The Positive Association of Plasma Levels of Vitamin C and Inverse Association of VCAM-1 and Total Adiponectin with Bone Mineral Density in Subjects with Diabetes
Abstract
1. Introduction
2. Materials and Methods
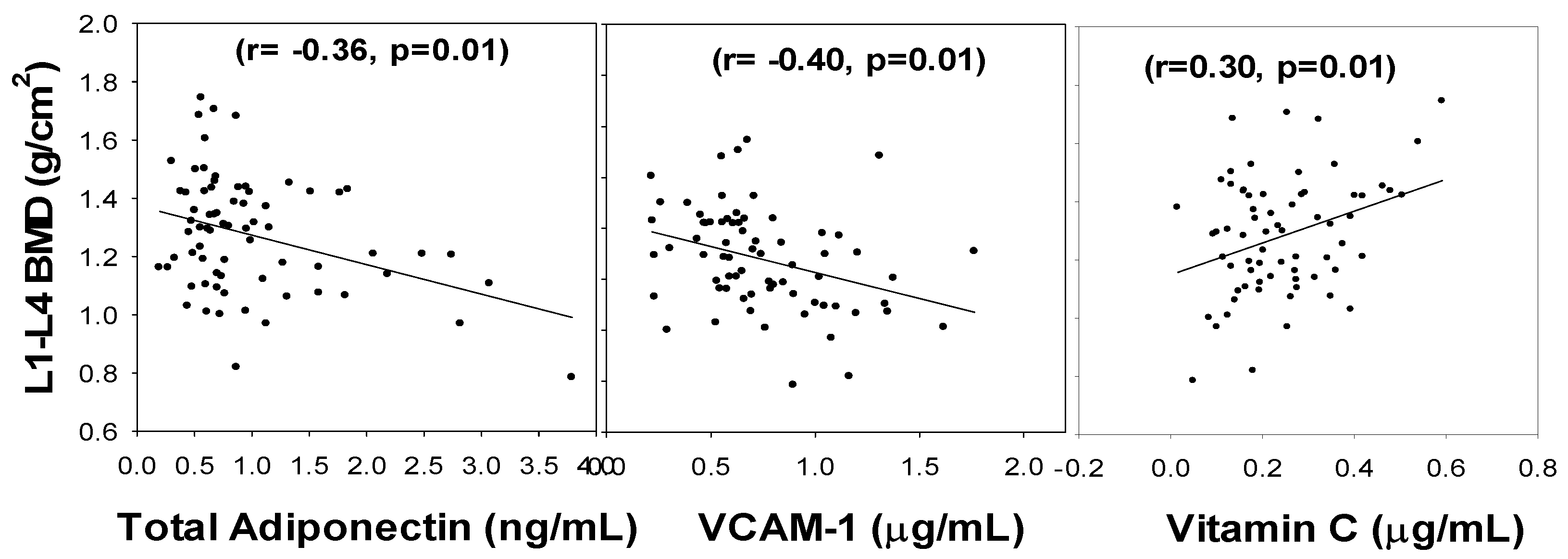
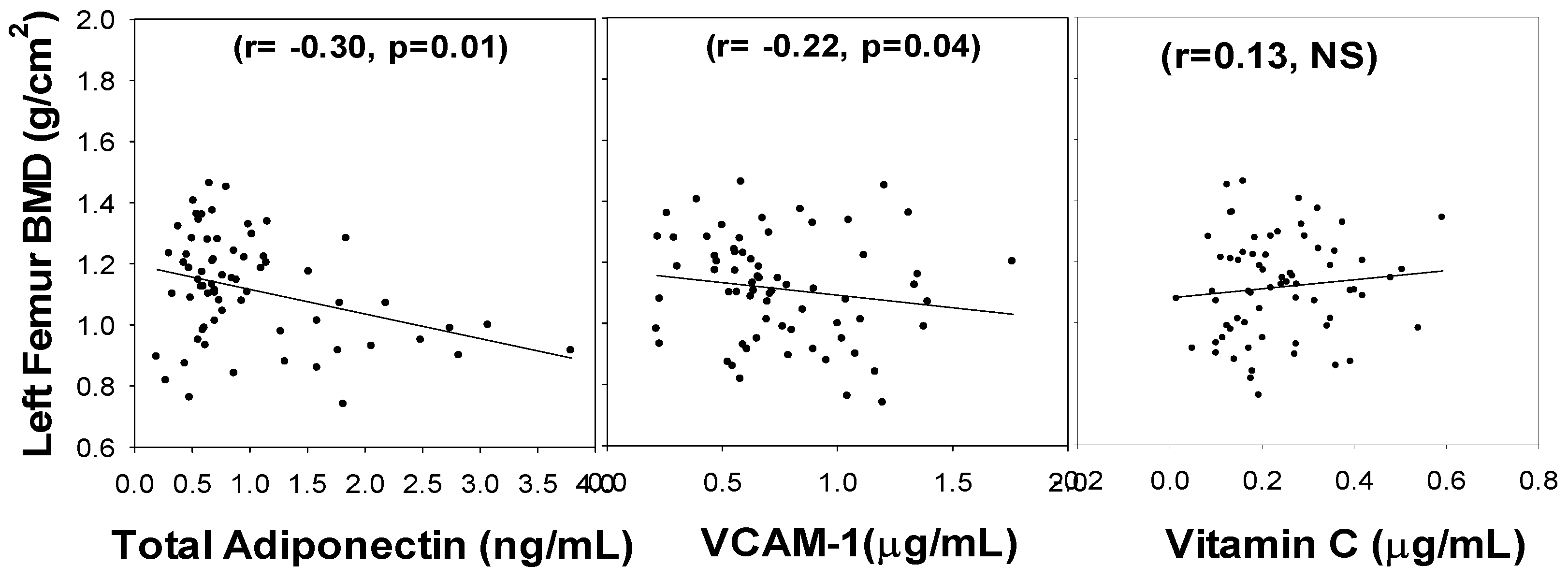
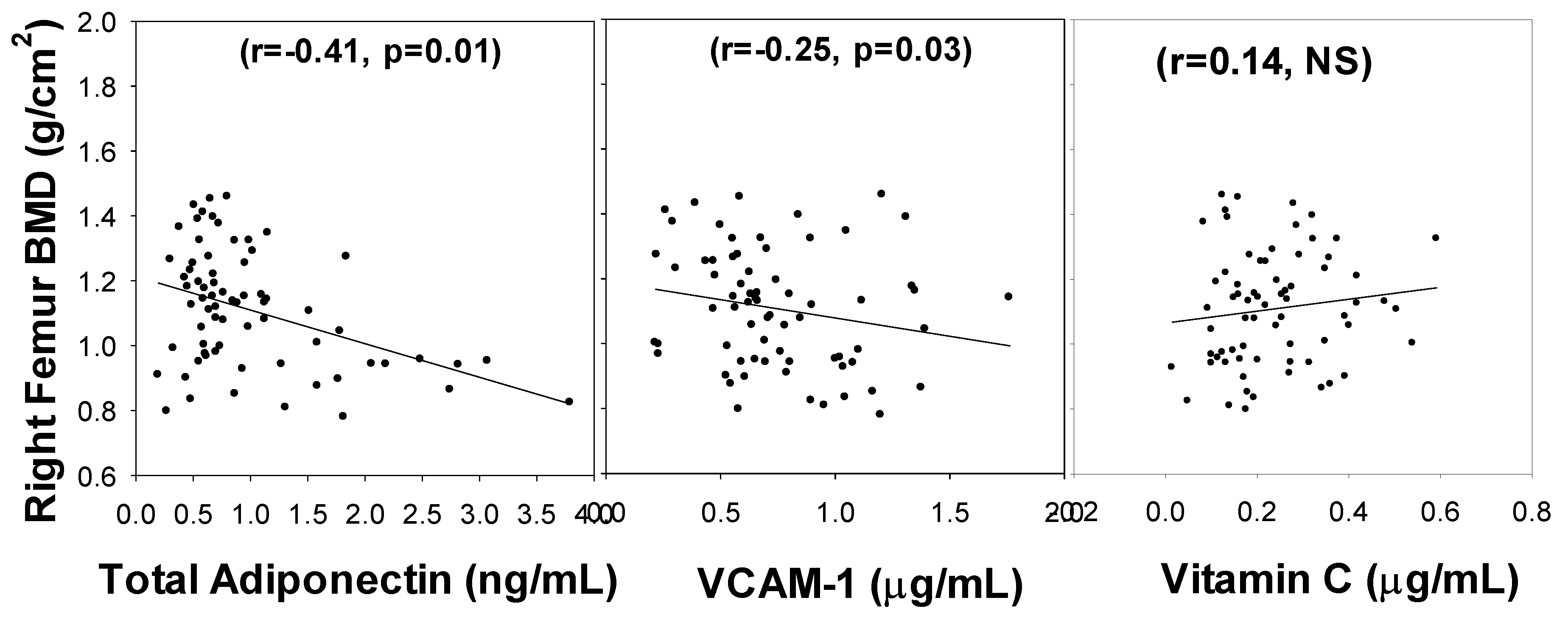
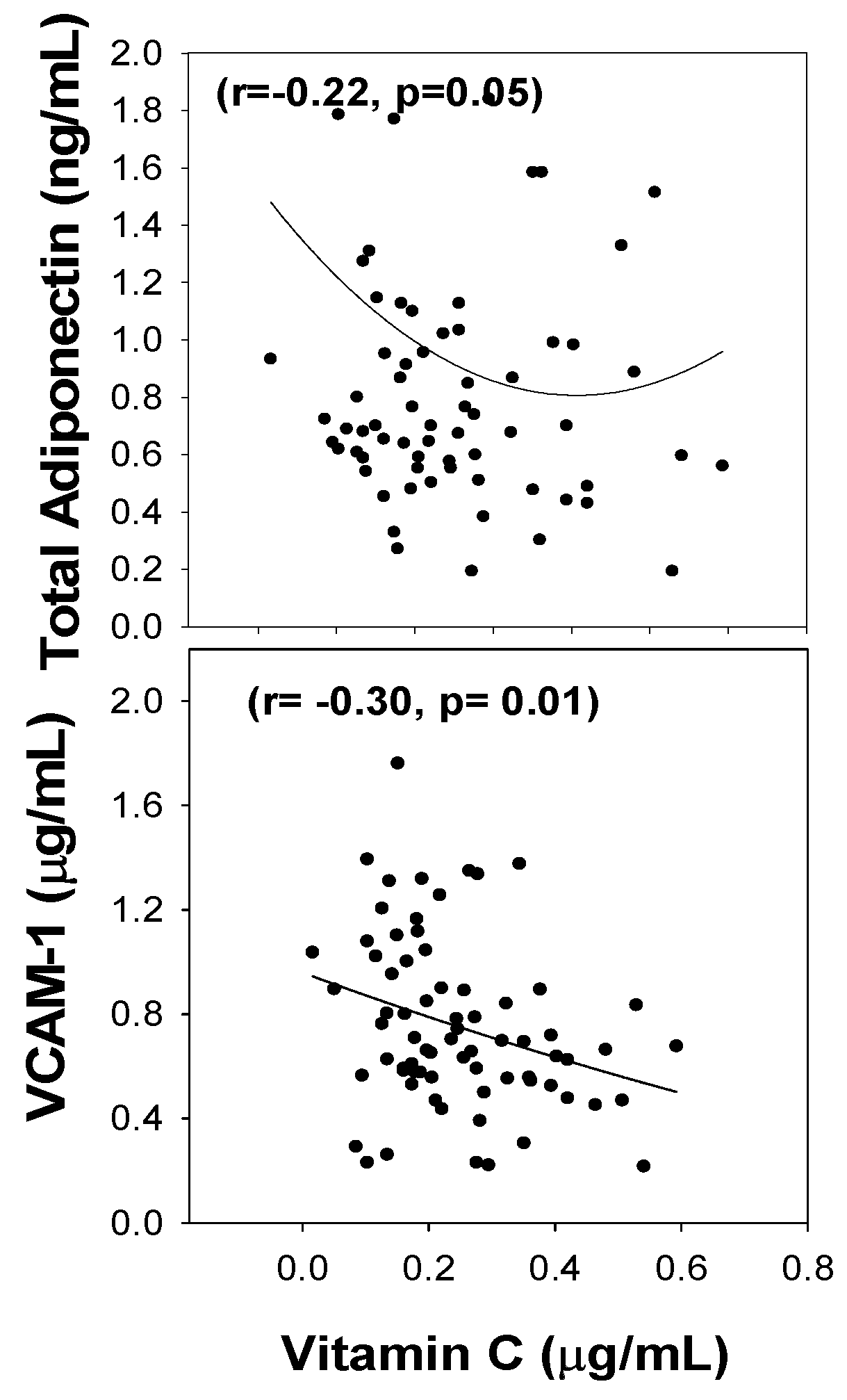
3. Results
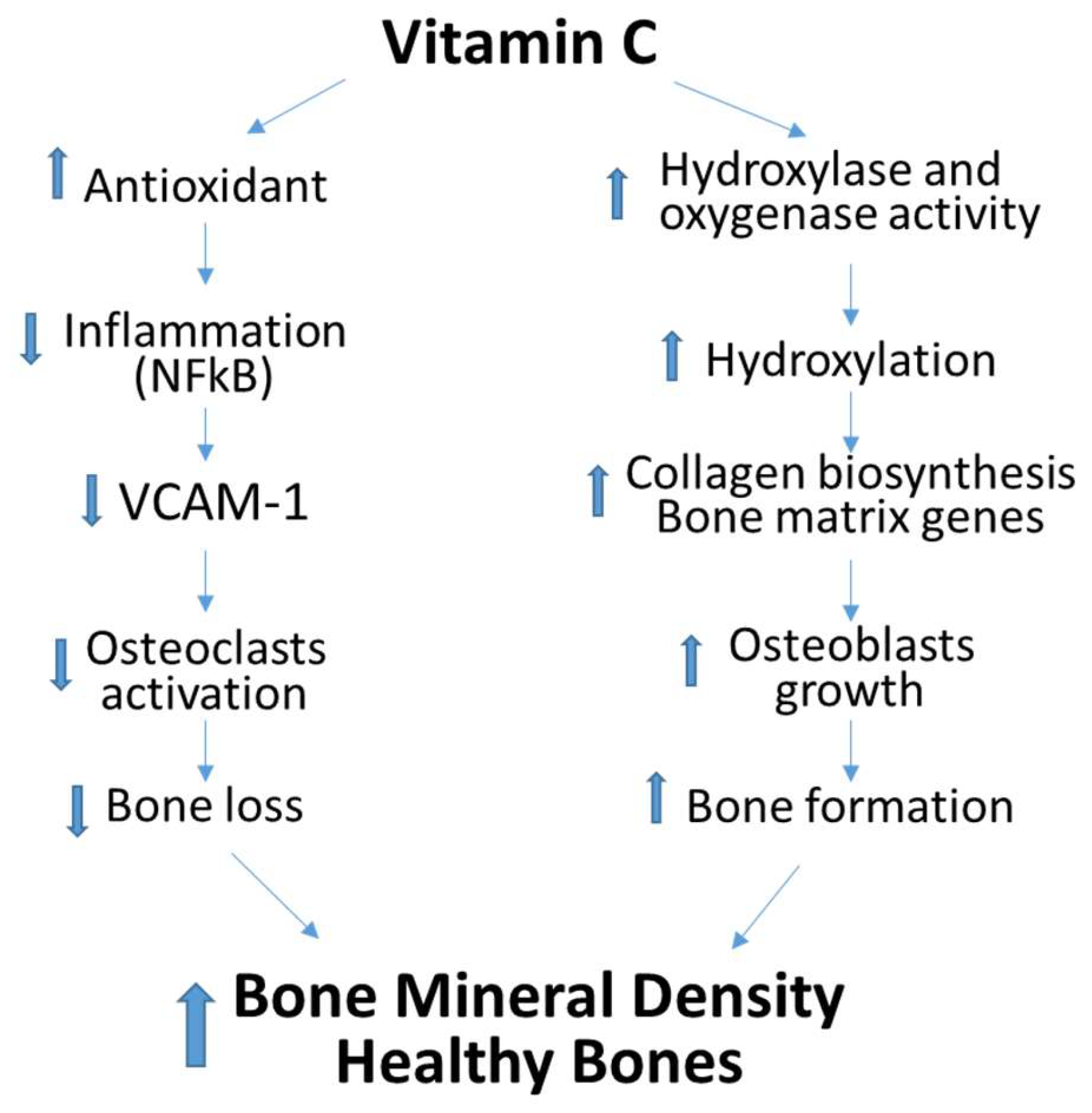
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J.; Mankin, C.J. Metabolic bone disease: A review and update. Instr. Course Lect. 2008, 57, 575–593. [Google Scholar] [PubMed]
- Hofbauer, L.C.; Busse, B.; Eastell, R.; Ferrari, S.; Frost, M.; Müller, R.; Burden, A.M.; Rivadeneira, F.; Napoli, N.; Rauner, M. Bone fragility in diabetes: Novel concepts and clinical implications. Lancet Diabetes Endocrinol. 2022, 10, 207–220. [Google Scholar] [CrossRef]
- Epstein, S.; Defeudis, G.; Manfrini, S.; Napoli, N.; Pozzilli, P. Diabetes and disordered bone metabolism (diabetic osteodystrophy): Time for recognition. Osteoporos. Int. 2016, 27, 1931–1951. [Google Scholar] [CrossRef] [PubMed]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, P.; Hall, S.; Wongworawat, M.D.; Mohan, S. The Roles and Mechanisms of Actions of Vitamin C in Bone: New Developments. J. Bone Miner. Res. 2015, 30, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Li, M.; Hara, K.; Sasaki, M.; Tabata, C.; de Freitas, P.H.L.; Hongo, H.; Suzuki, R.; Kobayashi, M.; Inoue, K.; et al. Morphological assessment of bone mineralization in tibial metaphyses of ascorbic acid-deficient ODS rats. Biomed. Res. 2011, 32, 259–269. [Google Scholar] [CrossRef]
