Abstract
Triglyceride-bound fatty acids constitute the majority of lipids in human milk and may affect infant growth. We describe the composition of fatty acids in human milk, identify predictors, and investigate associations between fatty acids and infant growth using data from the Norwegian Human Milk Study birth cohort. In a subset of participants (n = 789, 30% of cohort), oversampled for overweight and obesity, we analyzed milk concentrations of detectable fatty acids. We modelled percent composition of fatty acids in relation to maternal body mass index, pregnancy weight gain, parity, smoking, delivery mode, gestational age, fish intake, and cod liver oil intake. We assessed the relation between fatty acids and infant growth from 0 to 6 months. Of the factors tested, excess pregnancy weight gain was positively associated with monounsaturated fatty acids and inversely associated with stearic acid. Multiparity was negatively associated with monounsaturated fatty acids and n-3 fatty acids while positively associated with stearic acid. Gestational age was inversely associated with myristic acid. Medium-chain saturated fatty acids were inversely associated with infant growth, and mono-unsaturated fatty acids, particularly oleic acid, were associated with an increased odds of rapid growth. Notably, excessive maternal weight gain was associated with cis-vaccenic acid, which was further associated with a threefold increased risk of rapid infant growth (OR = 2.9, 95% CI 1.2–6.6), suggesting that monounsaturated fatty acids in milk may play a role in the intergenerational transmission of obesity.
1. Introduction
Human milk provides nutrients for the optimal development of newborns and infants [1]. The major energy-providing component in human milk is fat, 98–99% of which is triglyceride fatty acids (TGFAs) that are passed into milk from the maternal diet and fat stores or synthesized by the mammary gland [2,3]. The quantity and composition of lipids to which infants are exposed affect growth, vision, inflammatory responses, the gut, and neurodevelopment [4,5,6,7,8,9,10,11,12]. The developmental origins of health and disease hypothesis suggests that these programming effects last beyond infancy, impacting longer-term health outcomes, such as obesity [13].
The importance of human milk TGFAs in determining long-term obesity outcomes is indicated in animal models where exposure to certain TGFAs during early development can change the production and use of lipids within the body [14]. This is supported by human studies that link certain maternal serum fatty acid profiles during pregnancy to infant health outcomes, including blood pressure and neuropsychiatric outcomes, although the evidence for infant growth and obesity is equivocal [8,9,11]. Milk TGFA composition differs across mothers based on geography, sociodemographic factors, and diet and, thus, infants’ exposures vary [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Despite the potential impact that exposure to these fatty acids may have on infant health, our knowledge of what determines the composition of milk is still evolving. In this study, we characterized the concentrations and percent composition of detectable TGFAs in human milk and studied the relation between maternal factors and these TGFAs. Further, we explored associations between TGFA milk composition and infant growth during the first six months of life.
2. Materials and Methods
2.1. Study Population
This study was based on a subset (n = 789, 30% of 2606) from the Norwegian Human Milk Study (HUMIS), a multi-center, Norway-wide cohort of mothers and newborns [34,35,36]. Briefly, new mothers were recruited in 2003–2009 by public health nurses during routine home visits around 2 weeks postpartum in 7 counties across Norway [34]. A subset of mothers was recruited in 2002–2005 by a pediatrician at the maternity ward in Østfold hospital in Southern Norway, two term births for every preterm birth [34,35,36].
Mothers were asked to save and freeze a hand- or pump-expressed sample of the morning’s first milk prior to infant feeding on each of 8 consecutive days between 2 weeks and 2 months postpartum [35]. Milk samples were sent to the Norwegian Institute of Public Health in Oslo, Norway, where they were frozen and stored. The milk was collected in accordance with protocols established by the World Health Organization for biomonitoring of chemicals in human milk [37]. Mothers completed questionnaires about the child’s weight and height, maternal weight, and relevant confounders. Data from the Norwegian Medical Birth Registry and mothers’ medical pregnancy journals were used to supplement information on participants.
The study subset was determined as illustrated in Figure 1. Singleton mothers who were overweight and obese (pre-pregnancy body mass index (BMI) > 30 kg/m2 or BMI at delivery >32 kg/m2 with a pre-pregnancy BMI of <27 kg/m2) were oversampled and represent 41.5% of the sample as compared to 34.0% of women in the whole HUMIS cohort [38].
