The Metabolome and the Gut Microbiota for the Prediction of Necrotizing Enterocolitis and Spontaneous Intestinal Perforation: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Type
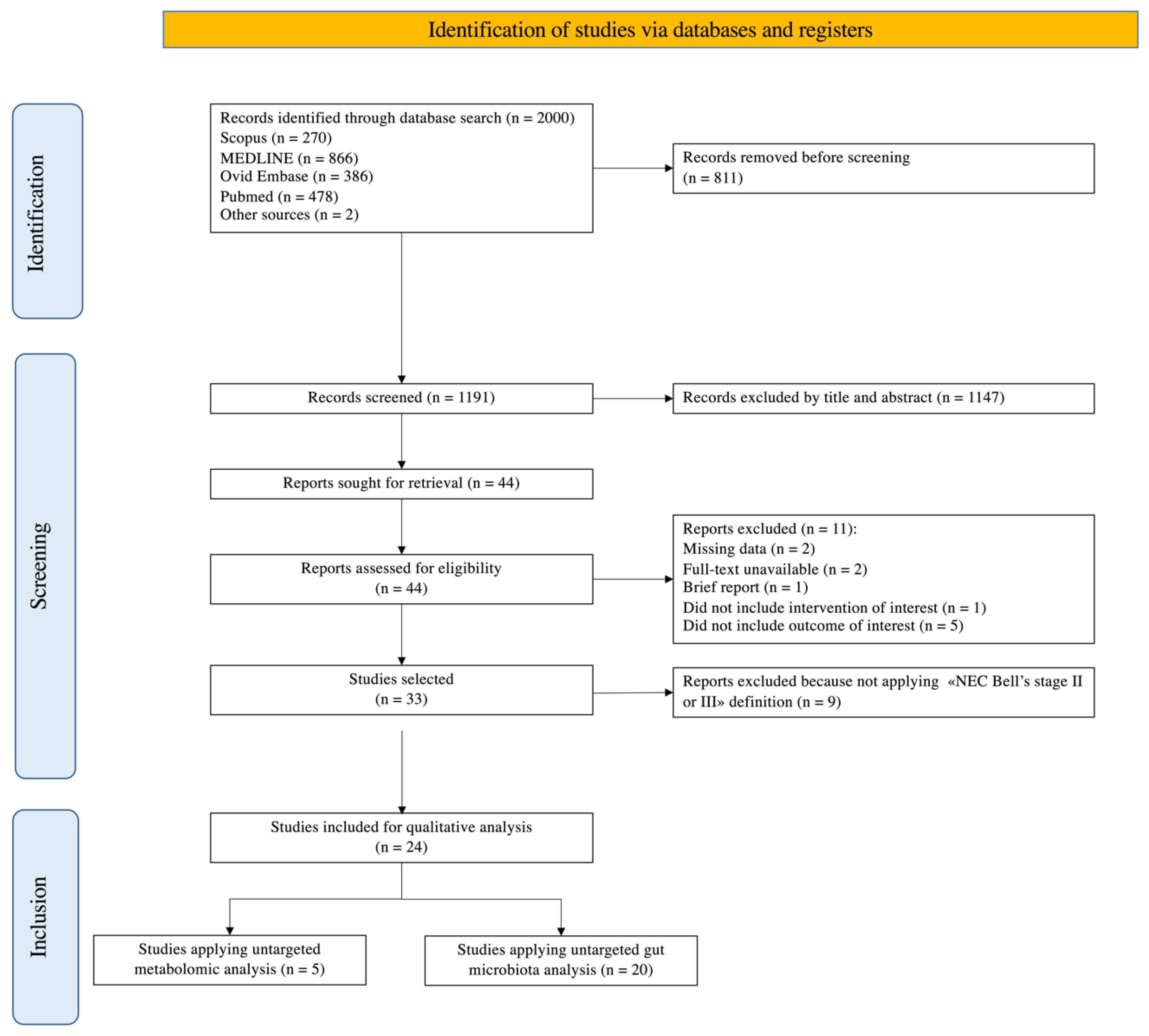
2.2. Literature Search
2.3. Inclusion Criteria
2.4. Outcomes
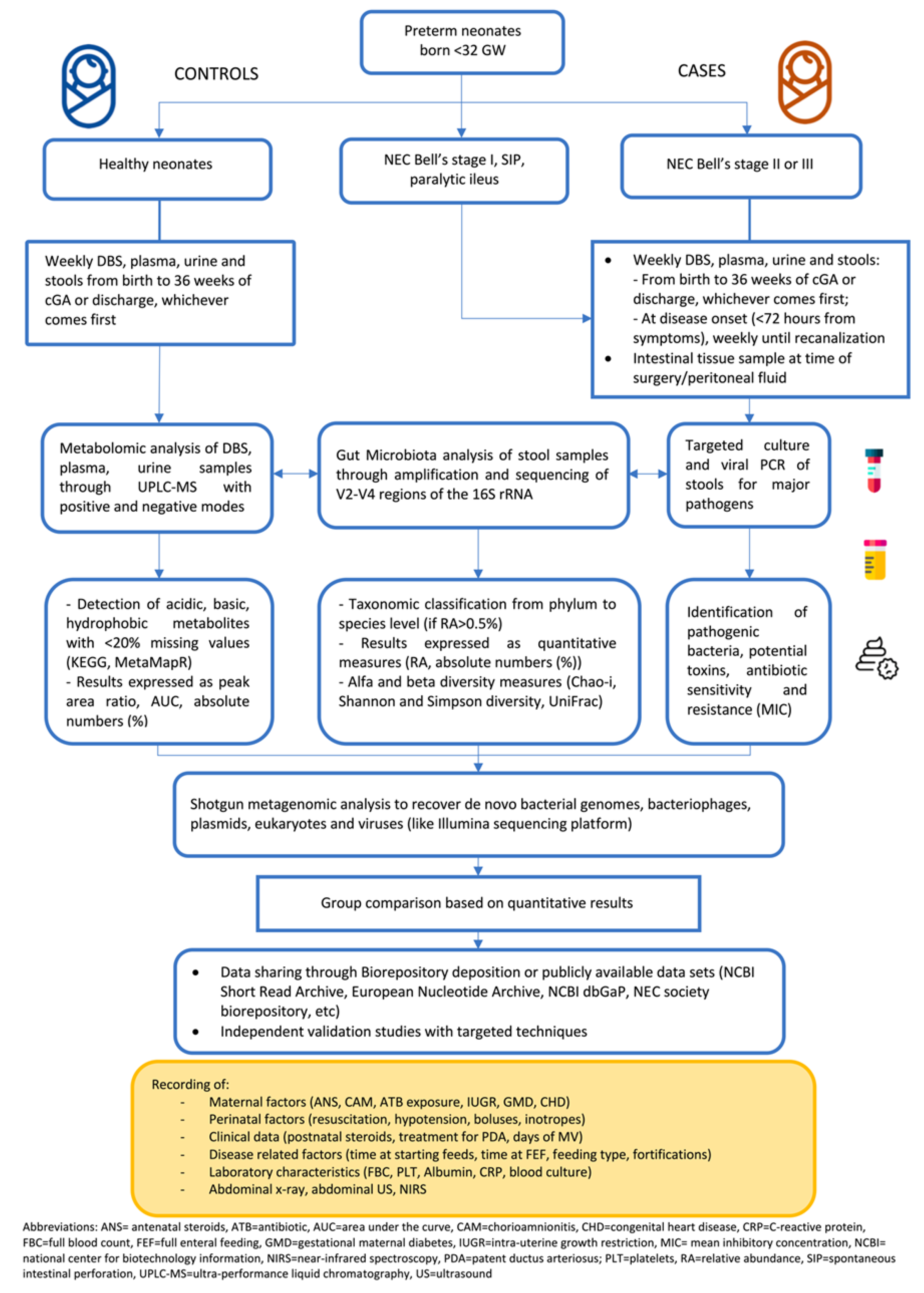
2.5. Data Extraction and Synthesis
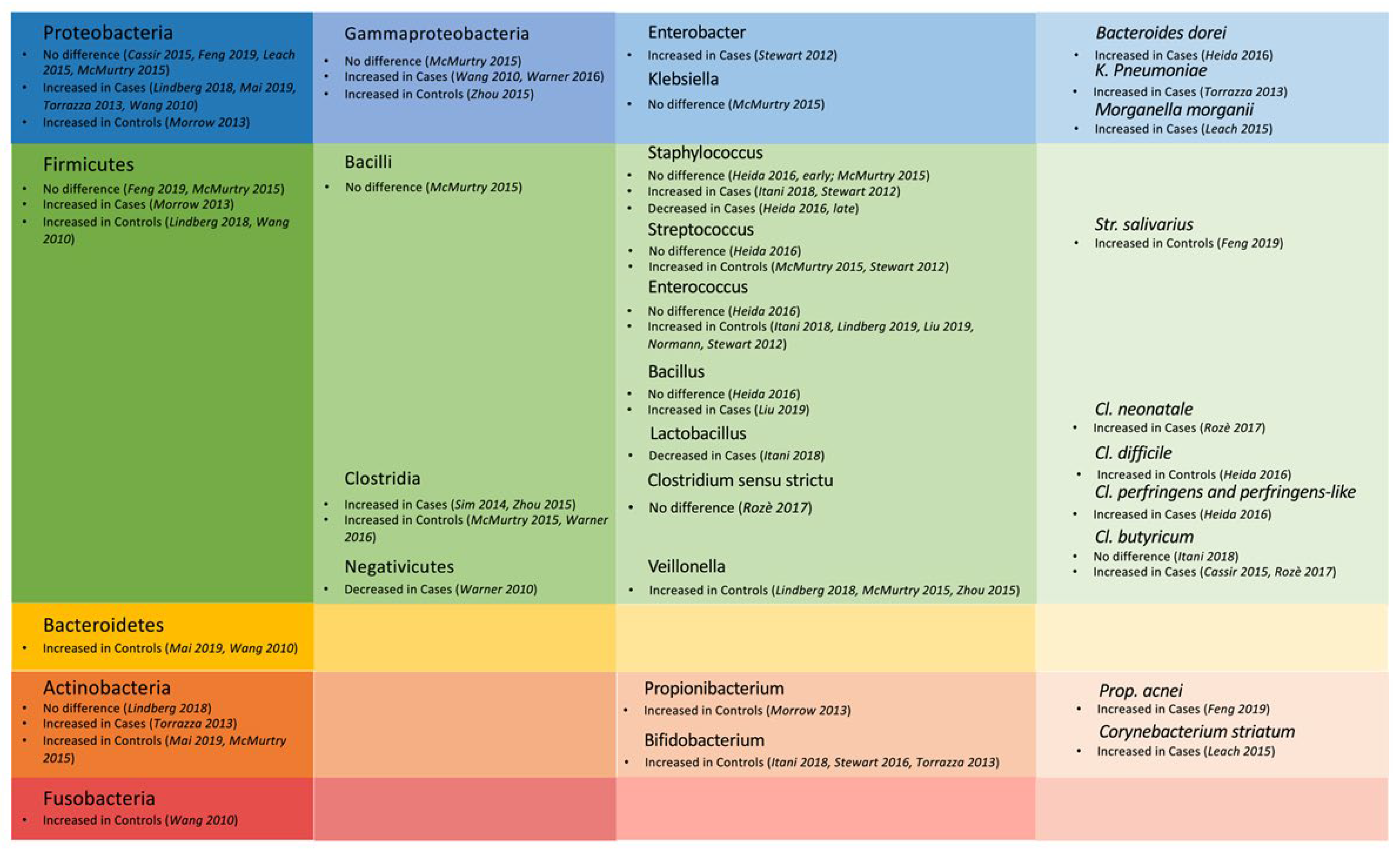
3. Results
3.1. Untargeted Metabolomic Analysis
3.2. Untargeted Metabolomic Analysis
3.3. Combined Untargeted Metabolomics and Gut Microbiota Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, X.; Zhang, L.; Jiang, S.; Li, M.; Yan, C.; Shen, C.; Yang, Y.; Lee, S.K.; Cao, Y. Epidemiology of necrotizing enterocolitis in preterm infants in China: A multicenter cohort study from 2015 to 2018. J. Pediatr. Surg. 2022, 57, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Alsaied, A.; Islam, N.; Thalib, L. Global incidence of necrotising enterocolitis: A systematic review and meta-analysis. BMC Pediatr. 2020, 20, 344. [Google Scholar] [CrossRef] [PubMed]
- Rysavy, M.A.; Mehler, K.; Oberthür, A.; Ågren, J.; Kusuda, S.; McNamara, P.J.; Giesinger, R.E.; Kribs, A.; Normann, E.; Carlson, S.J.; et al. An Immature Science: Intensive Care for Infants Born at ≤23 Weeks of Gestation. J. Pediatr. 2021, 233, 16–25.e1. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.H.; Hall, N.J. Contemporary Outcomes for Infants with Necrotizing Enterocolitis—A Systematic Review. J. Pediatr. 2020, 220, 86–91.e3. [Google Scholar] [CrossRef] [PubMed]
- Hau, E.M.; Meyer, C.S.; Berger, S.; Goutaki, M.; Kordasz, M.; Kessler, U. Gastrointestinal sequelae after surgery for necrotising enterocolitis: A systematic review and meta-analysis. Arch. Dis. Child. Fetal. Neonatal. Ed. 2019, 104, F265–F273. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotising enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- Walsh, M.C.; Kliegman, R.M. Necrotizing enterocolitis: Treatment based on staging criteria. Pediatr. Clin. N. Am. 1986, 33, 179–201. [Google Scholar] [CrossRef]
- Neu, J. Necrotising enterocolits: The future. Neonatology 2020, 117, 240–244. [Google Scholar] [CrossRef]
- Patel, R.M.; Ferguson, J.; McElroy, S.J.; Khashu, M.; Caplan, M.S. Defining necrotizing enterocolitis: Current difficulties and future opportunities. Pediatr. Res. 2020, 88, 10–15. [Google Scholar] [CrossRef]
- Agakidou, E.; Agakidis, C.; Gika, H.; Sarafidis, K. Emerging biomarkers for prediction and early diagnosis of necrotising enterocolitis in the era of metabolomics and proteomics. Front. Pediatr. 2020, 8, 602255. [Google Scholar] [CrossRef]
- Gordon, P.V.; Swanson, J.R.; MacQueen, B.C.; Christensen, R.D. A critical question for NEC researchers: Can we create a consensus definition of NEC that facilitates research progress? Semin. Perinatol. 2017, 41, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.D.; Sharma, D.; Bansal, A. Biomarkers of necrotizing enterocolitis: A review of literature. J. Matern. Fetal. Neonatal. Med. 2018, 31, 3051–3064. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, B.; Good, M.; Warner, B.B. The Microbiome and Biomarkers for Necrotizing Enterocolitis: Are We Any Closer to Prediction? J. Pediatr. 2017, 189, 40–47.e2. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C.; Ma, T.P.; Lam, H.S. The use of laboratory biomarkers for surveillance, diagnosis and prediction of clinical outcomes in neonatal sepsis and necrotising enterocolitis. Arch. Dis. Child. Fetal. Neonatal. Ed. 2015, 100, F448–F452. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Wilson, I.D. Opinion: Understanding ‘global’ systems biology: Metabonomics and the continuum of metabolism. Nat. Rev. Drug. Discov. 2003, 2, 668–676. [Google Scholar] [CrossRef]
- Baraldi, E.; Carraro, S.; Giordano, G.; Reniero, F.; Perilongo, G.; Zacchello, F. Metabolomics: Moving towards personalized medicine. Ital. J. Pediatr. 2009, 35, 30. [Google Scholar] [CrossRef]
- Morowitz, M.J.; Poroyko, V.; Caplan, M.; Alverdy, J.; Liu, D.C. Redefining the role of intestinal microbes in the pathogenesis of necrotizing enterocolitis. Pediatrics 2010, 125, 777–785. [Google Scholar] [CrossRef]
- Cuna, A.; Morowitz, M.J.; Ahmed, I.; Umar, S.; Sampath, V. Dynamics of the preterm gut microbiome in health and disease. Am. J. Physiol. Gastrointest. Liver. Physiol. 2021, 320, G411–G419. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 372, n71. [Google Scholar] [CrossRef]
- Pepe, M.S.; Feng, Z.; Janes, H.; Bossuyt, P.M.; Potter, J.D. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: Standards for study design. J. Natl. Cancer. Inst. 2008, 100, 1432–1438. [Google Scholar] [CrossRef]
- Vermont Oxford Network 2022 Manual of Operations: Part 2. Data Definitions & Infant Data Booklets. 26.2, Published February 2022. Available online: https://vtoxford.zendesk.com/hc/article_attachments/4520517619731/2022_Manual_of_Operations__Part_2__Release_26.2_PDF.pdf (accessed on 29 June 2022).
- Simpson, E. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. Shannon’s Formula as a Measure of Specific Diversity: Its Use and Misuse. Am. Nat. 1966, 100, 463–465. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Brehin, C.; Dubois, D.; Dicky, O.; Breinig, S.; Oswald, E.; Serino, M. Evolution of Gut Microbiome and Metabolome in Suspected Necrotizing Enterocolitis: A Case-Control Study. J. Clin. Med. 2020, 9, 2278. [Google Scholar] [CrossRef]
- Morrow, A.L.; Lagomarcino, A.J.; Schibler, K.R.; Taft, D.H.; Yu, Z.; Wang, B.; Altaye, M.; Wagner, M.; Gevers, D.; Ward, D.V.; et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome 2013, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Picaud, J.C.; De Magistris, A.; Mussap, M.; Corbu, S.; Dessì, A.; Noto, A.; Fanos, V.; Cesare Marincola, F. Urine NMR Metabolomics Profile of Preterm Infants with Necrotizing Enterocolitis Over the First Two Months of Life: A Pilot Longitudinal Case-Control Study. Front. Mol. Biosci. 2021, 8, 680159. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, B.; Jiang, X.; Sidhu, R.; Ory, D.S.; Warner, B.B.; Tarr, P.I. Gut Sphingolipid Composition as a Prelude to Necrotizing Enterocolitis. Sci. Rep. 2018, 8, 10984. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Embleton, N.D.; Marrs, E.C.; Smith, D.P.; Nelson, A.; Abdulkadir, B.; Skeath, T.; Petrosino, J.F.; Perry, J.D.; Berrington, J.E.; et al. Temporal bacterial and metabolic development of the preterm gut reveals specific signatures in health and disease. Microbiome 2016, 4, 67. [Google Scholar] [CrossRef]
- Stewart, C.J.; Nelson, A.; Treumann, A.; Skeath, T.; Cummings, S.P.; Embleton, N.D.; Berrington, J.E. Metabolomic and proteomic analysis of serum from preterm infants with necrotising entercolitis and late-onset sepsis. Pediatr. Res. 2016, 79, 425–431. [Google Scholar] [CrossRef]
- Thomaidou, A.; Chatziioannou, A.C.; Deda, O.; Benaki, D.; Gika, H.; Mikros, E.; Agakidis, C.; Raikos, N.; Theodoridis, G.; Sarafidis, K. A pilot case-control study of urine metabolomics in preterm neonates with necrotizing enterocolitis. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2019, 1117, 10–21. [Google Scholar] [CrossRef]
- Wandro, S.; Osborne, S.; Enriquez, C.; Bixby, C.; Arrieta, A.; Whiteson, K. The microbiome and metabolome of preterm infant stool are personalized and not driven by health outcomes, including necrotizing enterocolitis and late-onset sepsis. mSphere 2018, 3, e00104-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, W.; Wang, G.; Yu, M.; Zhong, J.; Xu, C.; Li, D.; Zhou, Y. Gas chromatography-mass spectrometry based serum metabolic analysis for premature infants and the relationship with necrotizing enterocolitis: A cross-sectional study. Ital. J. Pediatr. 2019, 45, 54. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, A.; Begley, P.; Stevens, A.; Whatmore, A.; Victor, S. The metabolomics of necrotising enterocolitis in preterm babies: An exploratory study. J. Matern. Fetal. Neonatal. Med. 2016, 29, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Cassir, N.; Benamar, S.; Khalil, J.B.; Croce, O.; Saint-Faust, M.; Jacquot, A.; Million, M.; Azza, S.; Armstrong, N.; Henry, M.; et al. Clostridium butyricum strains and dysbiosis linked to necrotizing enterocolitis in preterm neonates. Clin. Infect. Dis. 2015, 61, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Claud, E.C.; Keegan, K.P.; Brulc, J.M.; Lu, L.; Bartels, D.; Glass, E.; Chang, E.B.; Meyer, F.; Antonopoulos, D.A. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome 2013, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Dobbler, P.T.; Procianoy, R.S.; Mai, V.; Silveira, R.C.; Corso, A.L.; Rojas, B.S.; Roesch, L.F.W. Low microbial diversity and abnormal microbial succession is associated with necrotizing enterocolitis in preterm infants. Front. Microbiol. 2017, 8, 2243. [Google Scholar] [CrossRef]