- Sugimoto, T.; Nakada, M.; Fukase, M.; Imai, Y.; Kinoshita, Y.; Fujita, T. Effects of ascorbic acid on alkaline phosphatase activity and hormone responsiveness in the osteoblastic osteosarcoma cell line UMR-106. Calcif. Tissue Int. 1986, 39, 171–174. [Google Scholar] [CrossRef]
- Ratajczak, A.; Szymczak-Tomczak, A.; Skrzypczak-Zielińska, M.; Rychter, A.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Vitamin C Deficiency and the Risk of Osteoporosis in Patients with an Inflammatory Bowel Disease. Nutrients 2020, 12, 2263. [Google Scholar] [CrossRef]
- Nguyen, V.H. School-based nutrition interventions can improve bone health in children and adolescents. Osteoporos. Sarcopenia 2021, 7, 1–5. [Google Scholar] [CrossRef]
- Zeng, L.-F.; Luo, M.-H.; Liang, G.-H.; Yang, W.-Y.; Xiao, X.; Wei, X.; Yu, J.; Guo, D.; Chen, H.-Y.; Pan, J.-K.; et al. Can Dietary Intake of Vitamin C-Oriented Foods Reduce the Risk of Osteoporosis, Fracture, and BMD Loss? Systematic Review with Meta-Analyses of Recent Studies. Front. Endocrinol. 2019, 10, 844. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Han, A.; Park, Y. Association of Dietary Total Antioxidant Capacity with Bone Mass and Osteoporosis Risk in Korean Women: Analysis of the Korea National Health and Nutrition Examination Survey 2008–2011. Nutrients 2021, 13, 1149. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Shab-Bidar, S.; Djafarian, K. Vitamin C intake in relation to bone mineral density and risk of hip fracture and osteoporosis: A systematic review and meta-analysis of observational studies. Br. J. Nutr. 2018, 119, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Peroni, G.; Fossari, F.; Vecchio, V.; Faliva, M.; Naso, M.; Perna, S.; Di Paolo, E.; Riva, A.; Petrangolini, G.; et al. Evidence of a Positive Link between Consumption and Supplementation of Ascorbic Acid and Bone Mineral Density. Nutrients 2021, 13, 1012. [Google Scholar] [CrossRef]