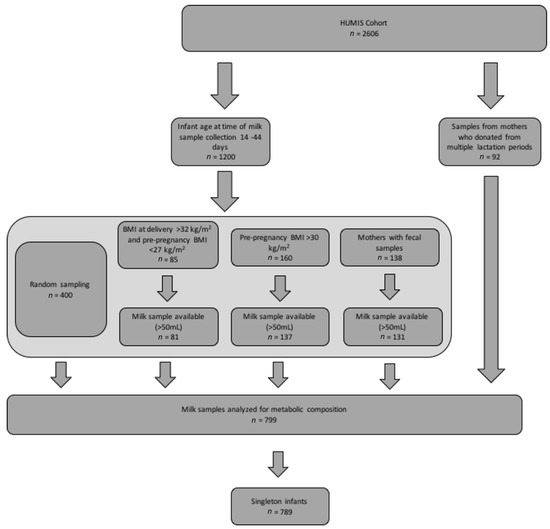
Figure 1.
Participant flow diagram for present analysis. Legend: This figure describes the sampling process by which the milk samples for the present analysis (n = 789) were selected from the Norwegian Human Milk Study (HUMIS) cohort (n = 2606). BMI stands for body mass index.
2.2. TGFA Composition Analysis
TGFAs were measured in pooled (per mother) milk samples collected at median 31 days (range: 14–108 days). Milk samples were shipped by air freight on dry ice to LMU Munich for analysis. TGFA composition was measured by gas chromatography (GC) as previously described [39]. Briefly, 100 µL milk was combined with 700 µL methanol and 600 µL tert-butyl-methyl-ether, vortexed for 30 seconds, and centrifuged at room temperature with 1500 g for 5 minutes to precipitate proteins. Next, 300 µL supernatant with the dissolved lipids were transferred into a 4 mL glass vial and combined with 1260 µL methanol, 140 µL hexane, and 400 µL methanolic hydrochloric acid. The content was mixed by shaking and heated to 100 °C for one hour to synthesize fatty acid methyl esters from free and lipid-bound fatty acids. Water (500 µL) and hexane (1000 µL) were added to achieve phase separation. An aliquot of the organic upper phase was used for GC after appropriate dilution for injection. GC separation of fatty acid methyl esters on a 60 m BPX-70 column and detection by flame ionization were performed as described elsewhere, with the modification that tri-undecanoin instead of tri-pentadecanoin was used as the internal standard for quantification [40]. TGFAs are presented as absolute concentrations (g/L) and percent of total fatty acid concentration by weight (%).
Thirty-two TGFAs with chain lengths of 8–22 carbon atoms could be quantified. As a control for analytical quality, 82 aliquots of a reference milk sample were distributed randomly among the study samples for the analysis. For TGFA concentrations, coefficients of variation were between 6% (C12:0) and 33% (C22:5n-6), with an average of 13%. For the percentage contributions of TGFAs, coefficients of variation ranged from 1% (C16:0) to 33% (C22:5n-6), with an average of 9%.
2.3. Predictors of TGFA Composition
We studied percent composition of 32 TGFAs, total saturated fatty acids (SFA), polyunsaturated fatty acids (PUFA), n-3 PUFAs, n-6 PUFAs, monounsaturated fatty acids (MUFA), trans-fatty acids, medium chain fatty acids (MCFA), and long-chain PUFAs (LC-PUFA). In addition, we studied SFA/PUFA and n-6/n-3 PUFA ratios (Table 1). For the purposes of this analysis, the intermediate-chain-length FA myristic acid (C14:0) was categorized as an MCFA due to its synthesis in the mammary gland and similar effects as MCFAs in the infant diet [1,41,42].

Table 1.
TGFAs included in each fatty acid sub-category 1.
We identified potential predictors of TGFAs from the literature [22,30,31,43,44,45,46,47,48] and included pre-pregnancy BMI (underweight versus normal/overweight versus obese) [49], excess weight gain in pregnancy (categorical) [50], parity (primiparous versus multiparous), smoking at the start of pregnancy (never/former smoker versus current smoker), gestational age at birth (days), delivery mode (vaginal versus cesarean section), fatty fish dinners in one year (continuous, in servings), and cod liver oil consumption in one year (continuous, in servings).
