Anti-Inflammatory Diets in Fertility: An Evidence Review
Abstract
1. Introduction
2. Assessing Dietary Patterns Using Diet Quality Indices
2.1. Quantifying Dietary Patterns
2.2. The Dietary Inflammatory Index
3. Inflammation in Relation to Diet and Fertility
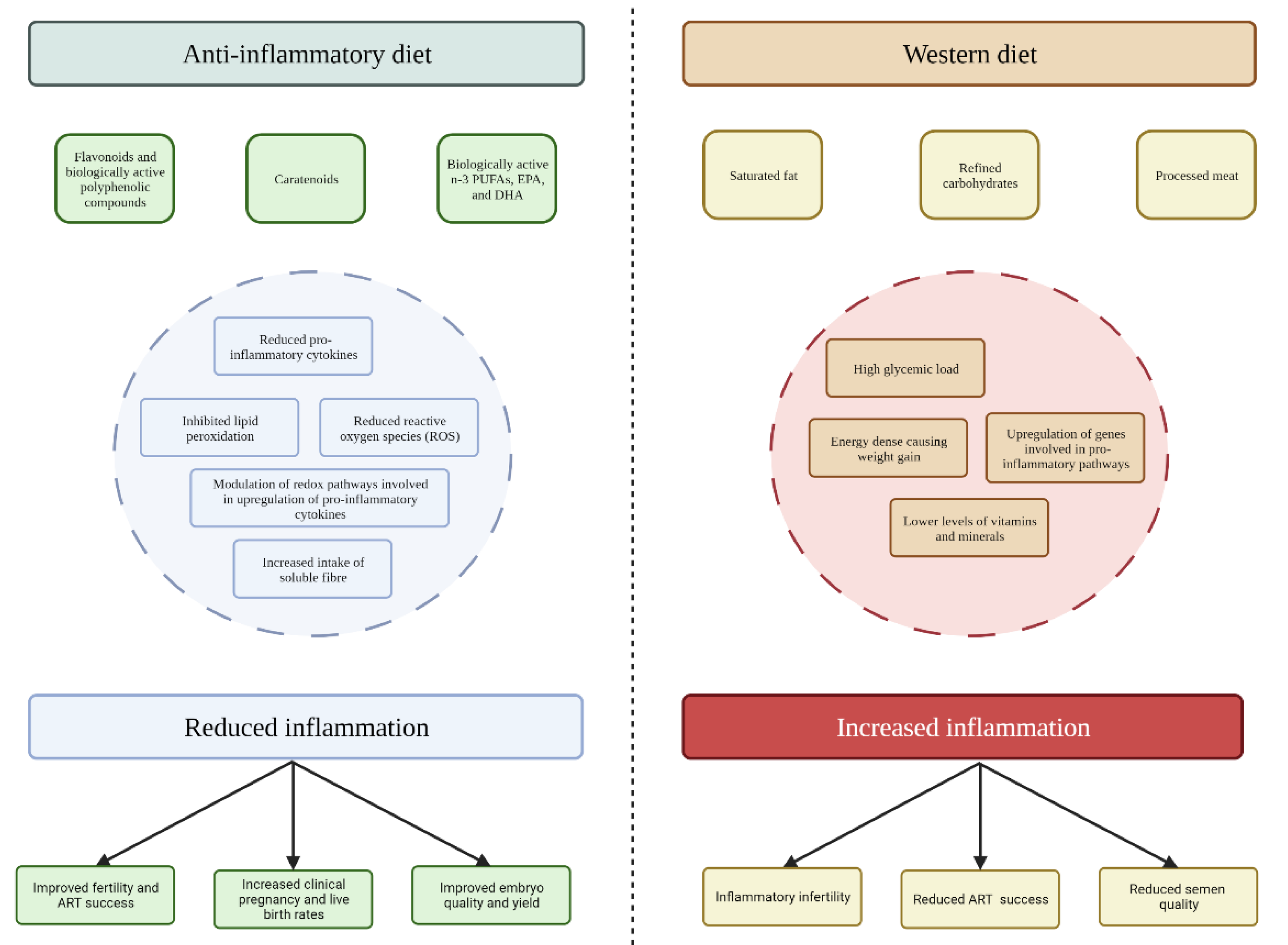
3.1. The Anti-Inflammatory Diet
3.2. The Western Diet
4. Anti-Inflammatory Diets and Female Fertility
4.1. Menstruation
4.2. Endometriosis
4.3. Polycystic Ovary Syndrome (PCOS)
4.4. Embryo Quality and Live Birth
5. Anti-Inflammatory Diets and Male Fertility
Sperm Quality
6. Limitations and Future Directions
7. Communicating Novel Approaches: Evidence to Integration
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tabong, P.T.; Adongo, P.B. Infertility and childlessness: A qualitative study of the experiences of infertile couples in Northern Ghana. BMC Pregnancy Childbirth 2013, 13, 72. [Google Scholar] [CrossRef]
- Organisation, W.H. Infertility. Available online: https://www.who.int/health-topics/infertility#tab=tab_1 (accessed on 10 August 2022).