- Duan, M.; Han, Z.; Huang, N. Changes of intestinal microflora in neonatal necrotizing enterocolitis: A single-center study. J. Int. Med. Res. 2020, 48, 300060520957804. [Google Scholar] [CrossRef]
- Feng, J.; He, Y.; Liu, D.; Li, L.; Chen, J.; Yu, J. The constitution and functional prediction of the microbiota in necrotizing enterocolitis with a gestational age of over 28 weeks. Medicine 2019, 98, e17206. [Google Scholar] [CrossRef]
- Heida, F.H.; Van Zoonen, A.G.J.F.; Hulscher, J.B.F.; Te Kiefte, B.J.C.; Wessels, R.; Kooi, E.M.W.; Bos, A.F.; Harmsen, H.; de Goffau, M.C. A necrotizing enterocolitis-associated gut microbiota is present in the meconium: Results of a prospective study. Clin. Infect. Dis. 2016, 62, 863–870. [Google Scholar] [CrossRef]
- Itani, T.; Ayoub Moubareck, C.; Melki, I.; Rousseau, C.; Mangin, I.; Butel, M.; Karam-Sarkis, D. Preterm infants with necrotising enterocolitis demonstrate an unbalanced gut microbiota. Acta Paediatr. 2018, 107, 40–47. [Google Scholar] [CrossRef]
- Leach, S.T.; Lui, K.; Naing, Z.; Dowd, S.E.; Mitchell, H.M.; Day, A.S. Multiple opportunistic pathogens, but not pre-existing inflammation, may be associated with necrotizing enterocolitis. Dig. Dis. Sci. 2015, 60, 3728–3734. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, T.P.; Caimano, M.J.; Hagadorn, J.I.; Bennett, E.M.; Maas, K.; Brownell, E.A.; Matson, A.P. Preterm infant gut microbial patterns related to the development of necrotizing enterocolitis. J. Matern. Fetal. Neonatal. Med. 2020, 33, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Feng, Y.; Pan, L.; Xie, Z.; Yan, Z.; Zhang, L.; Li, M.; Zhao, J.; Sun, J.; et al. Patterned progression of gut microbiota associated with necrotizing enterocolitis and late onset sepsis in preterm infants: A prospective study in a chinese neonatal intensive care unit. PeerJ 2019, 7, e7310. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.; Young, C.M.; Ukhanova, M.; Wang, X.; Sun, Y.; Casella, G.; Theriaque, D.; Li, N.; Sharma, R.; Hudak, M.; et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE 2011, 6, e20647. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, V.E.; Gupta, R.W.; Tran, L.; Blanchard, E.E.; Penn, D.; Taylor, C.M.; Ferris, M.J. Bacterial diversity and clostridia abundance decrease with increasing severity of necrotizing enterocolitis. Microbiome 2015, 3, 11. [Google Scholar] [CrossRef]
- Normann, E.; Fahlén, A.; Engstrand, L.; Lilja, H.E. Intestinal microbial profiles in extremely preterm infants with and without necrotizing enterocolitis. Acta Paediatr. 2013, 102, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Olm, M.R.; Bhattacharya, N.; Crits-Christoph, A.; Firek, B.A.; Baker, R.; Song, Y.S.; Morowitz, M.J.; Banfield, J.F. Necrotizing enterocolitis is preceded by increased gut bacterial replication, klebsiella, and fimbriae-encoding bacteria. Sci. Adv. 2019, 5, eaax5727. [Google Scholar] [CrossRef]
- Rozé, J.C.; Ancel, P.Y.; Lepage, P.; Martin-Marchand, L.; Al Nabhani, Z.; Delannoy, J.; Picaud, J.C.; Lapillonne, A.; Aires, J.; Durox, M.; et al. Nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very-preterm infants. Am. J. Clin. Nutr. 2017, 106, 821–830. [Google Scholar] [CrossRef]
- Sim, K.; Shaw, A.G.; Randell, P.; Cox, M.J.; McClure, Z.E.; Li, M.S.; Haddad, M.; Langford, P.R.; Cookson, W.O.; Moffatt, M.F.; et al. Dysbiosis anticipating necrotizing enterocolitis in very premature infants. Clin. Infect. Dis. 2015, 60, 389–397. [Google Scholar] [CrossRef]
- Stewart, C.J.; Marrs, E.C.; Magorrian, S.; Nelson, A.; Lanyon, C.; Perry, J.D.; Embleton, N.D.; Cummings, S.P.; Berrington, J.E. The preterm gut microbiota: Changes associated with necrotizing enterocolitis and infection. Acta Paediatr. 2012, 101, 1121–1127. [Google Scholar] [CrossRef]
- Stewart, C.J.; Marrs, E.C.; Nelson, A.; Lanyon, C.; Perry, J.D.; Embleton, N.D.; Cummings, S.P.; Berrington, J.E. Development of the preterm gut microbiome in twins at risk of necrotising enterocolitis and sepsis. PLoS One 2013, 8, e73465. [Google Scholar] [CrossRef] [PubMed]
- Torrazza, R.M.; Ukhanova, M.; Wang, X.; Sharma, R.; Hudak, M.L.; Neu, J.; Mai, V. Intestinal microbial ecology and environmental factors affecting necrotizing enterocolitis. PLoS One 2013, 8, e83304. [Google Scholar] [CrossRef]
- Wang, Y.; Hoenig, J.D.; Malin, K.J.; Qamar, S.; Petrof, E.O.; Sun, J.; Antonopoulos, D.A.; Chang, E.B.; Claud, E.C. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009, 3, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.V.; Scholz, M.; Zolfo, M.; Taft, D.H.; Schibler, K.R.; Tett, A.; Segata, N.; Morrow, A.L. Metagenomic sequencing with strain-level resolution implicates uropathogenic E. coli in necrotizing enterocolitis and mortality in preterm infants. Cell. Rep. 2016, 14, 2912–2924. [Google Scholar] [CrossRef] [PubMed]
- Warner, B.B.; Deych, E.; Zhou, Y.; Hall-Moore, C.; Weinstock, G.M.; Sodergren, E.; Shaikh, N.; Hoffmann, J.A.; Linneman, L.A.; Hamvas, A.; et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: A prospective case-control study. Lancet 2016, 387, 1928–1936. [Google Scholar] [CrossRef]
- Zhou, Y.; Shan, G.; Sodergren, E.; Weinstock, G.; Walker, W.A.; Gregory, K.E. Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis: A case-control study. PLoS One 2015, 10, e0118632. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadir, B.; Nelson, A.; Skeath, T.; Marrs, E.C.; Perry, J.D.; Cummings, S.P.; Embleton, N.D.; Berrington, J.E.; Stewart, C.J. Stool bacterial load in preterm infants with necrotising enterocolitis. Early. Hum. Dev. 2016, 95, 1–2. [Google Scholar] [CrossRef][Green Version]
- Barron, L.K.; Warner, B.B.; Tarr, P.I.; Shannon, W.D.; Deych, E.; Warner, B.W. Independence of gut bacterial content and neonatal necrotizing enterocolitis severity. J. Pediatr. Surg. 2017, 52, 993–998. [Google Scholar] [CrossRef]
- De Magistris, A.; Corbu, S.; Cesare Flamincola, F.; Gueye, M.; Pastor-Diez, B.; Dessì, A.; Noto, A.; Reali, A.; Fanos, V.; Puddu, M.; et al. NMR-based metabolomics analysis of urinary changes in neonatal enterocolitis. JPNIM 2015, 4, 37–38. [Google Scholar]
- Feng, J.-X.; Li, Y.; He, Y.; Xiao, S.; Ai, Q.; Wang, Z.-L.; Liu, Z.-Q.; Yu, J.-L. Diversity and dynamic changes of intestinal microbial community in preterm infants with necrotizing enterocolitis. Int. J. Clin. Exp. Med. 2017, 10, 6100–6107. [Google Scholar]
- Itani, T.; Ayoub Moubareck, C.; Mangin, I.; Butel, M.-J.; Karam Sarkis, D. Individual variations in intestinal microbiota were higher in preterm infants with necrotising enterocolitis than healthy controls. Acta Paediatr. 2019, 108, 2294–2295. [Google Scholar] [CrossRef] [PubMed]
- Raveh-Sadka, T.; Thomas, B.C.; Singh, A.; Firek, B.; Brooks, B.; Castelle, C.J.; Sharon, I.; Baker, R.; Good, M.; Morowitz, M.J.; et al. Gut bacteria are rarely shared by co-hospitalized premature infants, regardless of necrotizing enterocolitis development. Elife 2015, 4, e05477. [Google Scholar] [CrossRef] [PubMed]
- Romano-Keeler, J.; Shilts, M.H.; Tovchigrechko, A.; Wang, C.; Brucker, R.M.; Moore, D.J.; Fonnesbeck, C.; Meng, S.; Correa, H.; Lovvorn, H.N., 3rd; et al. Distinct mucosal microbial communities in infants with surgical necrotizing enterocolitis correlate with age and antibiotic exposure. PLoS One 2018, 13, e0206366. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.J.; Ye, C.; Chen, Y.; Zhang, D.; Li, T.; Ling, X.B.; Cohen, H.J.; Shaw, G.M.; Stevenson, D.K.; Chace, D.; et al. Progressive Metabolic Dysfunction and Nutritional Variability Precedes Necrotizing Enterocolitis. Nutrients 2020, 12, 1275. [Google Scholar] [CrossRef]