We identified covariates from the literature [15,16,17,18,19,20,21,22,23,24,25,26] and identified the minimally sufficient adjusted set for each predictor model using directed acyclic graphs (DAGs) [51]. Dependent on each predictor’s DAG, confounders included a combination of pre-pregnancy BMI, excess weight gain in pregnancy, parity, gestational age, smoking, maternal education (<12, 12, or >12 years), maternal age (years), and birthweight (grams) (Supplemental Table S1). We adjusted all models for infant age at time of milk collection (days) and whether formula had been introduced at time of milk collection (yes/no).
2.4. TGFA Associations with Infant Growth
The primary outcome for these analyses was infant growth defined as change in weight-for-age z-score from 0–6 months. Weight and height data were recorded in children’s health cards during regular health examinations and were parent reported in questionnaires at 1, 6, 12, and 24 months. Using these weight and height data, we estimated weight at exactly 6 months in a sex-specific multilevel (mixed) linear model fitted with cubic polynomials and random effects for infant [52]. For the period of 0–6 months, infants had up to 5 weight and height data points. For this time period, the distribution of rapid growth was no different between infants with less than 2 weight points available and those in the whole cohort. We calculated sex- and gestational-age-specific weight-for-age z-scores for birth and 6 months for the full HUMIS population (n = 2606) [53]. We then calculated the primary outcome change in weight-for-age z-score as the difference between birth and 6-month z-scores (continuous). As a secondary outcome, we evaluated rapid growth, a risk factor for obesity later in life [54,55], defined as a change in z-score greater than 0.67 (normal versus rapid) [52].
Exposures were TGFA percent composition and ratios. Again, we identified appropriate potential confounders through the literature and chose the minimally sufficient adjusted set identified in DAGs (Supplemental Figure S1). We adjusted all growth models for maternal age, smoking, education, pre-pregnancy BMI (continuous), gestational weight gain (continuous), parity, and sex.
2.5. Statistical Analysis
We summarized the data (exposure, covariate, and outcome variables) in the complete case data set. We imputed missing data among predictors (2–4%), covariates (13–16%), and growth outcomes (1%) using multiple imputation by chained equations [56]. There were no missing data among the TGFA variables. We used the STATA 14.0 “mi impute chained” function with a burn-in of 1000 to generate 10 imputed data sets.
For the predictor models, we used linear regressions for each predictor with TGFA percent composition and ratios as outcomes. We investigated the relation between percent composition of TGFAs and change in weight-for-age z-score using linear regression and with rapid growth using logistic regression. We ran all models using imputed data sets and the “mi estimate” STATA 14.0 function and Rubin’s rules to combine imputed data set results [56]. We present results as β-coefficients or odds ratios (ORs) with 95% confidence intervals (CI). All regression models were adjusted for the confounders described above.
2.6. Sensitivity Analyses
For TGFAs that were inversely associated with rapid growth, we ran a sensitivity analysis in the complete case set, testing for association with failure to thrive, defined as change in weight-for-age z-score < −0.67 [57]. Additionally, we assessed the sensitivity of our results by testing the complete case data.
We assessed all regression models for heteroscedasticity and normality of residuals and used the Hosmer–Lemeshow’s test to establish goodness of fit for the logistic regression models. We assessed the combination of high leverage and residuals to fit regression models with and without influential observations.
3. Results
3.1. Study Population and Milk Composition
Of 789 mothers, 41.5% were overweight or obese and 57.0% had excess pregnancy weight gain, while 18.2% of infants were rapid growers (Table 2). Comparison of our study subsample to the larger cohort and the general Norwegian population can be found in Supplemental Table S2. The most abundant TGFAs were palmitic acid (C16:0), oleic acid (C18:1n-9), and linoleic acid (C18:2n-6) (Table 3, Figure 2).

Table 2.
Characteristics of study population (n = 789).

Table 3.
Concentrations and percentages of fatty acids in milk (n = 789).
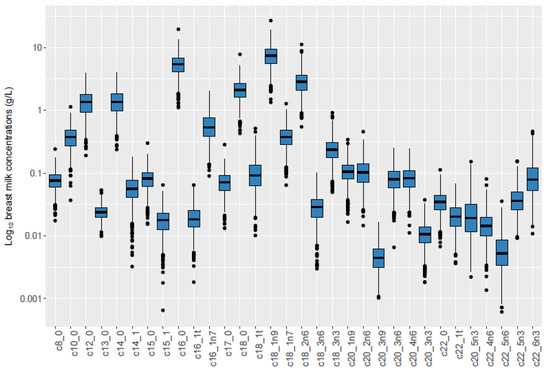
Figure 2.
