Association of Selenium Levels with Gestational Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Search Strategy
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Primary Outcome Definition
2.6. Assessment of Risk of Bias
2.7. Data Extraction
2.8. Statistical Analyses
3. Results
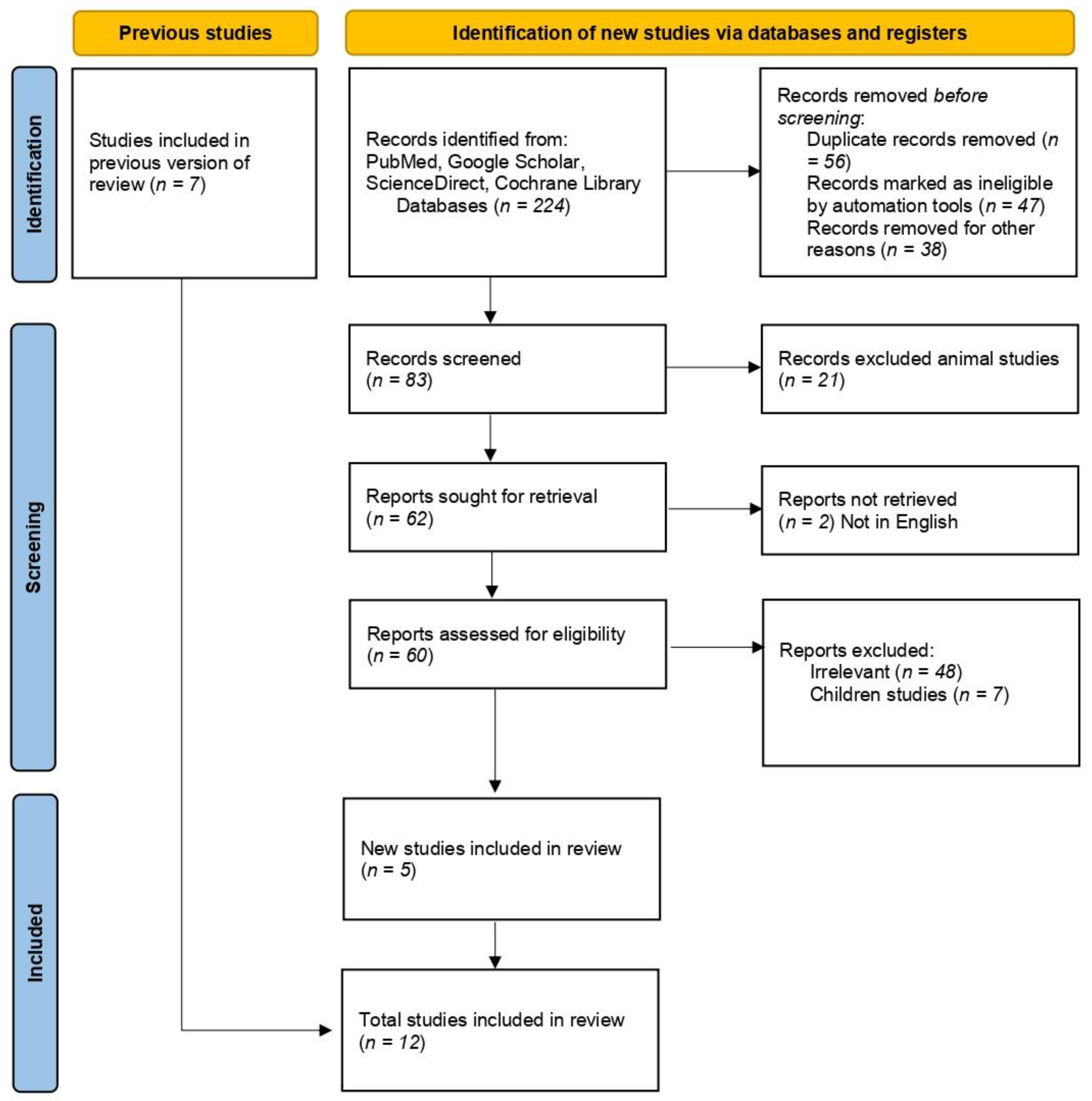
3.1. Studies Selection
3.2. Characteristics of the Included Studies
3.3. Overall Meta-Analysis
3.4. Stratified Meta-Analysis and Meta-Regression
3.5. Reporting Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Standards of Medical Care in Diabetes-2014. Diabetes Care 2014, 37, 14–80. [Google Scholar] [CrossRef] [PubMed]
- Kunasegaran, T.; Balasubramaniam, V.R.M.T.; Arasoo, V.J.T.; Palanisamy, U.D.; Ramadas, A. Gestational Diabetes Mellitus in Southeast Asia: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 1272. [Google Scholar] [CrossRef]
- Ornoy, A. Prenatal Origin of Obesity and Their Complications: Gestational Diabetes, Maternal Overweight and the Paradoxical Effects of Fetal Growth Restriction and Macrosomia. Reprod. Toxicol. 2011, 32, 205–212. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Xiang, A.H.; Page, K.A. Gestational Diabetes Mellitus: Risks and Management during and after Pregnancy. Nat. Rev. Endocrinol. 2012, 8, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ning, Y. Effect of Dietary and Lifestyle Factors on the Risk of Gestational Diabetes: Review of Epidemiologic Evidence. Am. J. Clin. Nutr. 2011, 94, 1975–1979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Rawal, S.; Chong, Y.S. Risk Factors for Gestational Diabetes: Is Prevention Possible? Diabetologia 2016, 59, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, U. Gestational Diabetes: A Clinical Update. World J. Diabetes 2015, 6, 1065–1072. [Google Scholar] [CrossRef]
- Ben-Haroush, A.; Yogev, Y.; Hod, M. Epidemiology of Gestational Diabetes Mellitus and Its Association with Type 2 Diabetes. Diabet. Med. 2004, 21, 103–113. [Google Scholar] [CrossRef]
- Mihailović, M.; Cvetković, M.; Ljubić, A.; Kosanović, M.; Nedeljković, S.; Jovanović, I.; Pešut, O. Selenium and Malondialdehyde Content and Glutathione Peroxidase Activity in Maternal and Umbilical Cord Blood and Amniotic Fluid. Biol. Trace Elem. Res. 2000, 73, 47–54. [Google Scholar] [CrossRef]