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Chambers, G.M.; Sullivan, E.A.; Shanahan, M.; Ho, M.T.; Priester, K.; Chapman, M.G. Is in vitro fertilisation more effective than stimulated intrauterine insemination as a first-line therapy for subfertility? A cohort analysis. Aust. N. Z. J. Obstet. Gynaecol. 2010, 50, 280–288. [Google Scholar] [CrossRef]
- Rooney, K.L.; Domar, A.D. The relationship between stress and infertility. Dialogues Clin. Neurosci. 2018, 20, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dag, Z.O.; Dilbaz, B. Impact of obesity on infertility in women. J. Turk. Ger Gynecol Assoc. 2015, 16, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Gavarkovs, A.; Tamez, M.; Mattei, J. The Influence of Diet on Fertility and the Implications for Public Health Nutrition in the United States. Front. Public Health 2018, 6, 211. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet. Gynecol. 2007, 110, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Herforth, A.; Arimond, M.; Alvarez-Sanchez, C.; Coates, J.; Christianson, K.; Muehlhoff, E. A Global Review of Food-Based Dietary Guidelines. Adv. Nutr. 2019, 10, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goldsmith, L.T.; Taylor, R.N.; Bellet, D.; Taylor, H.S. Inflammation in reproductive disorders. Reprod. Sci. 2009, 16, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Azenabor, A.; Ekun, A.O.; Akinloye, O. Impact of Inflammation on Male Reproductive Tract. J. Reprod. Infertil. 2015, 16, 123–129. [Google Scholar]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, E.M.; Hu, F.B. Dietary Patterns: From Nutritional Epidemiologic Analysis to National Guidelines; Nutr, A.J.C., Ed.; Oxford University Press: Oxford, UK, 2015; Volume 101, pp. 899–900. [Google Scholar]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef]
- Guenther, P.M.; Reedy, J.; Krebs-Smith, S. Development of the healthy eating index-2005. J. Am. Diet. Assoc. 2008, 108, 1896–1901. [Google Scholar] [CrossRef]
- Roy, R.; Hebden, L.; Rangan, A.; Allman-Farinelli, M. The development, application, and validation of a healthy eating index for Australian adults (HEIFA—2013). Nutrition 2016, 32, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Eng. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- Fung, T.T.; Chiuve, S.E.; McCullough, M.L.; Rexrode, K.M.; Logroscino, G.; Hu, F.B. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 2008, 168, 713–720. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Phillips, C.M.; Chen, L.-W.; Heude, B.; Bernard, J.Y.; Harvey, N.C.; Duijts, L.; Mensink-Bout, S.M.; Polanska, K.; Mancano, G.; Suderman, M. Dietary inflammatory index and non-communicable disease risk: A narrative review. Nutrients 2019, 11, 1873. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arellano, A.; Martínez-González, M.A.; Ramallal, R.; Salas-Salvadó, J.; Hébert, J.R.; Corella, D.; Shivappa, N.; Forga, L.; Schröder, H.; Muñoz-Bravo, C.J.C.N. Dietary inflammatory index and all-cause mortality in large cohorts: The SUN and PREDIMED studies. Clin. Nutr. 2019, 38, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Corley, J.; Shivappa, N.; Hébert, J.R.; Starr, J.; Deary, I. Associations between dietary inflammatory index scores and inflammatory biomarkers among older adults in the Lothian birth cohort 1936 study. J. Nutr. Health Aging 2019, 23, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Kotemori, A.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Shivappa, N.; Hebert, J.R.; Ishihara, J.; Inoue, M.; Tsugane, S. Validating the dietary inflammatory index using inflammatory biomarkers in a Japanese population: A cross-sectional study of the JPHC-FFQ validation study. Nutrition 2020, 69, 110569. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Agalliu, I.; Hingle, M.; Hou, L.; Hurley, T.G.; Jiao, L. Construct validation of the dietary inflammatory index among postmenopausal women. Ann. Epidemiol. 2015, 25, 398–405. [Google Scholar] [CrossRef]
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Giovannucci, E.L. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. J. Nutr. 2017, 147, 1567–1577. [Google Scholar] [CrossRef]
- Shivappa, N.; Hébert, J.R.; Rietzschel, E.R.; De Buyzere, M.L.; Langlois, M.; Debruyne, E.; Marcos, A.; Huybrechts, I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br. J. Nutr. 2015, 113, 665–671. [Google Scholar] [CrossRef]
- Moran, L.J.; Grieger, J.A.; Mishra, G.D.; Teede, H.J. The Association of a Mediterranean-Style Diet Pattern with Polycystic Ovary Syndrome Status in a Community Cohort Study. Nutrients 2015, 7, 8553–8564. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106, S1–S78. [Google Scholar] [CrossRef]
- Bahr, L.S.; Franz, K.; Mähler, A. Assessing the (anti)-inflammatory potential of diets. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 402–410. [Google Scholar] [CrossRef]
- Jahns, L.; Conrad, Z.; Johnson, L.K.; Whigham, L.D.; Wu, D.; Claycombe-Larson, K.J. A diet high in carotenoid-rich vegetables and fruits favorably impacts inflammation status by increasing plasma concentrations of IFN-α2 and decreasing MIP-1β and TNF-α in healthy individuals during a controlled feeding trial. Nutr. Res. 2018, 52, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Lapuente, M.; Estruch, R.; Shahbaz, M.; Casas, R. Relation of fruits and vegetables with major cardiometabolic risk factors, markers of oxidation, and inflammation. Nutrients 2019, 11, 2381. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Berthon, B.S.; Saedisomeolia, A.; Starkey, M.R.; Collison, A.; Wark, P.A.; Wood, L.G. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: A systematic literature review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 136–155. [Google Scholar] [CrossRef]