Boxplot of TGFA concentrations in study population (n = 789) 1. Legend: 1 X-axis represents TGFAs measured in milk samples. Y-axis represents log10 of breast milk concentration.
3.2. Associations between Predictors and Fatty Acid Composition
We found that the following maternal factors were associated with fatty acid composition: gestational age, cod liver oil intake, fatty fish intake, excess weight gain in pregnancy, parity, and obesity (Table 4). We found no association between maternal smoking, underweight BMI, or mode of delivery and TGFA percent composition (Supplemental Table S3).

Table 4.
Notable maternal predictors of triglyceride fatty acid percent composition in milk from Norwegian mothers 1.
3.3. Associations between TGFA Composition and Infant Growth
MCFAs, lauric acid (C12:0), and myristic acid (C14:0) were inversely associated with infant growth and rapid growth. MUFAs, palmitoleate, oleic acid, and cis-vaccenic acid were positively associated with infant growth and an increased odds of rapid growth (Table 5, Figure 3 and Figure 4). SFAs and SFA/PUFA were inversely associated with infant growth and odds of rapid growth (Table 4, Supplemental Figures S3 and S4). None of the TGFAs analyzed were associated with failure to thrive (Supplemental Table S4).

Table 5.
Associations between triglyceride fatty acid percent composition 1 in human milk and infant growth.
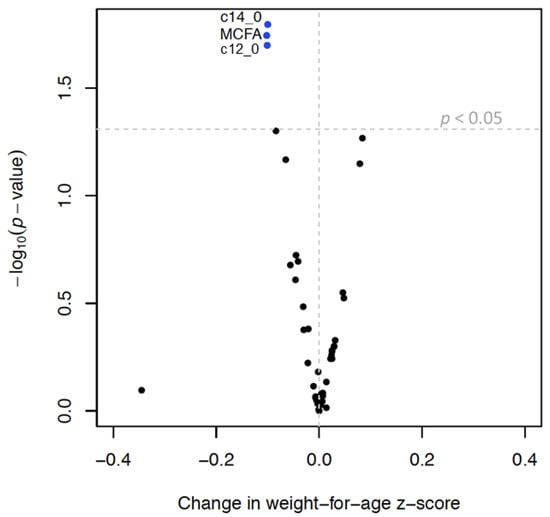
Figure 3.
Volcano plot 1 showing associations 2 between triglyceride fatty acid (TGFA) composition in milk and infant growth 3. Legend: 1 Each dot represents change in weight-for-age z-score (x axis) per interquartile range (IQR) increase in TGFA composition. Y-axis represents the −log10 of the p-value. The vertical dotted line represents a beta coefficient of 0 and the horizontal dotted line represents a p-value of 0.05, so that dots above that line represent associations that have a p-value < 0.05. 2 Models were run in the multiply imputed data set (n = 789) in 10 imputed sets and adjusted for maternal age, smoking, education, pre-pregnancy body mass index, gestational weight gain, parity, and child sex. 3 Infant growth defined as change in weight-for-age z-score between 0 and 6 months.
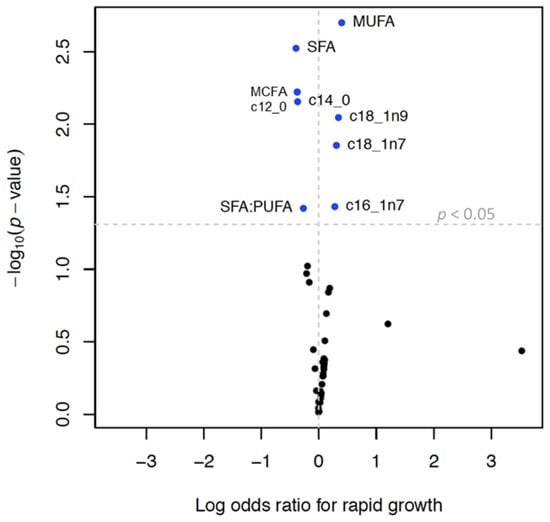
Figure 4.