- Chen, X.; Scholl, T.O.; Leskiw, M.J.; Donaldson, M.R.; Stein, T.P. Association of Glutathione Peroxidase Activity with Insulin Resistance and Dietary Fat Intake during Normal Pregnancy. J. Clin. Endocrinol. Metab. 2003, 88, 5963–5968. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, H.Z.; Hassan, A.A.; Adam, I. Minerals in Pregnancy and Newborns. In Molecular Nutrition: Mother and Infant; Vinciguerra, M., Sanchez, P.C., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 155–177. ISBN 9780128138625. [Google Scholar]
- Wolonciej, M.; Milewska, E.; Roszkowska-Jakimiec, W. Trace Elements as an Activator of Antioxidant Enzymes. Postepy Hig. Med. Dosw. 2016, 70, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, S. Selenium: An Insulin-Mimetic. Cell. Mol. Life Sci. 2000, 57, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, O. The Insulin-like Effects of Selenate in Rat Adipocytes. J. Biol. Chem. 1990, 265, 1124–1128. [Google Scholar] [CrossRef]
- Asemi, Z.; Jamilian, M.; Mesdaghinia, E.; Esmaillzadeh, A. Effects of Selenium Supplementation on Glucose Homeostasis, Inflammation, and Oxidative Stress in Gestational Diabetes: Randomized, Double-Blind, Placebo-Controlled Trial. Nutrition 2015, 31, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, T.; Wei, J.; Lei, G.; Zeng, C. Association between Serum Selenium Level and Type 2 Diabetes Mellitus: A Non-Linear Dose-Response Meta-Analysis of Observational Studies. Nutr. J. 2016, 15, 48. [Google Scholar] [CrossRef]
- Yadav, C.; Manjrekar, P.; Agarwal, A.; Ahmad, A.; Hegde, A.; Srikantiah, R. Association of Serum Selenium, Zinc and Magnesium Levels with Glycaemic Indices and Insulin Resistance in Pre-Diabetes: A Cross-Sectional Study from South India. Biol. Trace Elem. Res. 2017, 175, 65–71. [Google Scholar] [CrossRef]
- González de Vega, R.; Fernández-Sánchez, M.; Fernández, J.; Álvarez Menéndez, F.; Sanz-Medel, A. Selenium Levels and Glutathione Peroxidase Activity in the Plasma of Patients with Type II Diabetes Mellitus. J. Trace Elem. Med. Biol. 2016, 37, 44–49. [Google Scholar] [CrossRef]
- Tan, M.; Sheng, L.; Qian, Y.; Ge, Y.; Wang, Y.; Zhang, H.; Jiang, M.; Zhang, G. Changes of Serum Selenium in Pregnant Women with Gestational Diabetes Mellitus. Biol. Trace Elem. Res. 2001, 83, 231–237. [Google Scholar] [CrossRef]
- Kilinc, M.; Guven, M.A.; Ezer, M.; Ertas, I.E.; Coskun, A. Evaluation of Serum Selenium Levels in Turkish Women with Gestational Diabetes Mellitus, Glucose Intolerants, and Normal Controls. Biol. Trace Elem. Res. 2008, 123, 35–40. [Google Scholar] [CrossRef]