- Lutsey, P.L.; Jacobs, D.R.; Kori, S.; Mayer-Davis, E.; Shea, S.; Steffen, L.M.; Szklo, M.; Tracy, R. Whole grain intake and its cross-sectional association with obesity, insulin resistance, inflammation, diabetes and subclinical CVD: The MESA Study. Br. J. Nutr. 2007, 98, 397–405. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, Q.; Feng, J.; Du, L.; Li, K.; Zhou, Y. Whole grain diet reduces systemic inflammation: A meta-analysis of 9 randomized trials. Medicine 2018, 97, e12995. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.H.M.; Zulet, M.Á.; Abete, I.; Martínez, J.A. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur. J. Nutr. 2011, 50, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Saraf-Bank, S.; Esmaillzadeh, A.; Faghihimani, E.; Azadbakht, L.J.N. Effect of non-soy legume consumption on inflammation and serum adiponectin levels among first-degree relatives of patients with diabetes: A randomized, crossover study. Nutrition 2015, 31, 459–465. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Casas-Agustench, P.; Murphy, M.M.; López-Uriarte, P.; Bulló, M. The effect of nuts on inflammation. Asia Pac. J. Clin. Nutr. 2008, 17, 333–336. [Google Scholar] [PubMed]
- Liu, J.-F.; Liu, Y.-H.; Chen, C.-M.; Chang, W.-H.; Chen, C.O. The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: A randomized crossover controlled feeding trial. Eur. J. Nutr. 2013, 52, 927–935. [Google Scholar] [CrossRef]
- Zampelas, A.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Chrysohoou, C.; Skoumas, Y.; Stefanadis, C. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: The ATTICA study. J. Am. Coll. Cardiol. 2005, 46, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Sonoda, S.; Liu, H. Unprocessed red meat intakes are associated with increased inflammation, triglycerides and HDL cholesterol in past smokers. Nutr. Diet. 2020, 77, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Kengne, A.P.; George, E.S.; Siervo, M. The association of red meat intake with inflammation and circulating intermediate biomarkers of type 2 diabetes is mediated by central adiposity. Br. J. Nutr. 2021, 125, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Sun, Q.; Willett, W.C.; Eliassen, A.H.; Wu, K.; Pan, A.; Grodstein, F.; Hu, F.B. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am. J. Clin. Nutr. 2014, 99, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-T.; Kao, Y.-H.; Sothern, M.S.; Seal, D.W.; Lee, C.-H.; Lin, H.-Y.; Chen, T.; Tseng, T.-S. The association between sugar-sweetened beverages intake, body mass index, and inflammation in US adults. Int. J. Public Health 2020, 65, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Koebnick, C.; Black, M.H.; Wu, J.; Shu, Y.-H.; MacKay, A.W.; Watanabe, R.M.; Buchanan, T.A.; Xiang, A.H. A diet high in sugar-sweetened beverage and low in fruits and vegetables is associated with adiposity and a pro-inflammatory adipokine profile. Br. J. Nutr. 2018, 120, 1230–1239. [Google Scholar] [CrossRef]
- Juul, F.; Vaidean, G.; Parekh, N. Ultra-processed Foods and Cardiovascular Diseases: Potential Mechanisms of Action. Adv. Nutr. 2021, 12, 1673–1680. [Google Scholar] [CrossRef]
- Barrea, L.; Marzullo, P.; Muscogiuri, G.; Di Somma, C.; Scacchi, M.; Orio, F.; Aimaretti, G.; Colao, A.; Savastano, S. Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr. Res. Rev. 2018, 31, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Borst, S.E.; Conover, C.F. High-fat diet induces increased tissue expression of TNF-α. Life Sci. 2005, 77, 2156–2165. [Google Scholar] [CrossRef]
- González, F.; Considine, R.V.; Abdelhadi, O.A.; Acton, A.J. Inflammation triggered by saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2020, 105, 2152–2167. [Google Scholar] [CrossRef] [PubMed]
- González, F.; Considine, R.V.; Abdelhadi, O.A.; Xue, J.; Acton, A.J. Saturated fat ingestion stimulates proatherogenic inflammation in polycystic ovary syndrome. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E689–E701. [Google Scholar] [CrossRef] [PubMed]
- Bendsen, N.T.; Stender, S.; Szecsi, P.B.; Pedersen, S.B.; Basu, S.; Hellgren, L.I.; Newman, J.W.; Larsen, T.M.; Haugaard, S.B.; Astrup, A. Effect of industrially produced trans fat on markers of systemic inflammation: Evidence from a randomized trial in women. J. Lipid Res. 2011, 52, 1821–1828. [Google Scholar] [CrossRef]
- Valenzuela, C.A.; Baker, E.J.; Miles, E.A.; Calder, P.C. Eighteen-carbon trans fatty acids and inflammation in the context of atherosclerosis. Prog. Lipid Res. 2019, 76, 101009. [Google Scholar] [CrossRef] [PubMed]
- Esmaillzadeh, A.; Azadbakht, L. Dairy consumption and circulating levels of inflammatory markers among Iranian women. Public Health Nutr. 2010, 13, 1395–1402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panagiotakos, D.B.; Pitsavos, C.H.; Zampelas, A.D.; Chrysohoou, C.A.; Stefanadis, C.I. Dairy products consumption is associated with decreased levels of inflammatory markers related to cardiovascular disease in apparently healthy adults: The ATTICA study. J. Am. Coll. Nutr. 2010, 29, 357–364. [Google Scholar] [CrossRef]
- Nieman, K.M.; Anderson, B.D.; Cifelli, C.J. The effects of dairy product and dairy protein intake on inflammation: A systematic review of the literature. J. Am. Coll. Nutr. 2020, 4, 1371. [Google Scholar] [CrossRef]
- Almagor, M.; Hazav, A.; Yaffe, H. The levels of C-reactive protein in women treated by IVF. Hum. Reprod 2004, 19, 104–106. [Google Scholar] [CrossRef]