- Falch, J.A.; Mowe, M.; Bohmer, T. Low levels of serum ascorbic acid in elderly patients with hip fracture. Scand. J. Clin. Lab. Investig. 1998, 58, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Hannan, M.T.; Gagnon, D.; Blumberg, J.; Cupples, L.A.; Kiel, D.P.; Tucker, K.L. Protective effect of total and supplemental vitamin C intake on the risk of hip fracture—A 17-year follow-up from the Framingham Osteoporosis Study. Osteoporos. Int. 2009, 20, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Park, K.-A.; Ito, M.; Ikeda, K.; Takeshita, S. Osteoclast-derived complement component 3a stimulates osteoblast differentiation. J. Bone Miner. Res. 2014, 29, 1522–1530. [Google Scholar] [CrossRef]
- Yokota, K.; Sato, K.; Miyazaki, T.; Aizaki, Y.; Tanaka, S.; Sekikawa, M.; Kozu, N.; Kadono, Y.; Oda, H.; Mimura, T. Characterization and Function of Tumor Necrosis Factor and Interleukin-6-Induced Osteoclasts in Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1145–1154. [Google Scholar] [CrossRef]
- Lam, G.Y.; Desai, S.; Fu, J.; Hu, X.Y.; Jang, J.; Goshtasebi, A.; Kalyan, S.; Quon, B.S. IL-8 correlates with reduced baseline femoral neck bone mineral density in adults with cystic fibrosis: A single center retrospective study. Sci. Rep. 2021, 11, 15405. [Google Scholar] [CrossRef]
- Labouesse, M.A.; Gertz, E.R.; Piccolo, B.D.; Souza, E.C.; Schuster, G.U.; Witbracht, M.G.; Woodhouse, L.R.; Adams, S.H.; Keim, N.L.; Van Loan, M.D. Associations among endocrine, inflammatory, and bone markers, body composition and weight loss induced bone loss. Bone 2014, 64, 138–146. [Google Scholar] [CrossRef]