- Al-Saleh, E.; Nandakumaran, M.; Al-Shammari, M.; Al-Harouny, A. Maternal-Fetal Status of Copper, Iron, Molybdenum, Selenium and Zinc in Patients with Gestational Diabetes. J. Matern. Neonatal Med. 2004, 16, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Lezo, A.; Menato, G.; Gallo, M.-L.; Bardelli, C.; Signorile, A.; Berutti, C.; Massobrio, M.; Pagano, G.F. Gestational Hyperglycemia, Zinc, Selenium, and Antioxidant Vitamins. Nutrition 2005, 21, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, E.; Nandakumaran, M.; Al-Rashdan, I.; Al-Harmi, J.; Al-Shammari, M. Maternal-Foetal Status of Copper, Iron, Molybdenum, Selenium and Zinc in Obese Gestational Diabetic Pregnancies. Acta Diabetol. 2007, 44, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, H.Z.; Elbashir, L.M.; Hamdan, S.Z.; Elhassan, E.M.; Adam, I. Zinc and Selenium Levels in Women with Gestational Diabetes Mellitus at Medani Hospital, Sudan. J. Obstet. Gynaecol. 2014, 34, 567–570. [Google Scholar] [CrossRef]
- Molnar, J.; Garamvolgyi, Z.; Herold, M.; Adanyi, N.; Somogyi, A.; Rigo, J. Serum Selenium Concentrations Correlate Significantly with Inflammatory Biomarker High-Sensitive CRP Levels in Hungarian Gestational Diabetic and Healthy Pregnant Women at Mid-Pregnancy. Biol. Trace Elem. Res. 2008, 121, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Ma, L.; Chen, S.; Li, G.; Zhou, J. Serum Selenium Level and Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutr. J. 2016, 15, 94. [Google Scholar] [CrossRef]
- Askari, G.; Iraj, B.; Salehi-Abargouei, A.; Fallah, A.; Jafari, T. The Association between Serum Selenium and Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Trace Elem. Med. Biol. 2015, 29, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yao, A.; Ma, L.; Chen, X.; Yu, S.; Liu, Y.; Hou, Y. Associations of Serum Selenium Levels in the First Trimester of Pregnancy with the Risk of Gestational Diabetes Mellitus and Preterm Birth: A Preliminary Cohort Study. Biol. Trace Elem. Res. 2021, 199, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, H.; Örgül, G.; Tonyalı, N.; Biriken, D.; Polat, N.; Yücel, A.; Yazihan, N.; Şahin, D. The Role of Afamin and Other Trace Elements in the Prediction of GDM: A Tertiary Center Experience. Biol. Trace Elem. Res. 2021, 199, 4418–4422. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B.; Sajdak, S.; Lubiński, J. First Trimester Microelements and Their Relationships with Pregnancy Outcomes and Complications. Nutrients 2020, 12, 1108. [Google Scholar] [CrossRef]
- Hamdan, H.Z.; Hamdan, S.Z.; Adam, I. Maternal Serum Selenium and Zinc Levels and Gestational Diabetes Mellitus; Systematic Reviews and Meta-Analysis. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021239431 (accessed on 28 March 2021).