- Smith, B.; Bodé, S.; Petersen, B.L.; Jensen, T.K.; Pipper, C.; Kloppenborg, J.; Boyé, M.; Krogfelt, K.A.; Mølbak, L. Community analysis of bacteria colonizing intestinal tissue of neonates with necrotizing enterocolitis. BMC Microbiol. 2011, 11, 73. [Google Scholar] [CrossRef]
- Stewart, C.J.; Fatemizadeh, R.; Parsons, P.; Lamb, C.A.; Shady, D.A.; Petrosino, J.F.; Hair, A.B. Using formalin fixed paraffin embedded tissue to characterize the preterm gut microbiota in necrotising enterocolitis and spontaneous isolated perforation using marginal and diseased tissue. BMC Microbiol. 2019, 19, 52. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Feng, J.; Ai, Q.; Li, L.; He, Y.; Li, H.; Tang, X.; Yu, J. Application of laser capture microdissection and 16S rRNA gene polymerase chain reaction in the analysis of bacteria colonizing the intestinal tissue of neonates with necrotizing enterocolitis. Pediatr. Infect. Dis. J. 2015, 34, e279–e289. [Google Scholar] [CrossRef]
- Han, S.M.; Hong, C.R.; Knell, J.; Edwards, E.M.; Morrow, K.A.; Soll, R.F.; Modi, B.P.; Horbar, J.D.; Jaksic, T. Trends in incidence and outcomes of necrotizing enterocolitis over the last 12 years: A multicenter cohort analysis. J. Pediatr. Surg. 2020, 55, 998–1001. [Google Scholar] [CrossRef]
- Masi, A.C.; Stewart, C.J. The role of the preterm intestinal microbiome in sepsis and necrotising enterocolitis. Early. Hum. Dev. 2019, 138, 104854. [Google Scholar] [CrossRef]
- Renwick, V.L.; Stewart, C.J. Exploring functional metabolites in preterm infants. Acta Paediatr. 2022, 111, 45–53. [Google Scholar] [CrossRef]
- Dessì, A.; Pintus, R.; Marras, S.; Cesare Marincola, F.; De Magistris, A.; Fanos, V. Metabolomics in necrotizing enterocolitis: The state of the art. Expert. Rev. Mol. Diagn. 2016, 16, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Stronati, L.; Cucchiara, S.; De Curtis, M. Serum Markers of Necrotizing Enterocolitis: A Systematic Review. J. Pediatr. Gastroenterol. Nutr. 2017, 65, e120–e132. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, D.; Niemarkt, H.J.; de Boer, N.; Benninga, M.A.; de Meij, T. The potential of gut microbiota and fecal volatile organic compounds analysis as early diagnostic biomarker for necrotizing enterocolitis and sepsis in preterm infants. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tao, G.; Sun, Z.; Sylvester, K.G. Recent Potential Noninvasive Biomarkers in Necrotizing Enterocolitis. Gastroenterol. Res. Pract. 2019, 2019, 8413698. [Google Scholar] [CrossRef]
- Beck, L.C.; Granger, C.L.; Masi, A.C.; Stewart, C.J. Use of omic technologies in early life gastrointestinal health and disease: From bench to bedside. Expert. Rev. Proteom. 2021, 18, 247–259. [Google Scholar] [CrossRef]
- Pammi, M.; De Plaen, I.G.; Maheshwari, A. Recent Advances in Necrotizing Enterocolitis Research Strategies for Implementation in Clinical Practice. Clin. Perinatol. 2020, 47, 383–397. [Google Scholar] [CrossRef]
- Emwas, A.H.M.; Salek, R.M.; Griffin, J.L.; Merzaban, J. NMR-based metabolomics in human disease diagnosis: Applications, limitations, and recommendations. Metabolomics 2013, 9, 1048–1072. [Google Scholar] [CrossRef]
- Erben, V.; Poschet, G.; Schrotz-King, P.; Brenner, H. Evaluation of different stool extraction methods for metabolomics measurements in human faecal samples. BMJ Nutr. Prev. Health 2021, 4, 374–384. [Google Scholar] [CrossRef]
- Zhgun, E.S.; Ilina, E.N. Fecal Metabolites as Non-Invasive Biomarkers of Gut Diseases. Acta Naturae 2020, 12, 4–14. [Google Scholar] [CrossRef]
- Neu, J.; Pammi, M. Necrotising enterocolitis: The intestinal microbiome, metabolome and inflammatory mediators. Semin. Fetal. Neonatal. Med. 2018, 23, 400–405. [Google Scholar] [CrossRef]
- Tarracchini, C.; Milani, C.; Longhi, G.; Fontana, F.; Mancabelli, L.; Pintus, R.; Lugli, G.A.; Alessandri, G.; Anzalone, R.; Viappiani, A.; et al. Unraveling the Microbiome of Necrotizing Enterocolitis: Insights in Novel Microbial and Metabolomic Biomarkers. Microbiol. Spectr. 2021, 9, e0117621. [Google Scholar] [CrossRef] [PubMed]
- Nejrup, R.G.; Licht, T.R.; Hellgren, L.I. Fatty acid composition and phospholipid types used in infant formulas modifies the establishment of human gut bacteria in germ-free mice. Sci. Rep. 2017, 7, 3975. [Google Scholar] [CrossRef] [PubMed]
- Raba, A.A.; O’Sullivan, A.; Miletin, J. Pathogenesis of necrotising enterocolitis: The impact of the altered gut microbiota and antibiotic exposure in preterm infants. Acta Paediatrica 2021, 110, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Elgin, T.G.; Kern, S.L.; McElroy, S.J. Development of the Neonatal Intestinal Microbiome and Its Association with Necrotizing Enterocolitis. Clin Ther. 2016, 38, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Eckburg, P.B.; Bik, E.M.; Relman, D.A. Assembly of the human intestinal microbiota. Trends Ecol. Evol. 2006, 21, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, R.A.; Schanler, R.J.; Li, N.; Neu, J. The developing intestinal ecosystem: Implications for the neonate. Pediatr. Res. 2005, 58, 625–628. [Google Scholar] [CrossRef]
- Coscia, A.; Bardanzellu, F.; Caboni, E.; Fanos, V.; Peroni, D.G. When a Neonate Is Born, So Is a Microbiota. Life 2021, 11, 148. [Google Scholar] [CrossRef]
- Ward, T.L.; Hosid, S.; Ioshikhes, I.; Altosaar, I. Human milk metagenome: A functional capacity analysis. BMC Microbiol. 2013, 13, 116. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Pammi, M.; Cope, J.M.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Kuppala, V.S.; Meinzen-Derr, J.; Morrow, A.L.; Schibler, K.R. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J. Pediatr. 2011, 159, 720–725. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, C.; van Goudoever, J.B.; Szajewska, H.; Embleton, N.D.; Hojsak, I.; Reid, D.; Shamir, R.; ESPGHAN Working Group for Probiotics, Prebiotics & Committee on Nutrition. Probiotics for Preterm Infants: A Strain-Specific Systematic Review and Network Meta-analysis. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 103–122. [Google Scholar] [CrossRef]
- Morgan, R.L.; Preidis, G.A.; Kashyap, P.C.; Weizman, A.V.; Sadeghirad, B.; McMaster Probiotic, Prebiotic, and Synbiotic Work Group. Probiotics Reduce Mortality and Morbidity in Preterm, Low-Birth-Weight Infants: A Systematic Review and Network Meta-analysis of Randomized Trials. Gastroenterology 2020, 159, 467–480. [Google Scholar] [CrossRef]
- Repa, A.; Thanhaeuser, M.; Endress, D.; Weber, M.; Kreissl, A.; Binder, C.; Berger, A.; Haiden, N. Probiotics (Lactobacillus acidophilus and Bifidobacterium infantis) prevent NEC in VLBW infants fed breast milk but not formula [corrected]. Pediatr. Res. 2015, 77, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadir, B.; Nelson, A.; Skeath, T.; Marrs, E.C.; Perry, J.D.; Cummings, S.P.; Embleton, N.D.; Berrington, J.E.; Stewart, C.J. Routine Use of Probiotics in Preterm Infants: Longitudinal Impact on the Microbiome and Metabolome. Neonatology 2016, 109, 239–247. [Google Scholar] [CrossRef]
- Hosny, M.; Baptiste, E.; Levasseur, A.; La Scola, B. Molecular epidemiology of Clostridium neonatale and its relationship with the occurrence of necrotizing enterocolitis in preterm neonates. New Microbes New Infect. 2019, 32, 100612. [Google Scholar] [CrossRef]
- Gothefors, L.; Blenkharn, I. Clostridium butyricum and necrotising enterocolitis. Lancet 1978, 1, 52–53. [Google Scholar] [CrossRef]
- Alfa, M.J.; Robson, D.; Davi, M.; Bernard, K.; Van Caeseele, P.; Harding, G.K. An outbreak of necrotizing enterocolitis associated with a novel clostridium species in a neonatal intensive care unit. Clin. Infect. Dis. 2002, 35, S101–S105. [Google Scholar] [CrossRef]
- Dittmar, E.; Beyer, P.; Fischer, D.; Schäfer, V.; Schoepe, H.; Bauer, K.; Schlösser, R. Necrotizing enterocolitis of the neonate with Clostridium perfringens: Diagnosis, clinical course, and role of alpha toxin. Eur. J. Pediatr. 2008, 167, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Kliegman, R.M.; Fanaroff, A.A.; Izant, R.; Speck, W.T. Clostridia as pathogens in neonatal necrotizing enterocolitis. J. Pediatr. 1979, 95, 287–289. [Google Scholar] [CrossRef]