- Sjaarda, L.A.; Radin, R.G.; Silver, R.M.; Mitchell, E.; Mumford, S.L.; Wilcox, B.; Galai, N.; Perkins, N.J.; Wactawski-Wende, J.; Stanford, J.B.; et al. Preconception Low-Dose Aspirin Restores Diminished Pregnancy and Live Birth Rates in Women With Low-Grade Inflammation: A Secondary Analysis of a Randomized Trial. J. Clin. Endocrinol Metab 2017, 102, 1495–1504. [Google Scholar] [CrossRef]
- Weinhold, B. Epigenetics: The science of change. Environ. Health Perspect. 2006, 114, A160–A167. [Google Scholar] [CrossRef]
- Vanden Berghe, W. Epigenetic impact of dietary polyphenols in cancer chemoprevention: Lifelong remodeling of our epigenomes. Pharmacol. Res. 2012, 65, 565–576. [Google Scholar] [CrossRef]
- Gao, L.; Cheng, D.; Yang, J.; Wu, R.; Li, W.; Kong, A.N. Sulforaphane epigenetically demethylates the CpG sites of the miR-9-3 promoter and reactivates miR-9-3 expression in human lung cancer A549 cells. J. Nutr. Biochem. 2018, 56, 109–115. [Google Scholar] [CrossRef]
- Li, G.; Zhang, H.; Ma, H.; Qu, S.; Xing, Q.; Wang, G. MiR-221-5p is involved in the regulation of inflammatory responses in acute gouty arthritis by targeting IL-1beta. Int. J. Rheum. Dis. 2021, 24, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.M.; Kim, T.J.; Choi, J.H.; Kim, M.J.; Cho, Y.N.; Nam, K.I.; Kee, S.J.; Moon, J.B.; Choi, S.Y.; Park, D.J.; et al. MicroRNA-155 as a proinflammatory regulator via SHIP-1 down-regulation in acute gouty arthritis. Arthritis Res. Ther. 2014, 16, R88. [Google Scholar] [CrossRef] [PubMed]
- Claycombe, K.J.; Brissette, C.A.; Ghribi, O. Epigenetics of inflammation, maternal infection, and nutrition. J. Nutr. 2015, 145, 1109S–1115S. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.S. Connecting Female Infertility to Obesity, Inflammation, and Maternal Gut Dysbiosis. Endocrinology 2016, 157, 1725–1727. [Google Scholar] [CrossRef] [PubMed]
- Komiya, S.; Naito, Y.; Okada, H.; Matsuo, Y.; Hirota, K.; Takagi, T.; Mizushima, K.; Inoue, R.; Abe, A.; Morimoto, Y. Characterizing the gut microbiota in females with infertility and preliminary results of a water-soluble dietary fiber intervention study. J. Clin. Biochem. Nutr. 2020, 67, 105–111. [Google Scholar] [CrossRef]
- Sears, B. Anti-inflammatory Diets. J. Am. Coll. Nutr. 2015, 34 (Suppl. S1), 14–21. [Google Scholar] [CrossRef]
- Ricker, M.A.; Haas, W.C. Anti-Inflammatory Diet in Clinical Practice: A Review. Nutr. Clin. Pract. 2017, 32, 318–325. [Google Scholar] [CrossRef]
- Krznaric, Z.; Karas, I.; Ljubas Kelecic, D.; Vranesic Bender, D. The Mediterranean and Nordic Diet: A Review of Differences and Similarities of Two Sustainable, Health-Promoting Dietary Patterns. Front. Nutr. 2021, 8, 683678. [Google Scholar] [CrossRef]
- Lacatusu, C.M.; Grigorescu, E.D.; Floria, M.; Onofriescu, A.; Mihai, B.M. The Mediterranean Diet: From an Environment-Driven Food Culture to an Emerging Medical Prescription. Int. J. Environ. Res. Public Health 2019, 16, 942. [Google Scholar] [CrossRef]
- Cannataro, R.; Caroleo, M.C.; Fazio, A.; La Torre, C.; Plastina, P.; Gallelli, L.; Lauria, G.; Cione, E. Ketogenic Diet and microRNAs Linked to Antioxidant Biochemical Homeostasis. Antioxidants 2019, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- McGrice, M.; Porter, J. The Effect of Low Carbohydrate Diets on Fertility Hormones and Outcomes in Overweight and Obese Women: A Systematic Review. Nutrients 2017, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Couture, P.; Desroches, S.; Lamarche, B. Effect of the Mediterranean diet with and without weight loss on markers of inflammation in men with metabolic syndrome. Obesity 2013, 21, 51–57. [Google Scholar] [CrossRef]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F. Effect of Mediterranean diet enriched in high quality extra virgin olive oil on oxidative stress, inflammation and gut microbiota in obese and normal weight adult subjects. Front. Pharmacol. 2019, 10, 1366. [Google Scholar] [CrossRef]
- Sureda, A.; Bibiloni, M.D.M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J.A. Adherence to the mediterranean diet and inflammatory markers. Nutrients 2018, 10, 62. [Google Scholar] [CrossRef]
- Billingsley, H.E.; Carbone, S. The antioxidant potential of the Mediterranean diet in patients at high cardiovascular risk: An in-depth review of the PREDIMED. Nutr. Diabetes 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Mantzioris, E.; Muhlhausler, B.S.; Villani, A. Impact of the Mediterranean Dietary pattern on n-3 fatty acid tissue levels-A systematic review. Prostaglandins Leukot Essent Fat. Acids 2022, 176, 102387. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Aguilera, C.M.; Mesa, M.D.; Gil, A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: A systematic review of randomised clinical trials. Br. J. Nutr. 2012, 107 (Suppl. S2), S159–S170. [Google Scholar] [CrossRef]
- Natto, Z.S.; Yaghmoor, W.; Alshaeri, H.K.; Van Dyke, T.E. Omega-3 Fatty Acids Effects on Inflammatory Biomarkers and Lipid Profiles among Diabetic and Cardiovascular Disease Patients: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 18867. [Google Scholar] [CrossRef]
- Yu, J.; Liu, L.; Zhang, Y.; Wei, J.; Yang, F. Effects of omega-3 fatty acids on patients undergoing surgery for gastrointestinal malignancy: A systematic review and meta-analysis. BMC Cancer 2017, 17, 271. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Gil, A. Omega 3 fatty acids in cardiovascular disease risk factors: An updated systematic review of randomised clinical trials. Clin. Nutr. 2018, 37, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S.; Tuñón, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Lowe, G.D.; Rumley, A.; Bruckdorfer, K.R.; Whincup, P.H. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am. J. Clin. Nutr. 2006, 83, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Upritchard, J.E.; Sutherland, W.; Mann, J.I. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care 2000, 23, 733–738. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- Cannataro, R.; Fazio, A.; La Torre, C.; Caroleo, M.C.; Cione, E. Polyphenols in the Mediterranean Diet: From Dietary Sources to microRNA Modulation. Antioxidants 2021, 10, 328. [Google Scholar] [CrossRef]