- Sturtzel, C. Endothelial Cells. Adv. Exp. Med. Biol. 2017, 1003, 71–91. [Google Scholar] [PubMed]
- Lu, X.; Mu, E.; Wei, Y.; Riethdorf, S.; Yang, Q.; Yuan, M.; Yan, J.; Hua, Y.; Tiede, B.J.; Lu, X.; et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 2011, 20, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Berner, H.S.; Lyngstadaas, S.P.; Spahr, A.; Monjo, M.; Thommesen, L.; Drevon, C.A.; Syversen, U.; Reseland, J.E. Adiponectin and its receptors are expressed in bone-forming cells. Bone 2004, 35, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-H.; Guo, L.-J.; Yuan, L.-Q.; Xie, H.; Zhou, H.-D.; Wu, X.-P.; Liao, E.-Y. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp. Cell Res. 2005, 309, 99–109. [Google Scholar] [CrossRef]
- Peng, X.-D.; Xie, H.; Zhao, Q.; Wu, X.-P.; Sun, Z.-Q.; Liao, E.-Y. Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin. Chim. Acta 2008, 387, 31–35. [Google Scholar] [CrossRef]
- Biver, E.; Salliot, C.; Combescure, C.; Gossec, L.; Hardouin, P.; Legroux-Gerot, I.; Cortet, B. Influence of adipokines and ghrelin on bone mineral density and fracture risk: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011, 96, 2703–2713. [Google Scholar] [CrossRef]
- Nakamura, Y.; Nakano, M.; Suzuki, T.; Sato, J.; Kato, H.; Takahashi, J.; Shiraki, M. Two adipocytokines, leptin and adiponectin, independently predict osteoporotic fracture risk at different bone sites in postmenopausal women. Bone 2020, 137, 115404. [Google Scholar] [CrossRef]
- Tai, T.-Y.; Chen, C.-L.; Tsai, K.-S.; Tu, S.-T.; Wu, J.-S.; Yang, W.-S. A longitudinal analysis of serum adiponectin levels and bone mineral density in postmenopausal women in Taiwan. Sci. Rep. 2022, 12, 8090. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhao, J.; Zhang, M.; Yin, L.; Quan, Z.; Ou, Y.; Huang, W. Causal roles of circulating adiponectin in osteoporosis and cancers. Bone 2022, 155, 116266. [Google Scholar] [CrossRef]