- de la Cochetiere, M.F.; Piloquet, H.; des Robert, C.; Darmaun, D.; Galmiche, J.P.; Roze, J.C. Early intestinal bacterial colonization and necrotizing enterocolitis in premature infants: The putative role of Clostridium. Pediatr. Res. 2004, 56, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Thänert, R.; Keen, E.C.; Dantas, G.; Warner, B.B.; Tarr, P.I. Necrotizing Enterocolitis and the Microbiome: Current Status and Future Directions. J. Infect. Dis. 2021, 223, S257–S263. [Google Scholar] [CrossRef]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; de Rinaldis, E.; Bell, J.T.; Venter, J.C.; et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10, 4505. [Google Scholar] [CrossRef]
- Nguyen, Q.P.; Karagas, M.R.; Madan, J.C.; Dade, E.; Palys, T.J.; Morrison, H.G.; Pathmasiri, W.W.; McRitche, S.; Sumner, S.J.; Frost, H.R.; et al. Associations between the gut microbiome and metabolome in early life. BMC Microbiol. 2021, 21, 238. [Google Scholar] [CrossRef]
- Embleton, N.; Berrington, J.; Cummings, S.; Dorling, J.; Ewer, A.; Frau, A.; Juszczak, E.; Kirby, J.; Lamb, C.; Lanyon, C.; et al. Lactoferrin Impact on Gut Microbiota in Preterm Infants with Late-Onset Sepsis or Necrotising Enterocolitis: The MAGPIE Mechanisms of Action Study; NIHR Journals Library: Southampton, UK, 2021. [Google Scholar]
- De Meij, T.G.; van der Schee, M.P.; Berkhout, D.J.; van de Velde, M.E.; Jansen, A.E.; Kramer, B.W.; Van Weissenbruch, M.M.; van Kaam, A.H.; Andriessen, P.; van Goudoever, J.B.; et al. Early Detection of Necrotizing Enterocolitis by Fecal Volatile Organic Compounds Analysis. J. Pediatr. 2015, 167, 562–567.e1. [Google Scholar] [CrossRef]
- Probert, C.; Greenwood, R.; Mayor, A.; Hughes, D.; Aggio, R.; Jackson, R.E.; Simcox, L.; Barrow, H.; García-Finana, M.; Ewer, A.K. Faecal volatile organic compounds in preterm babies at risk of necrotising enterocolitis: The DOVE study. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.; Bannaga, A.S.; Iriarte, R.; Mahmoud, M.; Arasaradnam, R.P. Utility of volatile organic compounds as a diagnostic tool in preterm infants. Pediatr. Res. 2021, 89, 263–268. [Google Scholar] [CrossRef]
- Swanson, J.R.; Hair, A.; Clark, R.H.; Gordon, P.V. Spontaneous intestinal perforation (SIP) will soon become the most common form of surgical bowel disease in the extremely low birth weight (ELBW) infant. J. Perinatol. 2022, 42, 423–429. [Google Scholar] [CrossRef]
- Irles, C.; González-Pérez, G.; Carrera Muiños, S.; Michel Macias, C.; Sánchez Gómez, C.; Martínez-Zepeda, A.; Cordero González, G.; Laresgoiti Servitje, E. Estimation of Neonatal Intestinal Perforation Associated with Necrotizing Enterocolitis by Machine Learning Reveals New Key Factors. Int. J. Environ. Res. Public Health 2018, 15, 2509. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, D.; Klaassen, P.; Niemarkt, H.J.; de Boode, W.P.; Cossey, V.; van Goudoever, J.B.; Hulzebos, C.V.; Andriessen, P.; van Kaam, A.H.; Kramer, B.W.; et al. Risk Factors for Necrotizing Enterocolitis: A Prospective Multicenter Case-Control Study. Neonatology 2018, 114, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.T.; Patel, R.M. A critical analysis of risk factors for necrotizing enterocolitis. Semin. Fetal. Neonatal. Med. 2018, 23, 374–379. [Google Scholar] [CrossRef] [PubMed]
| Author/Year/Country | Study Design, Technique Applied | Inclusion Criteria (GA/BW) n NEC Definition | Mean (SD)/Median (IQR/Range) GA (Weeks) | Mean (SD)/Median (IQR) BW (Grams) | Sample Type and Timing | Increased Metabolites Cases vs. Controls (Number of Cases/Total Cases) | Decreased Metabolites Cases vs. Controls (Number of Controls/Total Controls) | Comments (Male Gender, C-Section, Antenatal Steroids, Antepartum Antibiotics, Enteral Feeding) |
|---|---|---|---|---|---|---|---|---|
| Brehin 2020 France [25] | Cross-sectional; NMR spectroscopy | <34 w Cases: 11 NEC Controls: 21 Suspected NEC by neonatologist (excluding CCC and SIP) | Cases: 28.4 (26–31) Controls: 30 (26.6–32) | Cases: 1150 (845–1815) Controls: 1360 (700–2105) | Stool every 10 days (1–10 d, 11–20 d, 21–30 d, >30 d) | None | Cases: lower Ethanol (>30 d); Serine (11–20 d); Leucine (>30 d) Controls: higher Serine (11–20 d); Ethanol (>30 d), Leucine (>30 d) | Cases: Male 9 (81.8%) CS NI ANS 11 (100%) Antepartum ATB 4 (36%) EBM NI Time of onset NI Controls: Male 13 (61.9%) CS NI ANS 19 (90%) Antepartum ATB 5 (24%) EBM NI |
| Morrow 2013 Ohio USA [26] | Cross-sectional; NMR spectroscopy | <29 w, <1200 g Cases: 11 NEC Controls: 21 NEC II or III Bell’s stage Subtypes of NEC based on ordination of day 4–9 samples: -NEC-I (n = 4) dominated by Firmicutes (Bacilli) -NEC-II (n = 5) dominated by Proteobacteria (Enterobacteriaceae) | Cases: 25.5 (1.8) Controls: 25.9 (1.5) | Cases: 791 (212) Controls: 839 (187) | Urine; T1 4–9 days; T2 10–16 days | T1: Increased Alanine and histidine normalized PI in NEC-I subtype compared to NEC-II subtype and controls; Cases: Alanine/histidine > 4 (9/11, 82%) Controls: A/H > 4 (5/20, 25%) | Controls: decreased alanine normalized PI; | Cases: Male 6 (54.6%) CS 7 (63.6%) ANS 10 (90.9%) Antepartum ATB 6 (54.6%) EBM (M or D) 11 (100%) Time of onset: NEC-I 7–21 d, NEC-II 19–39 d Controls: Male 8 (38.1%) CS 14 (66.7%) ANS 19 (90.5%) Antepartum ATB 10 (47.6%) EBM (M or D) 11 (100%) |
| Picaud 2021 France-Italy [27] | Cross-sectional; NMR spectroscopy | VLBW Cases: 6 NEC (3 early-onset, 3 late-onset) Controls: 12 (6 with feeding intolerance FI; 6 with good digestive tolerance GDT) NEC defined by clinical evidence fulfilling Bell’s stage criteria and with radiological pneumatosis intestinalis (stage ≥ II) | Cases: 27.1 (1.6) Controls 1: 27.2 (1.3) Controls 2: 27.7 (1.6) | Cases: 1016 (104) Controls 1: 920 (104) Controls 2: 950 (65) | Urine; Cases: before and at disease onset; Controls: at birth and as close as those of babies with NEC | Cases- Early-onset (<25 days): no differences; Cases-Late-onset (>40 days): increased lactate RI; | Controls GDT: increased N,N-DMG, betaine, myo-inositol, creatinine, urea RI; | Cases: Male 3 (50%) CS 2 (33.3%) EBM NI ANS, Antepartum ATB NI Time of onset < 25 days (3, 50%); >40 days (3, 50%) Controls 1: Male 3 (50%) CS 5 (83.3%) EBM NI ANS, Antepartum ATB NI Controls 2: Male 3 (50%) CS 3 (50%) EBM NI ANS, Antepartum ATB NI |
| Rusconi 2018 MO USA [28] | Prospective; UPLC-MS/MS for BRM, then targeted analysis of 14 ceramides and 7 sohingomyelins | Inclusion criteria NI Broad range metabolomics (BRM): Cases: 9 Controls: 19 Targeted: Cases: 23; Controls: 46 NEC Bell’s stage II and III, no SIP | BRM: Cases: 25.9 Controls: 25.1 Targeted: Cases: 25.9 (24.7–27.35) Controls: 25.5 (25–27.5) | BRM: Cases: 825.2 Controls: 787.3 Targeted: Cases: 800 (720–955) Controls: 840 (662.5–927.5) | BRM: stools closed to NEC onset (<5 days preceding it, not from the same day); Targeted: pre-event stool (1–3 days before NEC onset) | Cases (NEC II and III): increased sphingomyelins (not specified) Peak values expressed for the metabolites of the targeted analysis | Cases: low ceramides (not specified) | Targeted: Cases: Male 14 (61%) CS 19 (83.6%) EBM (M or D) 15 (65.2%) ANS NI, Antepartum ATB NI Time of onset 24 days Controls: Male 31 (67.2%) CS 29 (68.4%) EBM (M or D) 31 (67.4%) ANS NI Antepartum NI |
| Stewart Microbiome 2016 UK [29] | Prospective collection, retrospective analysis; UPLC-MS | <32 GW Cases: 7 NEC Controls: 28 Metabolomic analysis on 6 cases and 10 controls NEC “defined rigorously” by one senior clinician and two senior research clinicians, and classified as either surgical or medical, where pneumatosis was required for medical cases | Cases: 26 (23–30) Controls: 27 (24–30) | Cases: 760 (500–1470) Controls: 910 (545–1810) | Stools; (DOL) −14 (time point 1; TP1), −7 (TP2), 0 (TP3), +7 (TP4), and +14 (TP5) | TP3 (disease diagnosis): 5 metabolites with highest VIP scores: linoleate metabolism (2), C21-steroid hormone biosynthesis and linoleate metabolism (2), leukotriene metabolism and prostaglandin formation from arachidonate (1) | Cases: Male 3 (42.9%) CS 3 (42.9%) Time of onset 26.4 (14–42) days; ANS, EBM, Antepartum ATB NI Controls: Male 20 (71.4%) CS 15 (53.6%) ANS, EBM, Antepartum ATB NI | |