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Altman, D.G.; Booth, A.; et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Allot, A.; Lee, K.; Chen, Q.; Luo, L.; Lu, Z. LitSuggest: A Web-Based System for Literature Recommendation and Curation Using Machine Learning. Nucleic Acids Res. 2021, 49, W352–W358. [Google Scholar] [CrossRef] [PubMed]
- Coustan, D.; Carpenter, M. The Diagnosis of Gestational Diabetes. Diabetes Care 1998, 21, B5–B8. [Google Scholar]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 December 2021).
- Munn, Z.; Tufanaru, C.; Aromataris, E. JBI’s Systematic Reviews. AJN Am. J. Nurs. 2014, 114, 49–54. [Google Scholar] [CrossRef]
- Zhu, G.; Zheng, T.; Xia, C.; Qi, L.; Papandonatos, G.D.; Ming, Y.; Zeng, Z.; Zhang, X.; Zhang, H.; Li, Y. Plasma Levels of Trace Element Status in Early Pregnancy and the Risk of Gestational Diabetes Mellitus: A Nested Case-Control Study. J. Trace Elem. Med. Biol. 2021, 68, 126829. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based. Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Hedges, L.V. Distribution Theory for Glass’s Estimator of Effect Size and Related Estimators. J. Educ. Stat. 2016, 6, 107–128. [Google Scholar] [CrossRef]
- Sedgwick, P. Meta-Analyses: Heterogeneity and Subgroup Analysis. BMJ 2013, 346, f4040. [Google Scholar] [CrossRef]