- Lenchik, L.; Register, T.; Hsu, F.-C.; Lohman, K.; Nicklas, B.; Freedman, B.; Langefeld, C.; Carr, J.; Bowden, D. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 2003, 33, 646–651. [Google Scholar] [CrossRef]
- Azizieh, F.Y.; Shehab, D.; Al Jarallah, K.; Mojiminiyi, O.; Gupta, R.; Raghupathy, R. Circulatory pattern of cytokines, adipokines and bone markers in postmenopausal women with low BMD. J. Inflamm. Res. 2019, 12, 99–108. [Google Scholar] [CrossRef] [PubMed]
- DeFeudis, G.; Mazzilli, R.; Gianfrilli, D.; Lenzi, A.; Isidori, A.M. The CATCH checklist to investigate adult-onset hypogonadism. Andrology 2018, 6, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Colleluori, G.; Aguirre, L.; Napoli, N.; Qualls, C.; Villareal, D.T.; Armamento-Villareal, R. Testosterone Therapy Effects on Bone Mass and Turnover in Hypogonadal Men with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, e3058–e3068. [Google Scholar] [CrossRef]
- Yaturu, S.; Djedjos, S.; Alferos, G.; DePrisco, C. Bone mineral density changes on androgen deprivation therapy for prostate cancer and response to antiresorptive therapy. Prostate Cancer Prostatic Dis. 2006, 9, 35–38. [Google Scholar] [CrossRef][Green Version]
- Parish, R.C.; Todman, S.; Jain, S.K. Resting Heart Rate Variability, Inflammation, and Insulin Resistance in Overweight and Obese Adolescents. Metab. Syndr. Relat. Disord. 2016, 14, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.Y.; Kim, D.W.; Lee, W.G.; Rhee, K.H.; Youn, H.-S. Correlation between Helicobacter pylori infection and vitamin C levels in whole blood, plasma, and gastric juice, and the pH of gastric juice in Korean children. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 53–62. [Google Scholar] [CrossRef]