| Stewart 2016 Ped Res UK [30] | Prospective collection, retrospective analysis; UPLC-MS | Inclusion criteria NI Cases: 10 (6 NEC, 4 LOS) Controls: 9 NEC categorized independently by attending physician and blindly confirmed | Cases NEC: 26.3 Controls: 26.2 | Cases NEC: 922.5 Controls: 982.2 | Serum; Cases: 14 days (+/−7) prior and 14 days (+/−4) after diagnosis | No unique metabolites characterizing patients with NEC compared to controls | No unique metabolites characterizing patients with NEC compared to controls | Cases: CS 2 (33.3%) EBM 5 (83.3%) Time of onset 24.2 days (16–31) Male, ANS, Antepartum ATB NI Controls: CS 4 (44.4%) EBM 8 (88.9%) Male, ANS, Antepartum ATB NI |
| Thomaidou 2019 Greece [31] | Prospective cross-sectional; untargeted NMR spectroscopy and targeted LC-MS | Preterm neonates (GA not specified); Cases: 15 (5 NEC I, 10 NEC II/III) Controls: 15 NEC every grade; separate sub-group analysis after excluding stage I NEC cases | Cases: 34 (29–36) Controls: 33.5 (29–36) | Cases: 2030 (1100–2680) Controls: 1815 (1130–2640) | Urine at time of evaluation for NEC; Cases: 8 (4–22) d Controls: 9 (4–34) d | Cases: Low Tyrosine (FC −1.6, AUC 0.80), Proline (FC −0.26, AUC 0.83), Citrate (FC −1.3, AUC 0.85), 4-hydroxybenzoate (FC −1.09, AUC 0.86), Formate (FC −1.33, AUC 0.82), Succinate (FC −1.0, AUC 0.89), 4-hydroxyphenylacetate (FC −1.23, AUC 0.78), Fumarate (FC −1.54, AUC 0.82), Creatinine (FC −0.35, AUC 0.79), Myo inositol (FC −0.24, AUC 0.79), hippuric acid (FC −1.46, AUC 0.76) | Cases: Male 9 (60%) CS 10 (66.7%) ANS 9 (60%) Antepartum ATB, EBM, Time of onset NI Controls: Male 8 (53.3%) CS 12 (80%) ANS 10 (66.6%) Antepartum ATB, EBM NI | |
| Wandro 2018 California USA [32] | Retrospective; GC-MS | VLBW (<1500 g) Cases: 3 (NEC) Controls: 21 NEC definition NI | Cases 1: 25.6 Cases 2: 25.6 Controls: 27.4 | Cases 1: 920 Cases 2: 776.4 (1 missing) Controls: 1018.6 | Stools between days 7 and 75 of life (variable among patients) | No metabolites associated with NEC | No metabolites associated with NEC | Cases: Male NI CS 1 (33.3%) ANS, Antepartum ATB NI EBM 1 (33.3%) Mean time of onset 33 days Controls: Male NI CS 16 (76.2%) ANS, Antepartum ATB NI EBM 12 (57.1%) |
| Wang 2019 China [33] | Prospective collection, retrospective analysis; GC-MS | Cases (<35 GW, BW < 2.2 g): 19, 4 with NEC Controls (full-term): 20, 1 with NEC NEC Bell’s stage ≥1 | Cases: 29.6–34.4 Controls: NI | Cases: 1500–2100 Controls: NI | Serum; before feeding | No differences related to NEC diagnosis but only to prematurity | NI | No data specified |
| Wilcock 2015 UK [34] | Prospective collection, retrospective analysis; GC-MS | Cases 1 (GA at birth <30 GW, NEC): 7 Cases 2 (GA at birth <30 GW): 5 Controls (GA at birth ≥37 GW): 8 NEC definition NI | Cases 1: 25 (24–28) Cases 2: 27 (25–29) Controls: 38.5 (37–40) | Cases 1: 799 (653–942) Cases 2: 987 (855–1209) Controls: 2983 (2642–3820) | Serum; T1: first 7 days of life; T2: At full enteral feeds (≥180 mL/kg/die) | No differences at T1; T2: 16 different metabolites (p < 0.05), 10/16 aminoacids with a FCR 0.26–8.19, high false discovery rate (only 3 samples from NEC cases); among these glycine, L-serine, decanoic acid, methionine, phenylalanine, ornithine and lysine (interleukin−1β pathway) | No differences at T1; T2: 16 different metabolites (p < 0.05), 10/16 aminoacids with a FCR 0.26–8.19, high false discovery rate (only 3 samples from NEC cases); among these glycine, L-serine, decanoic acid, methionine, phenylalanine, ornithine and lysine | Cases 1: Male 3 (42.9%) CS, ANS, Antepartum ATB NI EBM at full feeds 3 (60%) Time of onset 28 (15–35) days Cases 2: Male 4 (80%) CS, ANS, Antepartum ATB NI EBM at full feeds 2 (28.6%) Controls: Male 5 (62.5%) CS, ANS, Antepartum ATB NI EBM at full feeds 7 (87.5%) |
| Author/Year/Country | Study Design, Technique Applied | Inclusion Criteria (GA/BW) n NEC Definition | Mean (SD)/Median (IQR) GA (Weeks) | Mean (SD)/Median (IQR) BW (Grams) | Sample Type and Timing | Increased Pathogens Cases Decreased Pathogens Cases | Increased Pathogens Controls Decreased Pathogens Controls | Diversity Index (Chao-i or Others) | Comments (Male Gender, C-Section, Antenatal Steroids, Antenatal ATB, Time at NEC Onset) |
|---|---|---|---|---|---|---|---|---|---|
| Brehin 2020 France [25] | Monocentric case-control; 16S bacterial DNA V3–V4 regions analysis by MiSeq | <34 w Cases: 11 NEC1 Controls: 21 Suspected NEC by neonatologist (excluding CCC and SIP) | Cases: 28.4 (26–31) Controls: 30 (26.6–32) | Cases: 1150 (845–1815) Controls: 1360 (700–2105) | Stool every 10 days (1–10 d, 11–20 d, 21–30 d, >30 d) | Increased Cases: Streptococcus spp., Staphylococcus spp., Micrococcales High intragroup variance | Increased Controls: Klebsiella spp. High intragroup variance | 1–10 d Cases: lower Chao-i, p < 0.05; 11–20 d Cases: increased Chao-i, p < 0.05; No difference in Shannon and Simpson | Cases: Male 9 (81.8%) CS NI ANS 11 (100%) Antepartum ATB 4 (36%) EBM NI Time of onset NI Controls: Male 13 (61.9%) CS NI ANS 19 (90%) Antepartum ATB 5 (24%) EBM NI |
| Cassir 2015 France [35] | Prospective case-control; V6 region of 16S rRNA pyrosequencing to develop specific qPCR assay for Clostridium butyricum tested on the 2nd cohort | Preterm neonates (not specified) First analysis: Cases: 15 NEC (10 stage II, 5 stage III) Controls: 15 Second confirmation analysis: Cases: 93 Controls: 270 NEC defined as Bell’s stage II or III | Cases: 28.2 (2.7) Controls: 29 (2.8) | Cases: 1127 (380) Controls: 1220 (506.9) | Stools on the day of symptoms’ onset and on the same matched day for controls | Increased Cases: Clostridium butyricum (11/15, p < 0.01) | Decreased Controls: C. butyricum (2/15) | Shannon diversity index lower in NEC than controls (p = 0.035); OTUs decreased in NEC than controls (p < 0.0001) | Cases: Male 10 (66.7%) CS 11 (73.3%) ANS, Antepartum ATB, EBM NI Mean time of onset 18.2 (12.6) d Controls: Male 9 (60%) CS 12 (80%) ANS, Antepartum ATB, EBM NI |
| Claud 2013 IL USA [36] | Cross-sectional; V3–V4 region of 16S rRNA gene sequencing; Shotgun metagenomics-based analyses to examine gene function; | GA 24–32 GW Cases: 5 (NEC) Controls 1: 5 preterms Controls 2: 8 full-term breast-fed infants NEC definition not specified | Cases: 26.8 (24.4–32) Controls 1: 24.4 (24.1–32) | NI | Stools; weekly from birth to 10 weeks of life | Increased Cases: Proteobacteria Decreased Cases: Firmicutes Gene sets differentially abundant between two twins: the one with NEC with more carbohydrate metabolism | Increased Controls: Proteobacteria and Firmicutes | NI | Cases: Male 2 (40%) CS 5 (100%) ANS, Antepartum ATB NI EBM 2 (40%) Time of onset NI Controls 1: Male 3 (60%) CS 5 (100%) ANS, Antepartum ATB NI EBM 2 (40%) |
| Dobbler 2017 Brazil [37] | Cross-sectional; V4 region of 16S rRNA gene sequencing; Metagenomic sequencing | GA ≤ 32 GW Cases: 11 NEC Controls: 29 NEC diagnosed by neonatologist based on clinical criteria (abdominal distention, gastric aspirates, bilious vomiting, bloody stools, lethargy, apnea, hypoperfusion); in 9 patients pneumatosis intestinalis, in 2 patients surgical diagnosis | Cases: 29.7 (2.2) Controls: 31.1 (1.6) | Cases: 1235 (411.1) Controls: 1529 (474.4) | Stools; weekly from first stool up to NEC diagnosis or 5 weeks of life | Increased Cases: Proteobacteria (Enterobacteriaceae: Citrobacter koseri, Klebsiella pneumoniae, E. Coli); Bacteroidetes, Actinobacteria Decreased cases: Bradirhizobiaceae (Bacteroides), Lactobacillus sp | Increased Controls: Firmicutes (Lactobacillus) | Cases: lower microbial diversity with abnormal succession of microbial community | Cases: Male NI CS 7 (63.6%) ANS NI Intrapartum ATB 5 (45.5%) EBM 1 (9%) Time of onset 8 (5–13) d Controls: Male NI CS 24 (82.7%) ANS NI Intrapartum ATB 15 (51.7%) EBM 2 (6.9%) |
| Duan 2020 China [38] | Prospective; PCR-DGGE combined with DNA sequencing of V3 region of 16S rDNA | GA < 37 GW Cases: 28 (16 Bell’s I, 11 Bell’s II, 1 Bell’s III) Controls: 30 NEC Bell’s stage ≥ I | Cases: 34.2 (1.3) Controls: 34.7 (1.6) | Cases: 2.2 (0.4) Controls: 2.4 (0.5) | Stools; days 1, 3, 5, 7, 9 after admission (admission = diagnosis?), and at discharge | Increased Cases: Bacteroides and Klebsiella (higher number of samples) Decreased Cases: E. Coli, Bifidobacterium, Lactobacillus | Increased Controls: E. Coli, Bifidobacterium, Lactobacillus | Cases: lower Shannon’s diversity index (1.97 (0.54) vs. 2.68 (0.31)) gradually increasing over time; Species richness 7.68 (0.73) vs. 15.47 (2.62) | Cases: Male 15 (53.6%) CS 14 (50%) ANS, Antepartum ATB, Time of onset NI EBM 16 (57%) Controls: Male 14 (46.7%) CS 15 (50%) ANS, Antepartum ATB NI EBM 17 (56.7%) |
