; Bouaziz-Amar, E.; Cognat, E.; Dumurgier, J.; Clergue-Duval, V.; Barré, T.; Amami, J.; Hispard, E.; Bellivier, F.; Paquet, C.; et al. Cerebrospinal Fluid Biomarkers in Patients With Alcohol Use Disorder and Persistent Cognitive Impairment. Alcohol. Clin. Exp. Res. 2021, 45, 561–565. [Google Scholar] [CrossRef]
- Wang, Z.-T.; Li, K.-Y.; Tan, C.-C.; Xu, W.; Shen, X.-N.; Cao, X.-P.; Wang, P.; Bi, Y.-L.; Dong, Q.; Tan, L.; et al. Associations of Alcohol Consumption with Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease Pathology in Cognitively Intact Older Adults: The CABLE Study. J. Alzheimers Dis. 2021, 82, 1045–1054. [Google Scholar] [CrossRef]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The Gut Microbiota-Derived Metabolite Trimethylamine N-Oxide Is Elevated in Alzheimer’s Disease. Alzheimers Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N-Oxide Promotes Brain Aging and Cognitive Impairment in Mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef]
- Falconi, C.A.; da Junho, C.V.C.; Fogaça-Ruiz, F.; Vernier, I.C.S.; da Cunha, R.S.; Stinghen, A.E.M.; Carneiro-Ramos, M.S. Uremic Toxins: An Alarming Danger Concerning the Cardiovascular System. Front. Physiol. 2021, 12, 686249. [Google Scholar] [CrossRef]
- Adesso, S.; Magnus, T.; Cuzzocrea, S.; Campolo, M.; Rissiek, B.; Paciello, O.; Autore, G.; Pinto, A.; Marzocco, S. Indoxyl Sulfate Affects Glial Function Increasing Oxidative Stress and Neuroinflammation in Chronic Kidney Disease: Interaction between Astrocytes and Microglia. Front. Pharmacol. 2017, 8, 370. [Google Scholar] [CrossRef]
- Adesso, S.; Ruocco, M.; Rapa, S.F.; Piaz, F.D.; Raffaele Di Iorio, B.; Popolo, A.; Autore, G.; Nishijima, F.; Pinto, A.; Marzocco, S. Effect of Indoxyl Sulfate on the Repair and Intactness of Intestinal Epithelial Cells: Role of Reactive Oxygen Species’ Release. Int. J. Mol. Sci. 2019, 20, 2280. [Google Scholar] [CrossRef] [PubMed]
- Bobot, M.; Thomas, L.; Moyon, A.; Fernandez, S.; McKay, N.; Balasse, L.; Garrigue, P.; Brige, P.; Chopinet, S.; Poitevin, S.; et al. Uremic Toxic Blood-Brain Barrier Disruption Mediated by AhR Activation Leads to Cognitive Impairment during Experimental Renal Dysfunction. J. Am. Soc. Nephrol. 2020, 31, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Karbowska, M.; Hermanowicz, J.M.; Tankiewicz-Kwedlo, A.; Kalaska, B.; Kaminski, T.W.; Nosek, K.; Wisniewska, R.J.; Pawlak, D. Neurobehavioral Effects of Uremic Toxin-Indoxyl Sulfate in the Rat Model. Sci. Rep. 2020, 10, 9483. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Li, J.-R.; Wang, Y.-Y.; Lin, S.-Y.; Ou, Y.-C.; Lin, C.-J.; Wang, J.-D.; Liao, S.-L.; Chen, C.-J. Indoxyl Sulfate Caused Behavioral Abnormality and Neurodegeneration in Mice with Unilateral Nephrectomy. Aging (Albany N. Y.) 2021, 13, 6681–6701. [Google Scholar] [CrossRef] [PubMed]
- Gache, P.; Michaud, P.; Landry, U.; Accietto, C.; Arfaoui, S.; Wenger, O.; Daeppen, J.-B. The Alcohol Use Disorders Identification Test (AUDIT) as a Screening Tool for Excessive Drinking in Primary Care: Reliability and Validity of a French Version. Alcohol Clin. Exp. Res. 2005, 29, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Cushman, P.J.; Forbes, R.; Lerner, W.; Stewart, M. Alcohol Withdrawal Syndromes: Clinical Management with Lofexidine. Alcohol. Clin. Exp. Res. 1985, 9, 103–108. [Google Scholar] [CrossRef]