- Yaturu, S.; Humphrey, S.; Landry, C.; Jain, S.K. Decreased bone mineral density in men with metabolic syndrome alone and with type 2 diabetes. Med. Sci. Monit. 2009, 15, CR5-9. [Google Scholar]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Carr, A.C.; Rowe, S. Factors Affecting Vitamin C Status and Prevalence of Deficiency: A Global Health Perspective. Nutrients 2020, 12, 1963. [Google Scholar] [CrossRef]
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Q. Trends in osteoporosis and mean bone density among type 2 diabetes patients in the US from 2005 to 2014. Sci. Rep. 2021, 11, 3693. [Google Scholar] [CrossRef] [PubMed]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 2011, 15, 1607–1638. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor, C.G.; Eisenga, M.F.; Neto, A.W.G.; Ozyilmaz, A.; Gans, R.O.B.; De Jong, W.H.A.; Zelle, D.M.; Berger, S.P.; Gaillard, C.A.J.M.; Navis, G.J.; et al. Vitamin C Depletion and All-Cause Mortality in Renal Transplant Recipients. Nutrients 2017, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.B.; Valdes, A.M.; Burling, K.; Perks, U.C.; Spector, T.D. Serum adiponectin and bone mineral density in women. J. Clin. Endocrinol. Metab. 2007, 92, 1517–1523. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Li, G.; Chong, Y.; Zhang, J.; Guo, X.; Li, B.; Bi, Z. Osteoclast regulation of osteoblasts via RANKRANKL reverse signal transduction in vitro. Mol. Med. Rep. 2017, 16, 3994–4000. [Google Scholar] [CrossRef]
- Wright, H.L.; McCarthy, H.S.; Middleton, J.; Marshall, M.J. RANK, RANKL and osteoprotegerin in bone biology and disease. Curr. Rev. Musculoskelet. Med. 2009, 2, 56–64. [Google Scholar] [CrossRef]