- Elce, A.; Amato, F.; Zarrilli, F.; Calignano, A.; Troncone, R.; Castaldo, G.; Canani, R. Butyrate modulating effects on pro-inflammatory pathways in human intestinal epithelial cells. Benef. Microbes 2017, 8, 841–847. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- McLoughlin, R.F.; Berthon, B.S.; Jensen, M.E.; Baines, K.J.; Wood, L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 930–945. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Lorente-Cebrian, S.; Costa, A.G.; Navas-Carretero, S.; Zabala, M.; Laiglesia, L.M.; Martinez, J.A.; Moreno-Aliaga, M.J. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J. Physiol. Biochem. 2015, 71, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Albers, R.; Antoine, J.M.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.A.; Folkerts, G.; Friedmann, P.S.; Frost, G.S.; Guarner, F.; et al. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009, 101 (Suppl. S1), S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Schulze, M.B.; Fung, T.T.; Meigs, J.B.; Rifai, N.; Manson, J.E.; Hu, F.B. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2004, 80, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Hankinson, S.E.; Hotamisligil, G.S.; Rifai, N.; Willett, W.C.; Rimm, E.B. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation 2003, 108, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Das, S.K.; Pieper, C.F.; Lewis, M.R.; Klein, S.; Dixit, V.D.; Gupta, A.K.; Villareal, D.T.; Bhapkar, M.; Huang, M.; et al. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: A randomized controlled trial in non-obese humans. Aging 2016, 8, 1416–1431. [Google Scholar] [CrossRef]
- Becker, G.F.; Passos, E.P.; Moulin, C.C. Short-term effects of a hypocaloric diet with low glycemic index and low glycemic load on body adiposity, metabolic variables, ghrelin, leptin, and pregnancy rate in overweight and obese infertile women: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.J.; Torres, S.J.; McNaughton, S.A.; Milte, C.M. Dietary patterns and associations with biomarkers of inflammation in adults: A systematic review of observational studies. Nutr. J. 2021, 20, 24. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Andreeva, V.A.; Kesse-Guyot, E.; Hercberg, S. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab. 2013, 39, 99–110. [Google Scholar] [CrossRef]
- Kopp, W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Guldan, G.S. Asian children’s obesogenic diets-time to change this part of the energy balance equation? Res. Sports Med. 2010, 18, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sobotka, T. Un tiers des femmes d‘Asie de l’Est resteront sans enfant. Popul. Sociétés 2021, 595, 1–4. [Google Scholar] [CrossRef]
- Anderson, T.; Kohler, H.P. Education Fever and the East Asian Fertility Puzzle: A case study of low fertility in South Korea. Asian Popul. Stud. 2013, 9, 196–215. [Google Scholar] [CrossRef] [PubMed]
- Patry, R.T.; Nagler, C.R. Fiber-poor Western diets fuel inflammation. Nat. Immunol. 2021, 22, 266–268. [Google Scholar] [CrossRef]
- Galland, L. Diet and inflammation. Nutr. Clin. Prac. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Norde, M.M.; Collese, T.S.; Giovannucci, E.; Rogero, M.M. A posteriori dietary patterns and their association with systemic low-grade inflammation in adults: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 331–350. [Google Scholar] [CrossRef]
- Han, M.S.; White, A.; Perry, R.J.; Camporez, J.P.; Hidalgo, J.; Shulman, G.I.; Davis, R.J. Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl. Acad. Sci. USA 2020, 117, 2751–2760. [Google Scholar] [CrossRef]
- Luger, M.; Lafontan, M.; Bes-Rastrollo, M.; Winzer, E.; Yumuk, V.; Farpour-Lambert, N. Sugar-sweetened beverages and weight gain in children and adults: A systematic review from 2013 to 2015 and a comparison with previous studies. Obes. Facts 2017, 10, 674–693. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Chavarro, J.E. Diet and fertility: A review. Am. J. Obstet. Gynecol. 2018, 218, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Tsagareli, V.; Noakes, M.; Norman, R. Altered preconception fatty acid intake is associated with improved pregnancy rates in overweight and obese women undertaking in vitro fertilisation. Nutrients 2016, 8, 10. [Google Scholar] [CrossRef]
- Firns, S.; Cruzat, V.F.; Keane, K.N.; Joesbury, K.A.; Lee, A.H.; Newsholme, P.; Yovich, J.L. The effect of cigarette smoking, alcohol consumption and fruit and vegetable consumption on IVF outcomes: A review and presentation of original data. Reprod. Biol. Endocrinol. 2015, 13, 1–13. [Google Scholar] [CrossRef]
- Braga, D.P.A.F.; Halpern, G.; Setti, A.S.; Figueira, R.C.S.; Iaconelli, A., Jr.; Borges, E., Jr. The impact of food intake and social habits on embryo quality and the likelihood of blastocyst formation. Reprod. Biomed. Online 2015, 31, 30–38. [Google Scholar] [CrossRef]
- Garruti, G.; Depalo, R.; De Angelis, M. Weighing the impact of diet and lifestyle on female reproductive function. Curr. Med. Chem. 2019, 26, 3584–3592. [Google Scholar] [CrossRef]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Mastrominas, M.; Yiannakouris, N. Adherence to the Mediterranean diet and IVF success rate among non-obese women attempting fertility. Hum. Reprod. 2018, 33, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Kiddy, D.S.; Hamilton-Fairley, D.; Bush, A.; Short, F.; Anyaoku, V.; Reed, M.J.; Franks, S. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin. Endocrinol. 1992, 36, 105–111. [Google Scholar] [CrossRef]