- Moshfeghy, Z.; Bashiri, K.; Dabbaghmanesh, M.H.; Akbarzadeh, M.; Asadi, N.; Sayadi, M. The Predictive Value of Selenium in Diagnosis of Gestational Diabetes: A Nested Case-Control Study. Int. J. Gen. Med. 2020, 13, 53–60. [Google Scholar] [CrossRef]
- Onat, T.; Demir Caltekin, M.; Turksoy, V.A.; Baser, E.; Aydogan Kirmizi, D.; Kara, M.; Yalvac, E.S. The Relationship Between Heavy Metal Exposure, Trace Element Level, and Monocyte to HDL Cholesterol Ratio with Gestational Diabetes Mellitus. Biol. Trace Elem. Res. 2021, 199, 1306–1315. [Google Scholar] [CrossRef]
- Alberti, K.; Zimmet, P. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus Provisional Report of a WHO Consultation. Diabet Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Xiang, A.H. Gestational Diabetes Mellitus. J. Clin. Invest. 2005, 115, 485–491. [Google Scholar] [CrossRef]
- Najib, F.S.; Poordast, T.; Nia, M.R.; Dabbaghmanesh, M.H. Effects of Selenium Supplementation on Glucose Homeostasis in Women with Gestational Diabetes Mellitus: A Randomized, Controlled Trial. Int. J. Reprod. Biomed. 2020, 18, 57–64. [Google Scholar] [CrossRef]
- Ferrer, E.; Alegría, A.; Barberá, R.; Farré, R.; Lagarda, M.J.; Monleon, J. Whole Blood Selenium Content in Pregnant Women. Sci. Total Environ. 1999, 227, 139–143. [Google Scholar] [CrossRef]