- Erlandson, K.M.; O’Riordan, M.; Labbato, D.; McComsey, G.A. Relationships between inflammation, immune activation, and bone health among HIV-infected adults on stable antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2014, 65, 290–298. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Cenci, S.; Rifas, L.; Haug, J.; Dipersio, J.; Pacifici, R. T Cell Activation Induces Human Osteoclast Formation via Receptor Activator of Nuclear Factor κB Ligand-Dependent and -Independent Mechanisms. J. Bone Miner. Res. 2001, 16, 328–337. [Google Scholar] [CrossRef]
- Kawai, T.; Matsuyama, T.; Hosokawa, Y.; Makihira, S.; Seki, M.; Karimbux, N.Y.; Goncalves, R.B.; Valverde, P.; Dibart, S.; Li, Y.-P.; et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 2006, 169, 987–998. [Google Scholar] [CrossRef]
- Wang, L.; He, Y.; Ning, W. Role of enhancer of zeste homolog 2 in osteoclast formation and periodontitis development by downregulating microRNA-101-regulated VCAM-1. J. Tissue Eng. Regen. Med. 2021, 15, 534–545. [Google Scholar] [CrossRef]
- Lee, H.-W.; Park, H.K.; Na, Y.J.; Kim, C.D.; Lee, J.H.; Kim, B.S.; Kim, J.B.; Lee, C.W.; Moon, J.O.; Yoon, S. RANKL stimulates proliferation, adhesion and IL-7 expression of thymic epithelial cells. Exp. Mol. Med. 2008, 40, 59–70. [Google Scholar] [CrossRef] [PubMed]
| T2D | Non-Diabetic Controls | |
|---|---|---|
| n | 74 | 26 |
| Age (years) | 49.6 ± 1.1 | 46.6 ± 1.9 |
| Gender (F/M) | 54/20 | 20/6 |
| BMI | 36.5 ± 1.03 | 29.8 ± 1.3 * |
| Duration of diabetes (years) | 4.2 ± 0.5 | NA |
| HbA1C (%) | 7.71 ± 0.20 | ND |
| Glucose (mg/dL) | 139 ± 6 | 93 ± 4 * |
| Calcium (mg/dL) | 9.33 ± 0.05 | 9.98 ± 0.42 |
| Lumbar (L1–L4) BMD (g/cm2) | 1.27 ± 0.02 | ND |