- Gower, B.A.; Chandler-Laney, P.C.; Ovalle, F.; Goree, L.L.; Azziz, R.; Desmond, R.A.; Granger, W.M.; Goss, A.M.; Bates, G.W. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clin. Endocrinol. 2013, 79, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Katsikis, I.; Kita, M.; Karkanaki, A.; Prapas, N.; Panidis, D. Anovulation and ovulation induction. Hippokratia 2006, 10, 120–127. [Google Scholar]
- Onieva-Zafra, M.D.; Fernandez-Martinez, E.; Abreu-Sanchez, A.; Iglesias-Lopez, M.T.; Garcia-Padilla, F.M.; Pedregal-Gonzalez, M.; Parra-Fernandez, M.L. Relationship between Diet, Menstrual Pain and other Menstrual Characteristics among Spanish Students. Nutrients 2020, 12, 1759. [Google Scholar] [CrossRef]
- Bajalan, Z.; Alimoradi, Z.; Moafi, F. Nutrition as a Potential Factor of Primary Dysmenorrhea: A Systematic Review of Observational Studies. Gynecol. Obstet. Investig. 2019, 84, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Barcikowska, Z.; Rajkowska-Labon, E.; Grzybowska, M.E.; Hansdorfer-Korzon, R.; Zorena, K. Inflammatory Markers in Dysmenorrhea and Therapeutic Options. Int. J. Environ. Res. Public Health 2020, 17, 1191. [Google Scholar] [CrossRef] [PubMed]
- Djuric, Z.; Turgeon, D.K.; Sen, A.; Ren, J.; Herman, K.; Ramaswamy, D.; Zhao, L.; Ruffin, M.T.t.; Normolle, D.P.; Smith, W.L.; et al. The Anti-inflammatory Effect of Personalized Omega-3 Fatty Acid Dosing for Reducing Prostaglandin E2 in the Colonic Mucosa Is Attenuated in Obesity. Cancer Prev. Res. 2017, 10, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Mantzioris, E.; James, M.J.; Gibson, R.A.; Cleland, L.G. Dietary substitution with an alpha-linolenic acid-rich vegetable oil increases eicosapentaenoic acid concentrations in tissues. Am. J. Clin. Nutr. 1994, 59, 1304–1309. [Google Scholar] [CrossRef]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef]
- Mohammed Rasheed, H.A.; Hamid, P. Inflammation to Infertility: Panoramic View on Endometriosis. Cureus 2020, 12, e11516. [Google Scholar] [CrossRef]
- Nirgianakis, K.; Egger, K.; Kalaitzopoulos, D.R.; Lanz, S.; Bally, L.; Mueller, M.D. Effectiveness of Dietary Interventions in the Treatment of Endometriosis: A Systematic Review. Reprod. Sci. 2022, 29, 26–42. [Google Scholar] [CrossRef]
- Saguyod, S.K.A.; Velarde, M.C. Diet and endometriosis-revisiting the linkages to inflammation. J. Endometr. Pelvic Pain Disord. 2018, 10, 51–58. [Google Scholar] [CrossRef]
- Chiang, Y.F.; Hung, H.C.; Chen, H.Y.; Huang, K.C.; Lin, P.H.; Chang, J.Y.; Huang, T.C.; Hsia, S.M. The Inhibitory Effect of Extra Virgin Olive Oil and Its Active Compound Oleocanthal on Prostaglandin-Induced Uterine Hypercontraction and Pain-Ex Vivo and In Vivo Study. Nutrients 2020, 12, 3012. [Google Scholar] [CrossRef]
- Parkinson, L.; Keast, R. Oleocanthal, a phenolic derived from virgin olive oil: A review of the beneficial effects on inflammatory disease. Int. J. Mol. Sci. 2014, 15, 12323–12334. [Google Scholar] [CrossRef]
- Mier-Cabrera, J.; Aburto-Soto, T.; Burrola-Mendez, S.; Jimenez-Zamudio, L.; Tolentino, M.C.; Casanueva, E.; Hernandez-Guerrero, C. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod. Biol. Endocrinol. 2009, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Mier-Cabrera, J.; Genera-Garcia, M.; De la Jara-Diaz, J.; Perichart-Perera, O.; Vadillo-Ortega, F.; Hernandez-Guerrero, C. Effect of vitamins C and E supplementation on peripheral oxidative stress markers and pregnancy rate in women with endometriosis. Int. J. Gynaecol. Obstet. 2008, 100, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.; Misso, M.; Balen, A.; Boyle, J.; Devoto, L.; Garad, R.; Hart, R.; Johnson, L.; Jordan, C.; Legro, R. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: Assessment and treatment of infertility. Hum. Reprod. Open 2019, 2019, hoy021. [Google Scholar] [CrossRef] [PubMed]
- Boulman, N.; Levy, Y.; Leiba, R.; Shachar, S.; Linn, R.; Zinder, O.; Blumenfeld, Z. Increased C-reactive protein levels in the polycystic ovary syndrome: A marker of cardiovascular disease. J. Clin. Endocrinol. Metab. 2004, 89, 2160–2165. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.; Davidson, C.R.; Billings, D.L. Dietary intake, eating behaviors, and quality of life in women with polycystic ovary syndrome who are trying to conceive. Hum. Fertil. 2015, 18, 16–21. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. A prospective study of dietary carbohydrate quantity and quality in relation to risk of ovulatory infertility. Eur. J. Clin. Nutr. 2009, 63, 78–86. [Google Scholar] [CrossRef]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef]
- Salama, A.A.; Amine, E.; Hesham, A.; Abd El-Fatteh, N. Effects of anti-inflammatory diet in the context of lifestyle modification (with or without metformin use) on metabolic, endocrine, inflammatory and reproductive profiles in overweight and obese women with polycystic ovary syndrome: Controlled clinical trial. Can. J. Clin. Nutr. 2018, 6, 81–106. [Google Scholar]
- Glueck, C.J.; Goldenberg, N.; Pranikoff, J.; Khan, Z.; Padda, J.; Wang, P. Effects of metformin-diet intervention before and throughout pregnancy on obstetric and neonatal outcomes in patients with polycystic ovary syndrome. Curr. Med. Res. Opin. 2013, 29, 55–62. [Google Scholar] [CrossRef]
- Lim, S.S.; Hutchison, S.K.; Van Ryswyk, E.; Norman, R.J.; Teede, H.J.; Moran, L.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019, 3, CD007506. [Google Scholar] [CrossRef]