| Feng 2019 China [39] | Case-control; V3–V4 region of 16S rRNA gene sequencing | GA > 28 GW Cases: 16 Controls: 16 NEC Bell’s stage II or III | Cases: 34.8 (33.4–36.1) Controls: 35.1 (33.1–36.5) | Cases: 2325 (2063–2575) Controls: 2345 (2025–2675) | Stool; average of <10 h after NEC diagnosis; controls at the same postnatal day | No significant difference Increased Cases: Actinobacteria (Propionibacteriales) and Bacteroides Decreased Cases: Lactobacillus, Phascolarctobacterium and Str. salivarius | No significant difference Increased Controls: Lactobacillus, Phascolarctobacterium, Str. Salivarius Decreased Controls: Bacteroidetes | No difference in total diversity as expressed by Chao-i (p = 0.40) | Cases: Male 8 (50%) CS 8 (50%) ANS, Antepartum ATB NI EBM 3 (18.7%) Mean time of onset 9 (5–16) d Controls: Male 8 (50%) CS 8 (50%) ANS, Antepartum ATB NI EBM 5 (31.2%) |
| Heida 2016 Netherlands [40] | Case-control in prospective cohort trial; V3–V4 region of 16S rRNA gene analysis | ≤30 GW, BW ≤ 1000 g or ≤32 GW and SGA with BW ≤ 1200 g, or with cardiovascular disease and reduced splanchnic blood flow, or neonates exposed to maternal indomethacin Cases: 11 NEC Controls: 22 NEC with pneumatosis intestinalis, or PVG, or both (Bell’s stage ≥II) | Cases: 27 (24–29) Controls: 26 (24–29) | Cases: 970 (560–1630) Controls: 995 (615–1735) | Stool; twice a week from meconium to last 2 faeces prior to NEC | Increased Cases: Cl. Perfrigens (8.4% and 6%) and Bacteroides dorei (0.9% and 0.7%) (meconium and 2 timepoints before NEC); Decreased Cases: Cl. Difficile (0.02%) (meconium), Staphylococci (0.5%) (1 timepoint before NEC); | Increased Controls: Cl. Difficile (2.7%) (meconium), and Staphylococci (23%) (1 timepoint before NEC) Decreased Controls: Cl. Perfrigens (0.1% and 0.003%) and Bacteroides (0.2% and 0.005%) | Microbial diversity (Simpson index) not associated with NEC development | Cases: Male 6 (54.5%) CS 4 (36.4%) ANS, EBM NI Peripartum ATB 3 (27%) Time of onset 12.5 (range 4–43) d Controls: Male 10 (45.5%) CS 13 (59%) ANS, EBM NI Peripartum ATB 5 (23%) |
| Itani 2018 Lebanon [41] | Case-control; quantitative PCR (qPCR); V3–V4 region of 16S rRNA analysis via TTGE; | GA < 36 GW (27–35 GW) Cases: 11 NEC Controls: 11 NEC Bell’s stage II or III | Cases: 31.2 (2.4) Controls: 31.4 (2.2) | Cases: 1516 (365) Controls: 1733 (481) | Stool; weekly from first meconium; Samples from NEC subjects divided into: before NEC diagnosis (TP1, >10 days before), at NEC diagnosis (TP2, <72 h from NEC), after NEC diagnosis (TP3, 7 to 10 days after); | qPCR: Increased cases: Staphylococci (p = 0.003); Decreased cases: lactobacilli (p = 0.048) and Enterococci (p = 0.039); TTGE: no clusterisation; | qPCR: Increased controls: Bifidobacterium and lactobacilli | TTGE: simple fecal microbiota in both groups (NMB 5.9, 1–10 vs. 6.7, 2–11) | Cases: Male 5 (45.5%) CS 11 (100%) ANS, Antepartum ATB NI EBM 4 (36.4%) Mean time of onset 22.6 (11.9, 10–50) d Controls: Male 8 (72.7%) CS 11 (100%) ANS, Antepartum ATB NI EBM 7 (63.6%) |
| Leach 2015 Australia [42] | Case-control; 16S rDNA gene analysis using next-generation sequencing techniques | GA 24–32 GW Cases: 4 NEC Controls: 18 NEC Bell’s stage II or III | Cases: 27.4 (2.5) Controls: 27.9 (0.7) | Cases: 1060 (346) Controls: 1204 (182.3) | Stools; first meconium, then daily for the first week, then weekly for the first 4 weeks; In cases, samples collected daily for the first week after diagnosis, then weekly until discharge; | Increased Cases: no consistency at the phyla level; Corynebacterium striatum (p = 0.01) and Morganella Morganii (p = 0.02), Ps. Aeruginosas, Corynebacterium amycolatum | Increased Controls: Proteobacteria | Species diversity prior to NEC equivalent or higher than controls of 27–32 GW (exact values NI) | Cases: Male 2 (50%) CS 3 (75%) ANS, Antepartum ATB NI EBM 2 (50%) Time of onset 34 (range 10–79) d Controls: Male 9 (50%) CS 10 (55.5%) ANS, Antepartum ATB NI EBM 10 (55.5%) |
| Lindberg 2018 CT, USA [43] | Prospective case-control; V4 of 16S rRNA gene sequencing | GA < 30 GW Cases: 5 NEC Controls: 5 NEC Bell’s stage II or III | Cases: 25.4 (24–27) Controls: 25 (24–27) | Cases: 695.8 (516–1026) Controls: 663.4 (485–860) | Stool; weekly from first meconium until discharge; | Increased Cases: Proteobacteria (Enterobacteriacee and Trabulsiella) | Increased Controls: Firmicutes (Veillonella and Enterococcus) | Number of OTUs and Simpson diversity index not different | Cases: Male 4 (80%) CS 4 (80%) ANS, Antepartum ATB NI Time of onset 38.5 (range 24–50) d EBM (M or D) 5 (100%) Controls: Male 4 (80%) CS 4 (80%) ANS, Antepartum ATB NI EBM (M or D) 4 (80%) |
| Liu 2019 China [44] | Prospective; V3-V4 regions of 16S rRNA gene sequencing and quantification with QuantiFluor-ST | GA < 33 GW BW > 950 g Cases: 4 NEC Controls: 17 NEC Bell’s stage II or III | Cases: 29 (29–30) Controls: 31 (28–33) | Cases: 1416.3 (773.4–2149.1) Controls: 1527.4 (1391.6–1663.1) | Stools; weekly, from meconium until death or discharge; Timepoints: TP1/EPP within 3 DOL; TP2/EPO from TP1 to 4 days before NEC; TP3/LPO from TP2 and onset; TP4/ED first third interval of disease span; TP5/MD; TP6/LD; TP7/PD from end to discharge | Increased Cases: Bacilli (TP1); Peptoclostridium; rapid surge in Enterococcus, Staphylococcus, Peptoclostridium and Streptococcus after disease onset Decreased Cases: TP1: Enterococcus (0.51%); TP1-TP4 decrease in Lactococcus (24.54 > 0.94%) | Increased Controls: Firmicutes (Veillonella), progressive increase in Klebsiella, Escherichia and Shigella Decreased Controls: TP1 to TP4: decrease in Lactobacillus, Pseudomonas and Enterococcus | Decreasing trend in microbiome richness (Sobs and Shannon-i) over time in both groups (NEC: p = 0.044, 3.14 > 0.58, p = 0.01; Controls: p < 0.01, 2.77 > 1.004, p = 0.04); Shannon-i significantly lower in NEC at TP4 (p = 0.025) | Cases: Male 1 (25%) CS 4 (100%) ANS, Antepartum ATB, EBM NI Time of onset 16 (11–19) d Controls: Male 8 (47%) CS 17 (100%) ANS, Antepartum ATB, EBM NI |
| Mai 2011 Florida, USA [45] | Prospective; DNA extraction via DGGE and V6–V8 region of 16S rRNA sequencing | GA ≤ 32 GW BW ≤ 1250 g Cases: 9 NEC Controls: 9 NEC Bell’s stage II or III | Cases: 26.2 (23–29) Controls: 27.5 (26–30) | Cases: 913.6 (570–1225) Controls: 1058 (652–1269) | Stools; weekly from meconium until discharge, and within 72 h from NEC | Increased Cases: Proteobacteria from 1 week up to <72 h from NEC (34% increase); y-Proteobacteria (related to Enterobacteriaceae) Decreased Cases: Actinobacteria and Bacteroidetes; decrease in Firmicutes from 1 week up to <72 h from NEC (32% decrease) | Increased Controls: Firmicutes, Bacteroides Decreased Controls: Proteobacteria | Total number of OUTs (estimated by Chao-i curves) not different | Cases: Male 5 (55.5%) CS 4 (44.4%) ANS, Antepartum ATB NI Time of onset 23.7 (range 5–41) d Controls: Male 4 (44.4%) CS 4 (44.4%) ANS, Antepartum ATB NI Predominant milk reported |
| McMurtry 2015 Louisiana USA [46] | Prospective; V1 to V3 region of 16S rRNA gene sequencing | GA ≤ 34 GW BW ≤ 1500 g Cases: 21 NEC Controls: 74 NEC Bell’s stage II or III, divided into mild, severe, lethal | Cases: 27.2 (2.8) Controls: 28.3 (2.5) | Cases: 1037 (397) Controls: 1111 (370) | Stools; 1–5 days prior to NEC symptoms’ onset, or on day of diagnosis; controls with matched specimens | Decreased Cases: Actinobacteria (1.24, p = 0.009) and Firmicutes (Clostridia, 5.76, p = 0.004); Veillonella (0.71, p = 0.007) and Streptococcus (0.96, p = 0.002)) | Increased Controls: Actinobacteria (1.67) and Firmicutes (Clostridia 18.8), Veillonella (6.61, p = 0.007) and Streptococcus (4.32, p = 0.002) | Low bacterial diversity in cases (Chao-i p < 0.0001, Shannon-i p < 0.0002) | Cases: Male 11 (52.4%) CS 12 (57.1%) ANS 14 (66.7%) Antepartum ATB NI EBM 7 (33.3%) Time of onset (time of samples) 26.7 (14.9) days Controls: Male 38 (51.4%) CS 56 (75.7%) ANS 42 (56.7%) Antepartum ATB NI EBM 17 (30%) |