- Hawkes, W.C.; Alkan, Z.; Lang, K.; King, J.C. Plasma Selenium Decrease during Pregnancy Is Associated with Glucose Intolerance. Biol. Trace Elem. Res. 2004, 100, 19–29. [Google Scholar] [CrossRef]
- Whiting, P.H.; Kalansooriya, A.; Holbrook, I.; Haddad, F.; Jennings, P.E. The Relationship between Chronic Glycaemic Control and Oxidative Stress in Type 2 Diabetes Mellitus. Br. J. Biomed. Sci. 2008, 65, 71–74. [Google Scholar] [CrossRef]
- Wilschefski, S.C.; Baxter, M.R. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef]
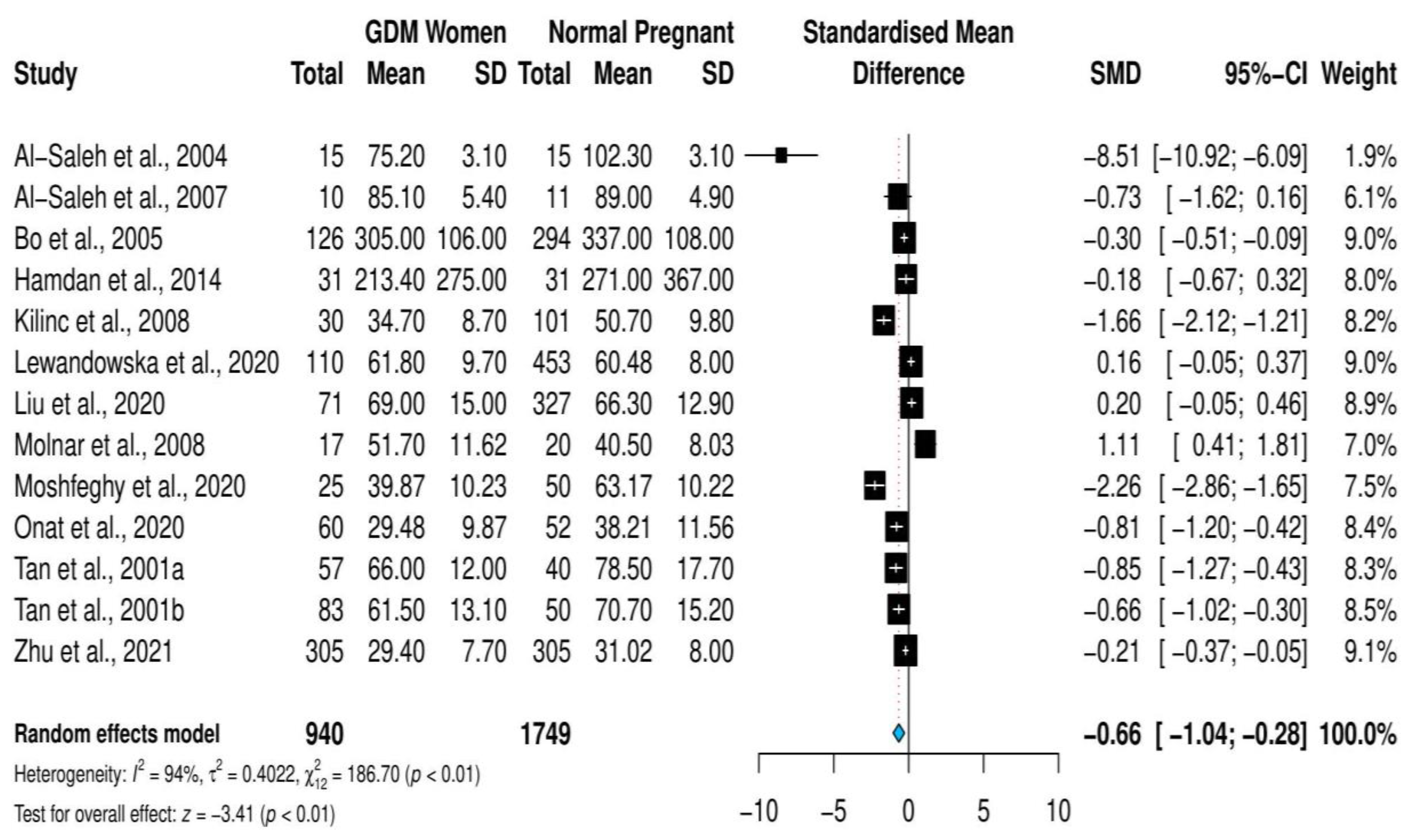
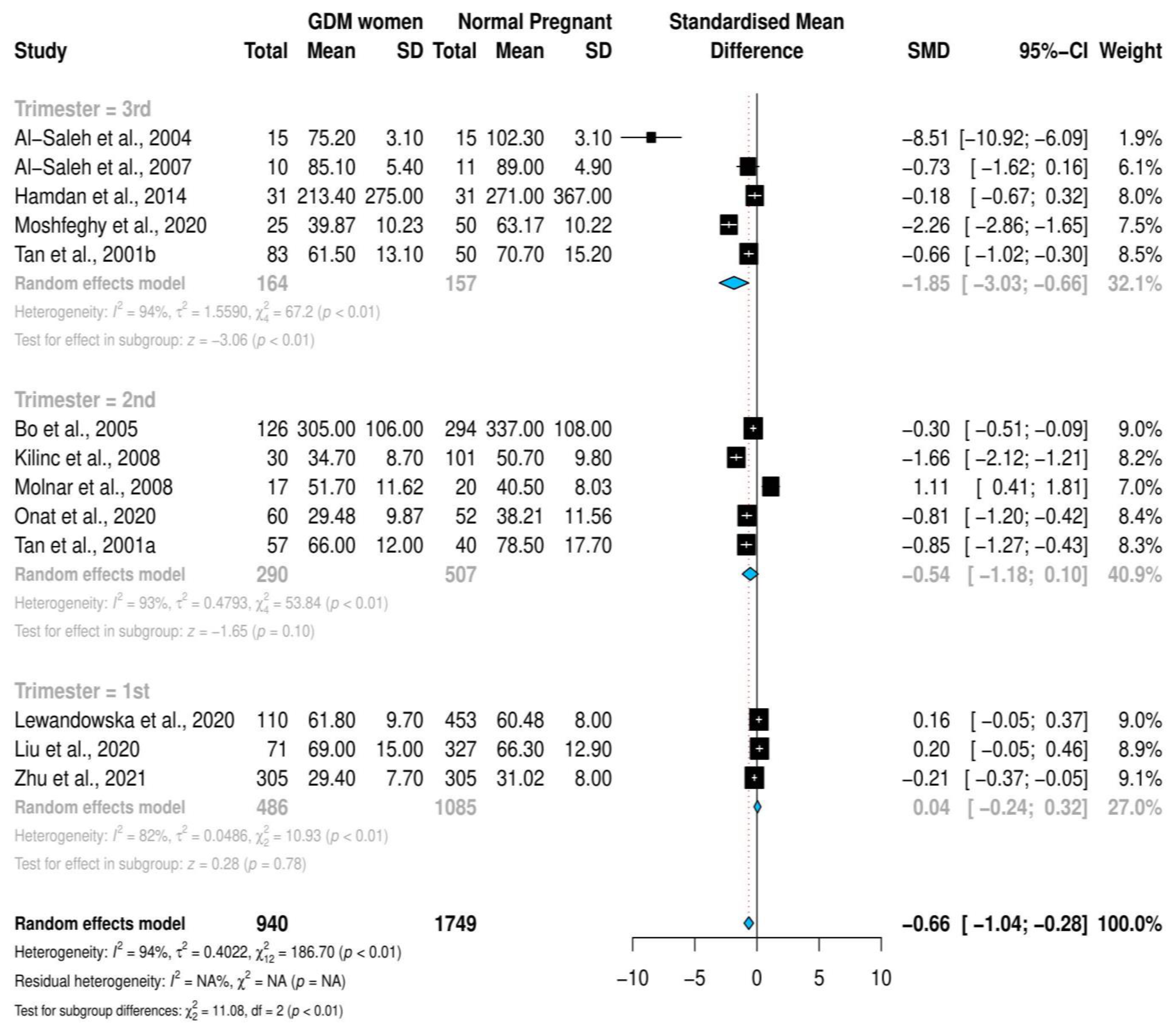
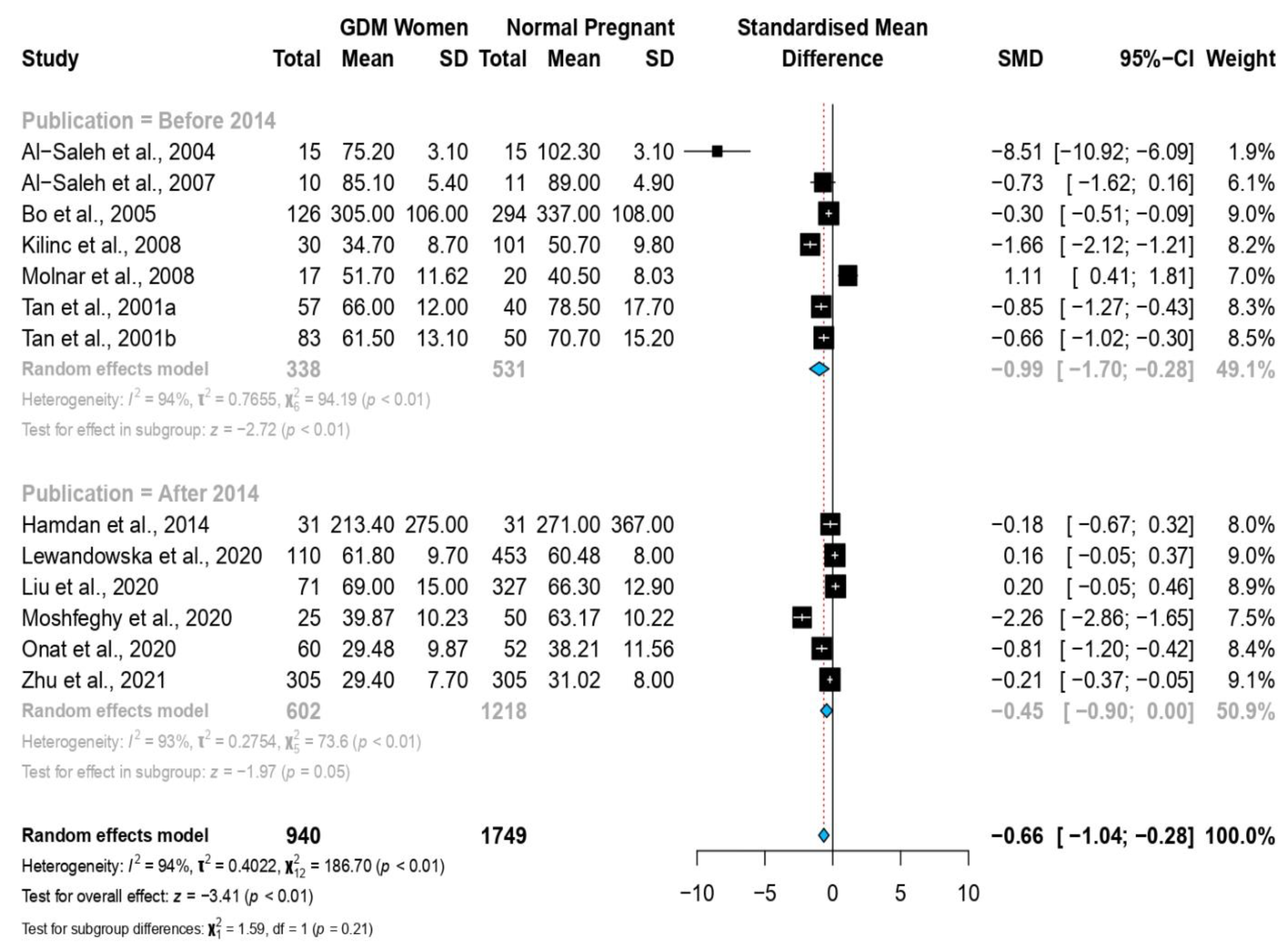
| Study, Year (Study Type) | Study Location | Diagnostic Criteria | Selenium Measurement Methods | GDM Group Sample Size Selenium Level Mean (SD) µg/L | Control Group Sample Size Selenium Level Mean (SD) µg/L | ||
|---|---|---|---|---|---|---|---|
| Al-Saleh et al., 2004 [22] (Case–control) | Kuwait | NA | AAS | 15 | 75.2 (3.1) | 15 | 102.3 (3.1) |
| Al-Saleh et al., 2007 [24] (Case–control) | Kuwait | NA | AAS | 10 | 85.1 (5.4) | 11 | 89 (4.9) |
| Bo et al., 2005 [23] (Cohort) | Italy | Carpenter and Coustan | AAS | 126 | 305 (106) | 294 | 337 (108) |
| Hamdan et al., 2014 [25] (Case–control) | Sudan | Carpenter and Coustan | AAS | 31 | 213.4 (275) | 31 | 271 (367) |