- Hoek, J.; Schoenmakers, S.; Baart, E.B.; Koster, M.P.; Willemsen, S.P.; van Marion, E.S.; Steegers, E.A.; Laven, J.S.; Steegers-Theunissen, R. Preconceptional Maternal Vegetable Intake and Paternal Smoking Are Associated with Pre-implantation Embryo Quality. Reprod. Sci. 2020, 27, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, L.; Lin, J.; Lu, X.; Song, N.; Cai, R.; Kuang, Y. Adherence to healthy dietary patterns and outcomes of assisted reproduction: A systematic review and meta-analysis. Int. J. Food Sci. Nutr. 2021, 72, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Kermack, A.J.; Calder, P.C.; Houghton, F.D.; Godfrey, K.M.; Macklon, N.S. A randomised controlled trial of a preconceptional dietary intervention in women undergoing IVF treatment (PREPARE trial). BMC Womens Health 2014, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Kermack, A.J.; Lowen, P.; Wellstead, S.J.; Fisk, H.L.; Montag, M.; Cheong, Y.; Osmond, C.; Houghton, F.D.; Calder, P.C.; Macklon, N.S.; et al. Effect of a 6-week “Mediterranean” dietary intervention on in vitro human embryo development: The Preconception Dietary Supplements in Assisted Reproduction double-blinded randomized controlled trial. Fertil. Steril. 2020, 113, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Diba-Bagtash, F.; Shahnazi, M.; Ghasemzadeh, A.; Jahanjoo, F.; Dolatkhah, N.; Farshbaf-Khalili, A. Association between dietary inflammatory index and inflammatory biomarkers with outcomes of in vitro fertilization treatment. J. Obstet. Gynaecol. Res. 2021, 47, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Sanderman, E.A.; Willis, S.K.; Wise, L.A. Female dietary patterns and outcomes of in vitro fertilization (IVF): A systematic literature review. Nutr. J. 2022, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Nassan, F.L.; Chiu, Y.-H.; Arvizu, M.; Williams, P.L.; Keller, M.G.; Souter, I.; Hauser, R.; Chavarro, J.E.; Team, E.S. Dietary patterns and outcomes of assisted reproduction. Am. J. Obstet. Gynecol. 2019, 220, 567.e1–567.e18. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lin, Y.; Lin, D.; Zou, C.; Zou, X.; Fu, L.; Meng, F.; Qian, W. Mediterranean diet improves embryo yield in IVF: A prospective cohort study. Reprod. Biol. Endocrinol. 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Ricci, E.; Bravi, F.; Noli, S.; Somigliana, E.; Cipriani, S.; Castiglioni, M.; Chiaffarino, F.; Vignali, M.; Gallotti, B.; Parazzini, F. Mediterranean diet and outcomes of assisted reproduction: An Italian cohort study. Am. J. Obstet. Gynecol. 2019, 221, 627.e1–627.e14. [Google Scholar] [CrossRef]
- Oostingh, E.C.; de Vos, I.; Ham, A.C.; Brouwer-Brolsma, E.M.; Willemsen, S.P.; Eggink, A.J.; Steegers, E.A.; Steegers-Theunissen, R. No independent associations between preconception paternal dietary patterns and embryonic growth; the Predict Study. Clin. Nutr. 2019, 38, 2333–2341. [Google Scholar] [PubMed]
- Wong, W.Y.; Thomas, C.M.; Merkus, J.M.; Zielhuis, G.A.; Steegers-Theunissen, R. Male factor subfertility: Possible causes and the impact of nutritional factors. Fertil. Steril. 2000, 73, 435–442. [Google Scholar] [CrossRef]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical relevance of oxidative stress in male factor infertility: An update. Am. J. Reprod. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, A. The Western-style diet: A major risk factor for impaired kidney function and chronic kidney disease. Am. J. Physiol. Renal Physiol. 2011, 301, F919–F931. [Google Scholar] [CrossRef]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Douka, L.; Mastrominas, M.; Yiannakouris, N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum. Reprod. 2017, 32, 215–222. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Babio, N.; Carrell, D.T.; Bulló, M.; Salas-Salvadó, J. Adherence to the Mediterranean diet is positively associated with sperm motility: A cross-sectional analysis. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Ricci, E.; Bravi, F.; Noli, S.; Ferrari, S.; De Cosmi, V.; La Vecchia, I.; Cavadini, M.; La Vecchia, C.; Parazzini, F. Mediterranean diet and the risk of poor semen quality: Cross-sectional analysis of men referring to an Italian Fertility Clinic. Andrology 2019, 7, 156–162. [Google Scholar] [CrossRef]
- Efrat, M.; Stein, A.; Pinkas, H.; Unger, R.; Birk, R. Dietary patterns are positively associated with semen quality. Fertil. Steril. 2018, 109, 809–816. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Rosique-Esteban, N.; Becerra-Tomás, N.; Vizmanos, B.; Bulló, M.; Salas-Salvadó, J. The effect of nutrients and dietary supplements on sperm quality parameters: A systematic review and meta-analysis of randomized clinical trials. Adv. Nutr. 2018, 9, 833–848. [Google Scholar] [CrossRef]
- Alahmar, A.T. The impact of two doses of coenzyme Q10 on semen parameters and antioxidant status in men with idiopathic oligoasthenoteratozoospermia. Clin. Exp. Reprod. Med. 2019, 46, 112–118. [Google Scholar] [CrossRef]
- Safarinejad, M.R. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J. Urol. 2009, 182, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R.; Safarinejad, S. The roles of omega-3 and omega-6 fatty acids in idiopathic male infertility. Asian J. Androl. 2012, 14, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. 2017, 23, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Nassan, F.L.; Jensen, T.K.; Priskorn, L.; Halldorsson, T.I.; Chavarro, J.E.; Jorgensen, N. Association of Dietary Patterns With Testicular Function in Young Danish Men. JAMA Netw. Open 2020, 3, e1921610. [Google Scholar] [CrossRef]