| Morrow 2013 Ohio USA [26] | Cross-sectional; V3 to V5 regions of 16S rRNA gene sequencing by 454 FLX Titanium | <29 w, <1200 g Cases: 11 (8 Bell’s stage II and 3 Bell’s stage III) Controls: 21 NEC II or III Bell’s modified; Subtypes of NEC based on ordination of day 4–9 samples: -NEC-I (n = 4) dominated by Firmicutes (Bacilli) -NEC-II (n = 5) dominated by Proteobacteria (Enterobacteriaceae) | Cases: 25.5 (1.8) Controls: 25.9 (1.5) | Cases: 791 (212) Controls: 839 (187) | Stools; TP1 4–9 days; TP2 10–16 days | Increased Cases: TP1: Firmicutes (4/9, NEC-I: Enterococcus and Staphylococcus 98%), -TP2: Proteobacteria (5/9, NEC-II, Enterobacteriaceae) PPV of Firmicutes or Proteobacteria 88% Decreased Cases: Firmicutes and Actinobacteria, Propionibacterium (TP1) | Increased Controls: Propionibacterium (10/18, 56%), 80% Proteobacteria (12/18: Enterobacter, Escherichia), 20% Firmicutes (Enterococcus, Staphylococcus); Decreased Controls: TP1: Firmicutes (2/18: Enterococcus 62% and Staphylococcus 73%); | T1: Chao-i: Cases 9.2, Controls 18.4, p = 0.086; similar Simpson index (p = 0.221) | Cases: Male 6 (54.6%) CS 7 (63.6%) ANS 10 (90.9%) Antepartum ATB 6 (54.6%) EBM (M or D) 11 (100%) Time of onset: NEC-I 7–21 d, NEC-II 19–39 d Controls: Male 8 (38.1%) CS 14 (66.7%) ANS 19 (90.5%) Antepartum ATB 10 (47.6%) EBM (M or D) 11 (100%) |
| Normann 2013 Sweden [47] | Prospective case-control; V3-V4 regions of the 16S rRNA gene amplification and barcoded pyrosequencing | <28 GW Cases: 10 NEC Controls: 10 (+6 in sub-analysis) NEC Bell’s stage II or III with radiological pneumatosis and/or portal venous gas or intraoperative histopathology confirmation | Cases: 23.5 (22–25.5) Controls: 23.5 (22–25.6) | Cases: 582 (487–965) Controls: 570 (440–892) | Stools; weekly for first 7 weeks or until NEC diagnosis | Increased Cases: Bacillales and Enterobacteriaceae in the 1st week; Enterobacteriaceae in the 2nd week | Increased Controls: Firmicutes (Enterococcus and Bacilli) | No difference in Shannon-i between cases and controls | Cases: Male NI CS 4 (40%) ANS NI Antepartum ATB 9 (90%) EBM (M or D) 100% Time of onset 5–48 daysI Controls: Male NI CS 4 (40%) ANS NI Antepartum ATB 8/16 (50%) EBM (M or D) 100% |
| Olm 2019 CA USA [48] | Prospective; Extensive computational analyses to recover genome de novo, phylogeny, metabolic potential and replication rates; | Criteria NI Cases: 34 NEC Controls: 126 NEC NI | Cases: 28 (2.5) Controls: 29 (2.2) | Cases: 1154.5 (465.3) Controls: 1217 (388.5) | Stools; mostly first month of life (average 7.2 samples per infant), with focus on those immediately before NEC onset (<2 days before, “pre-NEC”) | Increased Cases: Enterobacteriaceae (p = 8.9 × 10−7) and Bacteroidetes after NEC development; K. pneumoniae 52% pre-NEC (p = 0.008) Decreased Cases: Firmicutes (p = 3.7 × 10−7) after NEC | Increased controls: Firmicutes Decreased Controls: Enterobacteriaceae (K. pneumoniae 23%) | NI | Cases: Male 15 (44.1%) CS 25 (73.5%) ANS, Antepartum ATB NI EBM 14 (41.2%) Mean time of onset 9 (9.8) d Controls: Male 61 (48.4%) CS 93 (73.8%) ANS, Antepartum ATB NI EBM 35 (27.8%) |
| Rozé 2017 France Clinical data from EPIPAGE 2; Microbial data from EPIFLORE [49] | Prospective; V3–V4 region of 16S rRNA gene pyrosequencing | 24–31 GW, >7 DOL Cases: 106 NEC Controls: 3055 NEC Bell’s stage II or III | NI (divided into 3 subclasses) | Data for whole cohort Cases: NI Controls: 1175 (348) | Stools; at 7 and 28 DOL, at discharge (controls), at NEC diagnosis (cases); Culture-independent microbiota analysis on 15 cases and 57 controls | Increased Cases: Cl. sensu stricto (20.6%, p = 0.08) (neonatale and butyricum); Gammaproteobacteria (Enterobacteriaceae) Decreased Cases: Gammaproteobacteria (Klebsiella), Staphylococcus sp., Enterococcus faecalis | Increased Controls: Staphylococcus sp., Enterococcus faecalis Decreased Controls: Cl. sensu stricto (11.7%) | NI | Data for whole cohort: Cases: Male 63 (59.4%) CS, ANS, EBM, Antepartum ATB NI Median time of onset 26 (IQR 20–42) d Controls: Male 1548 (52.3%) CS, ANS, EBM, Antepartum ATB NI |
| Sim 2014 UK [50] | Prospective; V3-V5 regions of 16S rRNA gene sequen cing | <32 GW Cases: 12 NEC Bell’s stage II or III (analysed), 8 suspected NEC Controls: 44 (36 analysed) NEC according to VON criteria and staged according to Bell | Cases: 27 (25.7–28.4) Controls: 27.5 (25.4–29) | Cases: 845.4 (685–898.8) Controls: 1005.9 (755–1239.5) | Stools, every sample from recruitment until discharge | Increased Cases: Clostridia (Confirmed NEC, p = 0.006) | Increased Controls: Klebsiella, Staphylococcus, Enterobacteriaceae, Enterococcus, and Bifidobacterium | NI | Cases: Male 5 (41.7%) CS 7 (58.3%) ANS, EBM NI Peripartum ATB 2 (16.7%) Time of onset 27.5 (IQR 20.8–37.5) days Controls: Male 17 (47.2%) CS 18 (50%) ANS, EBM NI Peripartum ATB 11 (30.6%) |
| Stewart 2012 UK [51] | Prospective; V3 region of 16S rRNA gene amplification and profiling via DGGE | Preterm infants (criteria NI) Cases: 8 NEC Controls: 22 NEC categorized by 2 neonatologists as medical (pneumatosis, no surgery) or surgical | Cases: 25.7 (1.7) Controls: 27.2 (2.3) | Cases: 842.5 (227.4) Controls; 1027 (338.4) | Stools; weekly from meconium 7 NEC cases with molecular data | Increased Cases: Staphylococcus spp. (CONS 45%), Enterobacter spp. Decreased Cases: Ent. Faecalis (31%) | Increased Controls: Enterococcus spp. (Ent. Faecalis 57%) and Streptococcus spp. Decreased Controls: CONS (30%) | Low bacterial diversity increasing over time for whole cohort | Cases: Male 7 (87.5%) CS 4 (50%) ANS, Antepartum ATB, EBM NI Time of onset 16.7 (4.8) d Controls: Male 13 (59%) CS 11 (50%) ANS, Antepartum ATB, EBM NI |
| Stewart 2013 UK [52] | Prospective; PCR-DGGE analysis of targeted V3 region of 16S rRNA gene and pyrosequencing | GA < 32 GW from multiple birth Total cohort: 27 (12 twin pairs and 1 triple set), 5 of which developed NEC NEC confirmed by two neonatologists (definition NI) | Cases: 27.6 Controls: 27.2 | Cases: 1106 Controls: 975.2 | Stools, from birth to discharge | Increased Cases: Escherichia sp. | - | Reduced diversity in cases | Cases: Male 4 CS 3 ANS, Antepartum ATB, EBM NI Time of onset 25.4 (range 16–45) d Controls: Male 16 CS 17 ANS, Antepartum ATB, EBM NI |
| Stewart 2016 UK [29] | Prospective; V4 region of 16S rRNA gene sequencing | <32 GW Cases: 7 Controls: 28 NEC “defined rigorously” by one senior clinician and two senior research clinicians, and classified as either surgical or medical, where pneumatosis was required for medical cases | Cases: 26 (23–30) Controls: 27 (24–30) | Cases: 760 (500–1470) Controls: 910 (545–1810) | Stools; (DOL) −14 (TP1), −7 (TP2), 0 (TP3), +7 (TP4), and +14 (TP5) “Pre-NEC” > 10 days from onset | No clear causative organism diagnostic for NEC; No PGCTs assigned to preNEC samples PGCT 2 (Klebsiella and Enterococcus) and PGCT 5 (Escherichia) most associated with preNEC samples; Decreased Cases: PGCT 6 (Bifidobacterium) | Increased Controls: PGCT 6 (Bifidobacterium predominance) | Controls: higher alpha diversity and Shannon diversity (PGCT 6) compared to Cases; progressive lower acquisition of diversity | Cases: Male 3 (42.9%) CS 3 (42.9%) ANS, Antepartum ATB, EBM NI Time of onset 26.4 (14–42) days; Controls: Male 20 (71.4%) CS 15 (53.6%) ANS, Antepartum ATB, EBM NI |
| Torrazza 2013 Florida USA [53] | Prospective; V6–V8 region of 16S rRNA gene analysed by DGGE and PCR amplification, sequencing | ≤32 GW Cases: 18 NEC Controls: 35 NEC with clinical and radiologic signs or necrotic bowel at surgery | Cases: 27.4 (2.6) Controls: 28.5 (2.2) | Cases: 1073 (394) Controls: 1246 (350) | Stools; from birth (meconium) until discharge, analysed: TP1 2 weeks prior to NEC, TP2 1 week prior to NEC, TP3 closest to NEC diagnosis; matched for controls; | Increased Cases: -TP1: Proteobacteria (61%, p < 0.001)) -TP2: Actinobacteria 3% and Proteobacteria (p < 0.001) -TP3: Firmicutes 72%; Klebsiella granulomatis, Klebsiella pneumoniae and Clostridium perfringens, St. epidermidis Lower Cases: -TP1: Bacteroidetes | Decreased Controls: -TP1: Proteobacteria (19%) -TP2: Actinobacteria 0.4% | Similar Chao-i (species richness as alpha diversity) at all TPs; Significant different beta diversity (UNIFRA metric) at TP1 (p < 0.05) indicating a similar total number of species but different bacteria and proportions between cases and controls | Cases: Male 12 (66.7%) CS 9 (50%) ANS 11 (61%) Antepartum ATB 13 (72.2%) EBM 27.8% Time of onset 17.8 (12.8) Controls: Male 17 (48.6%) CS 23 (65.7%) ANS 20 (57%) Antepartum ATB 29 (82.9%) EBM 57.1% |