| Kilinc et al., 2008 [21] (Cross-sectional) | Turkey | Carpenter and Coustan | AAS | 30 | 34.7 (8.7) | 101 | 50.7 (9.8) |
| Lewandowska et al., 2020 [31] (Cohort) | Poland | IADAPSG | ICPMS | 110 | 61.8 (9.7) | 453 | 60.48 (8) |
| Liu et al., 2020 [29] (Cohort) | China | IADAPSG | ICPMS | 71 | 69 (15) | 327 | 66.3 (12.9) |
| Molnar et al., 2008 [26] (Case–control) | Hungary | WHO | AAS | 17 | 51.7 (11.62) | 20 | 40.5 (8.03) |
| Moshfeghy et al., 2020 [43] (Case–control) | Iran | Carpenter and Coustan | AAS | 25 | 39.87 (10.23) | 50 | 63.17 (10.22) |
| Onat et al., 2020 [44] (Case–control) | Turkey | Carpenter and Coustan | ICPMS | 60 | 29.48 (9.87) | 52 | 38.21 (11.56) |
| Tan et al., 2001a [20] (Case–control) | China | NA | AFS | 57 | 66 (12) | 40 | 78.5 (17.7) |
| Tan et al., 2001b [20] (Case–control) | China | NA | AFS | 83 | 61.5 (13.1) | 50 | 70.7 (15.2) |
| Zhu et al., 2021 [38] (Case–control) | China | IADAPSG | ICPMS | 305 | 29.4 (7.7) | 305 | 31.02 (8) |
| Covariate | Coefficient | 95% Confidence Interval | Standard Error | p-Value |
|---|---|---|---|---|
| Continent Non-Europe | −0.848 | (−5.213, 3.516) | 2.227 | 0.703 |
| Trimester of Selenium Measurement 2nd Trimester 3rd Trimester | −2.871 −3.283 | (−10.294, 4.552) (−10.173, 3.606) | 3.787 3.515 | 0.448 0.350 |
| Economic Classification Middle and Low Income | −0.162 | (−3.510, 3.186) | 1.708 | 0.924 |