- Alarcon, R.; Nalpas, B.; Pelletier, S.; Perney, P. MoCA as a Screening Tool of Neuropsychological Deficits in Alcohol-Dependent Patients. Alcohol. Clin. Exp. Res. 2015, 39, 1042–1048. [Google Scholar] [CrossRef]
- Ritz, L.; Lannuzel, C.; Boudehent, C.; Vabret, F.; Bordas, N.; Segobin, S.; Eustache, F.; Pitel, A.-L.; Beaunieux, H. Validation of a Brief Screening Tool for Alcohol-Related Neuropsychological Impairments. Alcohol. Clin. Exp. Res. 2015, 39, 2249–2260. [Google Scholar] [CrossRef]
- Adams, L.; Bulsara, M.; Rossi, E.; DeBoer, B.; Speers, D.; George, J. Hepascore: An Accurate Validated Predictor of Liver Fibrosis in Chronic Hepatitis C Infection. Clin. Chem. 2005, 51, 1867–1873. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Brydges, C.R.; Fiehn, O.; Mayberg, H.S.; Schreiber, H.; Dehkordi, S.M.; Bhattacharyya, S.; Cha, J.; Choi, K.S.; Craighead, W.E.; Krishnan, R.R.; et al. Indoxyl Sulfate, a Gut Microbiome-Derived Uremic Toxin, Is Associated with Psychic Anxiety and Its Functional Magnetic Resonance Imaging-Based Neurologic Signature. Sci. Rep. 2021, 11, 21011. [Google Scholar] [CrossRef]
- Millwood, I.Y.; Walters, R.G.; Mei, X.W.; Guo, Y.; Yang, L.; Bian, Z.; Bennett, D.A.; Chen, Y.; Dong, C.; Hu, R.; et al. Conventional and Genetic Evidence on Alcohol and Vascular Disease Aetiology: A Prospective Study of 500,000 Men and Women in China. Lancet 2019, 393, 1831–1842. [Google Scholar] [CrossRef]
- Burton, K.J.; Krüger, R.; Scherz, V.; Münger, L.H.; Picone, G.; Vionnet, N.; Bertelli, C.; Greub, G.; Capozzi, F.; Vergères, G. Trimethylamine-N-Oxide Postprandial Response in Plasma and Urine Is Lower after Fermented Compared to Non-Fermented Dairy Consumption in Healthy Adults. Nutrients 2020, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Caudill, M.A. Trimethylamine-N-Oxide: Friend, Foe, or Simply Caught in the Cross-Fire? Trends Endocrinol. Metab. 2017, 28, 121–130. [Google Scholar] [CrossRef]
- Griffin, L.E.; Djuric, Z.; Angiletta, C.J.; Mitchell, C.M.; Baugh, M.E.; Davy, K.P.; Neilson, A.P. A Mediterranean Diet Does Not Alter Plasma Trimethylamine N-Oxide Concentrations in Healthy Adults at Risk for Colon Cancer. Food Funct. 2019, 10, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.A.; Gillevet, P.M.; Rangwala, H.; Sikaroodi, M.; Naqvi, A.; Engen, P.A.; Kwasny, M.; Lau, C.K.; Keshavarzian, A. Colonic Microbiome Is Altered in Alcoholism. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G966–G978. [Google Scholar] [CrossRef]
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.M.; Jamar, F.; Stärkel, P.; Windey, K.; Tremaroli, V.; Bäckhed, F.; Verbeke, K.; et al. Intestinal Permeability, Gut-Bacterial Dysbiosis, and Behavioral Markers of Alcohol-Dependence Severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–E4493. [Google Scholar] [CrossRef]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial Tryptophan Metabolites Regulate Gut Barrier Function via the Aryl Hydrocarbon Receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef]