| Wandro 2018 California USA [32] | Retrospective; 16S rRNA gene sequencing | VLBW (<1500 g) Cases: 3 NEC Controls: 21 NEC definition NI | Cases: 25.6 Controls: 27.4 | Cases: 920 Controls: 1018.6 | Stools; over first 6 weeks of life (variable timepoints, between 7 and 75 DOL) | No single bacterial OUT or community composition consistent of NEC or LOS | Lower bacterial abundances in infants developing NEC or LOS (p < 0.001); Alpha diversity (Shannon-i) increasing overall with age | Cases: Male NI CS 1 (33.3%) ANS, Antepartum ATB NI EBM 1 (33.3%) Mean time of onset 33 days Controls: Male NI CS 16 (76.2%) ANS, Antepartum ATB NI EBM 12 (57.1%) | |
| Wang 2010 IL, USA [54] | Prospective case-control; 16S rRNA gene sequencing | 25–32 GW Cases: 10 NEC Controls: 10 NEC Bell’s stage II or III | Cases: 25–32 Controls: 26–32 | Birth weight NI | Stools; at NEC diagnosis (<1 day) (range 4–49 days) | Increased Cases: Proteobacteria (90.7% RA; p = 0.001; Gammaproteobacteria); at OTU level: Klebsiella pneumoniae, Shigella dysenteriae, Enterobacter hormaechei and Escherichia coli. Decreased Cases: Firmicutes (9.1%, p = 0.001) | Increased Controls: Firmicutes (57.8%), Bacteroidetes (2.4%), Fusobacteria (0.5%); at OTU level: Veillonella sp., Escherichia coli, Enterococcus sp., Staphylococcus sp., Enterobacter aerogenes (all 90%) Decreased Controls: Proteobacteria (34.9% RA, Gammaproteobacteria) | Low diversity in preterm infants, especially those with NEC: Shannon-i by T-RFLP cases 1.13 vs. controls 1.88, p = 0.035; OTUs cases 10.4 (6.1) vs. controls 19 (6.7), p = 0.008; Shannon-i by library cloning csses 1.19 (0.62) vs. controls 1.99 (0.55), p = 0.005 | Cases: Male 6 (60%) CS 8 (80%) ANS, Antepartum ATB NI EBM 4 (40%) Mean time of onset 5–49 days Controls: Male 7 (70%) CS 9 (90%) ANS, Antepartum ATB NI EBM 6 (60%) |
| Ward 2016 OH, USA [55] | Prospective caso-control; Shotgun metagenomic sequence analysis and pangenome-based computational analysis for E. Coli-specific gene content | Cases: 27 NEC (<30 GW) Controls 1: 117 (<30 GW) Controls 2: 22 (>37 GW) NEC Bell’s stage II or III (SIP excluded) | Cases: 26 (23–28) Controls 1: 26 (23–29) Controls 2: 39 (38–41) | Cases: 850 (415–1340) Controls 1: 904 (520–1741) Controls 2: 3476 (2217–4173) | Stools; between 3 and 22 DOL: TP1 3–9 DOL, TP2 10–16 DOL, TP3 17–22 DOL; | TP1 (8 NEC cases): taxa similar to preterm without NEC (Firmicutes Bacilli with S. epidermidis, Lactobacillales with E. fecalis, Gammaproteobacteria with Enterobacter) TP2 (15 NEC cases): not different from controls 1 (S. epidermidis, E. faecalis, E. cloacum, S. marcescens) TP3 (7 NEC cases): E.faecalis and Streptococcus; E. Coli; decreased Veillonella | TP1: controls 2: Actinobacteria (Bifidobacterium spp) and Bacteroidetes, Firmicutes, Negativicutes (Veillonella) TP2: controls 2 increased in all taxa except S. epidermidis, E. faecalis, E. cloacum, S.marcescens TP3: controls 2: all taxa except E.faecalis and Streptococcus | TP1: similar Shannon-i between cases and controls 1 (1.1 (0.79) vs. 0.96 (0.56), p =0.59) TP2: similar Shannon-i between cases and controls 1 (1.01 (0.92) vs. 1.15 (0.72), p = 0.52) TP3: Cases with less diversity than controls 1, but not significantly (SI 0.87 (0.63) vs. 1.32 (0.68), p =0.12) | Cases: Male 15 (56%) CS 16 (59%) ANS NI Peripartum ATB 12 (44%) Human milk ≥ 75% in first month 17 (63%) Mean time of onset 21 (7.4) days Controls 1: Male 61 (52%) CS 70 (60%) ANS NI Peripartum ATB 79 (68%) Human milk ≥ 75% in first month 86 (74%) Controls 2: Male 11 (50%) CS 10 (45%) |
| Warner 2016 MO USA [56] | Prospective; V3 to V5 regions of 16S rRNA genes pyrosequencing | VLBWI ≤ 1500 g, >7 days of life; Cohort 1: Cases: 28 NEC Controls: 94 Cohort 2: Cases: 18 NEC Controls: 26 NEC Bell’s stage II or III (SIP and CHD excluded) | Cohort 1: Cases: 26 (24.7–27.9) Controls: 27 (25.9–28.7) | Cohort 1: Cases: 795 (720–980) Controls: (940 (800–1500) | Stools; up to and including the day before NEC or at 60 days of age (whichever came first), divided into TP of 15 days | Increased Cases: Gammaproteobacteria (p = 0.001) (E. Coli, Enterobacter, Klebsiella) Decreased Cases: Anaerobic bacteria (Negativicutes, p = 0.0013; Clostridia-Negativicutes, p = 0.005) | Increased Controls: Negativicutes and Clostridia-Negativicutes | Shannon-i increasing in stools from controls, not from cases, with significantly discordant trend (p = 0.0004) | Cases: Male 18 (64%) CS 20 (71%) (75%) ANS, Antepartum ATB NI Exposure to human milk >50% 21 (75%) Time of onset 24 (19–48) days Controls: Male 45 (48%) CS 72 (77%) (53%) ANS, Antepartum ATB NI Exposure to human milk >50% 50 (53%) |
| Zhou 2015 MO USA [57] | Prospective case-control; V3–V5 region of 16S rRNA gene sequencing | <32 GW Cases: 12 NEC (6 medical, 6 surgical) Controls: 26 NEC Bell’s stage II or III (SIP excluded) | Cases: 27.8 (24–31) Controls: 27.9 (24–31) | Cases: 1048 (940–1860) Controls: 1092 (520–1800) | Stools, from birth to discharge or 60 DOL (median sampling interval of 3 days) | Increased Cases: Clostridia, in particular Cl. Sensu stricto (early onset NEC, <22 DOL); Gammaproteobacteria (Pseudomonas, Pasteurella,m Serratia, Klebsiella) and Escherichia, Shigella, Cronobacter, (Late onset NEC, >22 DOL) More differences at 2 weeks of life | Increased Controls: Veillonella (1–3 days prior to NEC, p = 0.005) Decreased Controls: Pasteurella | Increased richness in controls (p = 0.03); progressive increase in richness and Shannon-i over 2 months of life in cases (p<0.05) | Cases: Male 7 (58%) CS 9 (75%) ANS, EBM NI Antepartum ATB 4 (33%) Time of onset 25.5 (IQR 16.8–37) days Controls: Male 14 (54%) CS 17 (65%) ANS, EBM NI Antepartum ATB 6 (23%) |
| Study | Reason for Exclusion |
|---|---|
| Abdulkadir et al. 2016 [58] | Missing data |
| Barron et al. 2017 [59] | Missing data |
| De Magistris et al. 2015 [60] | Full text unavailable |
| Feng et al. 2017 [61] | Full text unavailable |
| Itani et al. 2019 [62] | Same population of Itani et al. 2018 [40]; brief report |
| Raveh-Sadka et al. 2015 [63] | Missing data; no comparison between NEC and Controls |
| Romano-Keeler et al. 2018 [64] | Comparison of NEC and surgical patients; no comparison with healthy controls |
| Sinclair et al. 2020 [65] | Targeted metabolome analysis |
| Smith et al. 2011 [66] | Analysis of inflamed intestinal tissue; no controls |
| Stewart et al. 2019 [67] | Comparison between NEC and SIP, no controls |
| Yang et al. 2015 [68] | Comparison between NEC and congenital intestinal atresia, no controls |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moschino, L.; Verlato, G.; Duci, M.; Cavicchiolo, M.E.; Guiducci, S.; Stocchero, M.; Giordano, G.; Fascetti Leon, F.; Baraldi, E. The Metabolome and the Gut Microbiota for the Prediction of Necrotizing Enterocolitis and Spontaneous Intestinal Perforation: A Systematic Review. Nutrients 2022, 14, 3859. https://doi.org/10.3390/nu14183859
Moschino L, Verlato G, Duci M, Cavicchiolo ME, Guiducci S, Stocchero M, Giordano G, Fascetti Leon F, Baraldi E. The Metabolome and the Gut Microbiota for the Prediction of Necrotizing Enterocolitis and Spontaneous Intestinal Perforation: A Systematic Review. Nutrients. 2022; 14(18):3859. https://doi.org/10.3390/nu14183859
Chicago/Turabian StyleMoschino, Laura, Giovanna Verlato, Miriam Duci, Maria Elena Cavicchiolo, Silvia Guiducci, Matteo Stocchero, Giuseppe Giordano, Francesco Fascetti Leon, and Eugenio Baraldi. 2022. "The Metabolome and the Gut Microbiota for the Prediction of Necrotizing Enterocolitis and Spontaneous Intestinal Perforation: A Systematic Review" Nutrients 14, no. 18: 3859. https://doi.org/10.3390/nu14183859
APA StyleMoschino, L., Verlato, G., Duci, M., Cavicchiolo, M. E., Guiducci, S., Stocchero, M., Giordano, G., Fascetti Leon, F., & Baraldi, E. (2022). The Metabolome and the Gut Microbiota for the Prediction of Necrotizing Enterocolitis and Spontaneous Intestinal Perforation: A Systematic Review. Nutrients, 14(18), 3859. https://doi.org/10.3390/nu14183859