- Haeri, F.; Pourmasoumi, M.; Ghiasvand, R.; Feizi, A.; Salehi-Abargouei, A.; Marvast, L.D.; Clark, C.C.T.; Mirzaei, M. The relationship between major dietary patterns and fertility status in iranian men: A case-control study. Sci. Rep. 2021, 11, 18861. [Google Scholar] [CrossRef]
- Adoamnei, E.; Cutillas-Tolin, A.; Mendiola, J.; Lopez-Espin, J.J.; Shivappa, N.; Vioque, J.; Hebert, J.R.; Torres-Cantero, A.M. Associations between dietary inflammatory index and male reproductive parameters. Rev. Int. De Androl. 2018, 17, 79–87. [Google Scholar]
- Liu, F.-H.; Wang, X.-B.; Wen, Z.-Y.; Wang, H.-Y.; Zhang, M.; Zhang, S.; Jiang, Y.-T.; Zhang, J.-Y.; Sun, H.; Pan, B.-C. Dietary Inflammatory Index and Risk of Asthenozoospermia: A Hospital-Based Case-Controlled Study in China. Front. Nutr. 2021, 8, 706869. [Google Scholar] [CrossRef]
- Hutchins-Wiese, H.L.; Bales, C.W.; Starr, K.N.P. Mediterranean diet scoring systems: Understanding the evolution and applications for Mediterranean and non-Mediterranean countries. Br. J. Nutr. 2021, 1–22. [Google Scholar] [CrossRef]
- Martínez-González, M.Á.; Hershey, M.S.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean diet to non-Mediterranean countries. What is and what is not the Mediterranean diet. Nutrients 2017, 9, 1226. [Google Scholar] [CrossRef]
- Villani, A.; Sultana, J.; Doecke, J.; Mantzioris, E. Differences in the interpretation of a modernized Mediterranean diet prescribed in intervention studies for the management of type 2 diabetes: How closely does this align with a traditional Mediterranean diet? Eur. J. Nutr. 2019, 58, 1369–1380. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean diet; a literature review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Hsu, M.; Krajbich, I.M.; Loewenstein, G.; McClure, S.M.; Wang, J.T.-Y.; Camerer, C.F. The wick in the candle of learning: Epistemic curiosity activates reward circuitry and enhances memory. Psychol. Sci. 2009, 20, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Schomaker, J.; Meeter, M. Short-and long-lasting consequences of novelty, deviance and surprise on brain and cognition. Neurosci. Biobehav. Rev. 2015, 55, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.; Wyness, L. ‘Don’t tell me what to eat!’–W ays to engage the population in positive behaviour change. Nutr. Bull. 2013, 38, 23–29. [Google Scholar] [CrossRef]
- Boylan, S.; Louie, J.C.Y.; Gill, T.P. Consumer response to healthy eating, physical activity and weight-related recommendations: A systematic review. Obes. Rev. 2012, 13, 606–617. [Google Scholar] [CrossRef]
- ASI. Britons Say No to Nanny! Modern Attitudes to Paternalism and State Provision; ASI: Fremont, CA, USA, 2012. [Google Scholar]
- Patterson, R.E.; Satia, J.A.; Kristal, A.R.; Neuhouser, M.L.; Drewnowski, A. Is there a consumer backlash against the diet and health message? J. Am. Diet. Assoc. 2001, 101, 37–41. [Google Scholar] [CrossRef]
- Penders, B. Why public dismissal of nutrition science makes sense: Post-truth, public accountability and dietary credibility. Br. Food J. 2018, 120, 1953–1964. [Google Scholar] [CrossRef]
- Eyles, H.C.; Mhurchu, C.N. Does tailoring make a difference? A systematic review of the long-term effectiveness of tailored nutrition education for adults. Nutr. Rev. 2009, 67, 464–480. [Google Scholar] [CrossRef]
- Cowan, S.; Sood, S.; Truby, H.; Dordevic, A.; Adamski, M.; Gibson, S. Inflaming Public Interest: A qualitative study of adult learners’ perceptions on nutrition and inflammation. Nutrients 2020, 12, 345. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. National Health Survey: Health Literacy; Australian Bureau of Statistics: Canberra, Australia, 2018.
- Wansink, B. Position of the American Dietetic Association: Food and nutrition misinformation. J. Am. Diet. Assoc. 2005, 106, 601–607. [Google Scholar]
| Diet | Eat/Drink Often | Eat/Drink in Moderation | Eat/Drink Rarely | Do Not Eat/Drink |
|---|---|---|---|---|
| Mediterranean | Vegetables, fruits, cereals and grains, nuts, seeds, low-fat, dairy, olive oil, and low-fat dairy | White (fish or chicken) and red meat, eggs, potatoes, and wine | High-fat foods or high-sugar feeds | |
| Nordic | Vegetables, fruits, whole grains, nuts, seeds, low-fat dairy, canola/rapeseed oil, low-fat dairy, potatoes, and fish and seafood | Game meats (bison, antelope, etc.), eggs, cheese, and yoghurt | Red meat | Processed or refined foods, added sugars (including sugar-sweetened beverages) |
| Okinawan | Vegetables, fruits, soy-based foods (tofu, miso, etc.), and grains | Fish, lean meats, and alcohol | Red meat, dairy, oils, herbs/spices, nuts, seeds, and refined carbohydrates | Processed or refined foods, all added sugars |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alesi, S.; Villani, A.; Mantzioris, E.; Takele, W.W.; Cowan, S.; Moran, L.J.; Mousa, A. Anti-Inflammatory Diets in Fertility: An Evidence Review. Nutrients 2022, 14, 3914. https://doi.org/10.3390/nu14193914
Alesi S, Villani A, Mantzioris E, Takele WW, Cowan S, Moran LJ, Mousa A. Anti-Inflammatory Diets in Fertility: An Evidence Review. Nutrients. 2022; 14(19):3914. https://doi.org/10.3390/nu14193914
Chicago/Turabian StyleAlesi, Simon, Anthony Villani, Evangeline Mantzioris, Wubet Worku Takele, Stephanie Cowan, Lisa J. Moran, and Aya Mousa. 2022. "Anti-Inflammatory Diets in Fertility: An Evidence Review" Nutrients 14, no. 19: 3914. https://doi.org/10.3390/nu14193914
APA StyleAlesi, S., Villani, A., Mantzioris, E., Takele, W. W., Cowan, S., Moran, L. J., & Mousa, A. (2022). Anti-Inflammatory Diets in Fertility: An Evidence Review. Nutrients, 14(19), 3914. https://doi.org/10.3390/nu14193914