| Right femur BMD (g/cm2) | 1.11 ± 0.02 | ND |
| Left femur BMD (g/cm2) | 1.12 ± 0.02 | ND |
| Vitamin C (µg/dL) | 0.25 ± 0.01 | 0.32 ± 0.03 * |
| Total adiponectin (ng/mL) | 1.03 ± 0.08 | 1.09 ± 0.22 |
| VCAM-1 (µg/mL) | 0.80 ± 0.04 | 0.65 ± 0.06 * |
| IL-8 (pg/mL) | 3.90 ± 0.23 | 2.59 ± 0.13 * |
| IL-1β (pg/mL) | 10.5 ± 0.4 | 7.5 ± 1.02 * |
| TNF-α (pg/mL) | 129.6 ± 12.1 | 68.1 ± 9.0 * |
| LDL/HDL-chol (ratio) | 2.23 ± 0.11 | 2.12 ± 0.17 |
| TG/HDL-chol (ratio) | 4.73 ± 0.60 | 1.90 ± 0.25 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, S.K.; McLean, W.E.; Stevens, C.M.; Dhawan, R. The Positive Association of Plasma Levels of Vitamin C and Inverse Association of VCAM-1 and Total Adiponectin with Bone Mineral Density in Subjects with Diabetes. Nutrients 2022, 14, 3893. https://doi.org/10.3390/nu14193893
Jain SK, McLean WE, Stevens CM, Dhawan R. The Positive Association of Plasma Levels of Vitamin C and Inverse Association of VCAM-1 and Total Adiponectin with Bone Mineral Density in Subjects with Diabetes. Nutrients. 2022; 14(19):3893. https://doi.org/10.3390/nu14193893
Chicago/Turabian StyleJain, Sushil K., William E. McLean, Christopher M. Stevens, and Richa Dhawan. 2022. "The Positive Association of Plasma Levels of Vitamin C and Inverse Association of VCAM-1 and Total Adiponectin with Bone Mineral Density in Subjects with Diabetes" Nutrients 14, no. 19: 3893. https://doi.org/10.3390/nu14193893
APA StyleJain, S. K., McLean, W. E., Stevens, C. M., & Dhawan, R. (2022). The Positive Association of Plasma Levels of Vitamin C and Inverse Association of VCAM-1 and Total Adiponectin with Bone Mineral Density in Subjects with Diabetes. Nutrients, 14(19), 3893. https://doi.org/10.3390/nu14193893