| Study Design Case–Control Studies | 1.467 | (−2.691, 5.624) | 2.121 | 0.489 |
| Selenium Detection Method AFS ICPMS | 3.058 −0.771 | (−1.850, 7.968) (−6.406, 4.864) | 2.504 2.875 | 0.222 0.788 |
| Year of Publication | −0.046 | (−0.514, 0.421) | 0.845 | 0.845 |
| Study NOS Quality Score | 1.794 | (−0.565, 4.154) | 0.136 | 0.136 |
| Sample Size | −0.003 | (−0.013, 0.007) | 0.555 | 0.555 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdan, H.Z.; Hamdan, S.Z.; Adam, I. Association of Selenium Levels with Gestational Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3941. https://doi.org/10.3390/nu14193941
Hamdan HZ, Hamdan SZ, Adam I. Association of Selenium Levels with Gestational Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis. Nutrients. 2022; 14(19):3941. https://doi.org/10.3390/nu14193941
Chicago/Turabian StyleHamdan, Hamdan Z., Sumaia Zaki Hamdan, and Ishag Adam. 2022. "Association of Selenium Levels with Gestational Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis" Nutrients 14, no. 19: 3941. https://doi.org/10.3390/nu14193941
APA StyleHamdan, H. Z., Hamdan, S. Z., & Adam, I. (2022). Association of Selenium Levels with Gestational Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis. Nutrients, 14(19), 3941. https://doi.org/10.3390/nu14193941