- Schulz, K.F.; Grimes, D.A. Multiplicity in randomised trials I: Endpoints and treatments. Lancet 2005, 365, 1591–1595. [Google Scholar] [CrossRef]

| AUD Patients (n = 30) | Healthy Controls (n = 15) | p | |
|---|---|---|---|
| Demographic and Clinical variables | |||
| Age (year) | 47.5 ± 10.1 | 46.2 ± 5.1 | NS |
| Sexe (M/F) | 24/6 | 12/3 | NS |
| Body Mass Index (kg/m2) | 24.1 ± 4.3 | 24.3 ± 4.3 | NS |
| AUDIT | 28.6 ± 7.8 | 2.6 ± 1.4 | p < 0.05 |
| Cushman’s score | 4.9 ± 2.2 | NA | ND |
| Benzodiazepin prescription (days) | 6.7 ± 5.3 | NA | ND |
| MoCA score (/30) | 24.5 ± 5.0 | 27.4 ± 1.4 | p < 0.05 |
| BEARNI score (/30) | 13.5 ± 6.1 | 19.1 ± 2.9 | p < 0.05 |
| Length of hospital stay (days) | 20.2 ± 5.6 | NA | ND |
| Biological measures | |||
| Glycemia (mmol/L) | 5.3 ± 0.9 | 5.3 ± 0.6 | NS |
| Glycated haemoglobin (%) | 5.4 ± 0.6 | 5.4 ± 0.3 | NS |
| Total serum protein (g/L) | 66.4 ± 6.9 | 70.3 ± 3.5 | p = 0.05 |
| Albumin (g/L) | 37.2 ± 4.7 | 43.2 ± 2.8 | p < 0.05 |
| Prealbumin (g/L) | 0.27 ± 0.08 | 0.31 ± 0.04 | p < 0.05 |
| γ-glutamyl-transpeptidase (U/L) | 442.2 ± 812.2 | 16.9 ± 10.5 | p < 0.05 |
| Ammonium (µmol/L) | 42.8 ± 23.3 | 28.1 ± 7.2 | p < 0.05 |
| ASAT (U/L) | 69.3 ± 60.3 | 19.6 ± 7.8 | p < 0.05 |
| ALAT (U/L) | 50.3 ± 71,7 | 21.1 ± 12.5 | p < 0.05 |
| ASAT/ALAT ratio | 2.2 ± 2.5 | 1.0 ± 0.3 | p < 0.05 |
| Bilirubin (µmol/L) | 25.1 ± 17.4 | 17.9 ± 10.5 | p < 0.05 |
| α2 macroglobulin (g/L) | 2.1 ± 0.6 | 1.7 ± 0.5 | p < 0.05 |
| Hyaluronic acid (µg/L) | 121.4 ± 320.7 | 15.2 ± 5.5 | p < 0.05 |
| Score of fibrosis HEPASCORE | 0.46 ± 0.31 | 0.22 ± 0.13 | p < 0.05 |
| Serum creatinine (µmol/L) | 61.9 ± 12.7 | 78.7 ± 12.8 | p < 0.05 |
| Urea (mmoles/L) | 2.7 ± 1.0 | 4.4 ± 0.8 | p < 0.05 |
| Haemoglobin (g/dL) | 14.1 ± 1.4 | 14.8 ± 0.7 | p < 0.05 |
| Mean Cellular Volume (µm3) | 98.0 ± 6.0 | 87.7 ± 4.3 | p < 0.05 |
| Platelets (G/L) | 205 ± 82 | 280 ± 61 | p < 0.05 |
| Prothrombin time (%) | 96.3 ± 8.4 | 96.9 ± 5.2 | NS |
| Trimethylamine N-oxide TMAO (µmol/L) | 12.1 ± 19.2 | 4.6 ± 2.5 | NS |
| Indoxyl sulfate IS (µmol/L) | 2.1 ± 1.0 | 3.9 ± 1.5 | p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coulbault, L.; Laniepce, A.; Segobin, S.; Boudehent, C.; Cabé, N.; Pitel, A.L. Trimethylamine N-Oxide (TMAO) and Indoxyl Sulfate Concentrations in Patients with Alcohol Use Disorder. Nutrients 2022, 14, 3964. https://doi.org/10.3390/nu14193964
Coulbault L, Laniepce A, Segobin S, Boudehent C, Cabé N, Pitel AL. Trimethylamine N-Oxide (TMAO) and Indoxyl Sulfate Concentrations in Patients with Alcohol Use Disorder. Nutrients. 2022; 14(19):3964. https://doi.org/10.3390/nu14193964
Chicago/Turabian StyleCoulbault, Laurent, Alice Laniepce, Shailendra Segobin, Céline Boudehent, Nicolas Cabé, and Anne Lise Pitel. 2022. "Trimethylamine N-Oxide (TMAO) and Indoxyl Sulfate Concentrations in Patients with Alcohol Use Disorder" Nutrients 14, no. 19: 3964. https://doi.org/10.3390/nu14193964
APA StyleCoulbault, L., Laniepce, A., Segobin, S., Boudehent, C., Cabé, N., & Pitel, A. L. (2022). Trimethylamine N-Oxide (TMAO) and Indoxyl Sulfate Concentrations in Patients with Alcohol Use Disorder. Nutrients, 14(19), 3964. https://doi.org/10.3390/nu14193964