Volcano plot 1 showing associations 2 between triglyceride fatty acid (TGFA) composition in milk and rapid growth 3 Legend: 1 Each dot represents change in the log of the odds ratio (OR) for rapid growth (x axis) per interquartile range (IQR) increase in TGFA. Y-axis represents the −log10 of the p-value. The vertical dotted line represents an OR of 1 and the horizontal dotted line represents a p-value of 0.05, so that dots above that line represent associations that have a p-value < 0.05. 2 Models were run in the multiply imputed data set (n = 789) in 10 imputed sets and adjusted for maternal age, smoking, education, pre-pregnancy body mass index, gestational weight gain, parity, and child sex. 3 Defined as change in weight-for-age z-score between 0 and 6 months > 0.67.
The non-heteroscedasticity and normality of residual assumptions of our models held. Effect estimates were comparable in models with and without the inclusion of extreme values and outliers, from analyses with the complete case set and multiply imputed sets and in unadjusted and adjusted models. Due to the large number of analyses, some of our results may have been random findings.
4. Discussion
We found that gestational age, maternal obesity, excess pregnancy weight gain, parity, and fatty fish and cod liver oil intake were associated with TGFA composition in human milk. Medium-chain saturated fatty acids were inversely associated with infant growth. MUFAs, including oleic acid, palmitoleate, and cis-vaccenic acid, were all associated with increased odds of rapid growth.
4.1. Medium-Chain Fatty Acids
The mammary gland secretes MCFAs directly into milk, rather than mobilizing them from maternal diet or adipose tissue. Milk TGFAs are typically SFAs, with carbon chains ranging from 8 to 14 atoms. Among adults, MCFAs are associated with increased cholesterol as a result of their roles in desaturase [58] and lipoprotein regulation [59]. However, among infants, they are an important source of energy because of their relatively shorter chain length as compared to LC-PUFAs and unique absorption pattern through the liver, independent of mitochondrial transport pathways [60,61]. MCFAs also play a role in developing the infant gut microbiome and have antibacterial and antiviral properties [60].
MCFAs are proportionally higher in the milk of mothers of premature and low-birth-weight infants and they are easier for these infants with immature gastrointestinal tracts to utilize than longer-chain TGFAs [18,21,62]. Our findings suggest a dose–response relation between gestational age and myristic acid that continues through the whole gestational spectrum, including among term babies.
We found an inverse, though non-significant, relation between MCFAs in human milk and infant growth. As noted above, compared to LC-PUFAs, MCFAs are more readily oxidized for energy rather than being stored in adipose tissue for later use, resulting in less weight gain [61,63]. In animal models, breastfeeding pups of mice fed high-MCFA diets of virgin coconut oil showed significantly lower body weight [64].
4.2. Monounsaturated Fatty Acids
Excess maternal weight gain in pregnancy was associated with increased palmitoleate and cis-vaccenic acid and inversely associated with stearic acid, expanding on the findings of previous studies [16]. The stearoyl-CoA desaturase-1 (SCD1) pathway converts palmitic acid to palmitoleate, which is then elongated to cis-vaccenic acid. Stearic acid competes with the SCD1 pathway for palmitic acid as it is elongated from palmitic acid [65]. SCD1 activity is a marker for obesity in adults [65,66,67] and might also be increased in women with excess weight gain in pregnancy. If this was the case, increased SCD1 activity might explain increased palmitoleate and cis-vaccenic acid and concurrent decreased stearic acid associated with excessive pregnancy weight gain.
A recent study in the CHILD cohort in Canada had opposite findings, with maternal obesity associated with decreased overall combined MUFAs [30]. This difference in results might reflect different dietary fat choices among obese women in Norway and Canada, respectively, as the CHILD cohort had a higher percent composition of oleic acid as compared to our cohort.
Multiparity showed inverse associations for these MUFAs, even when controlling for maternal BMI. The majority of MUFAs and PUFAs in milk derive from maternal adipose tissue [68], and women with previous pregnancies and lactation periods may have depleted stores with each successive pregnancy. Since stearic acid is highly represented in dietary sources, it may not deplete so readily and, therefore, be more abundant in these women’s milk [21].
Infants exposed to higher proportions of MUFAs, palmitoleate, oleic acid, and cis-vaccenic acid had increased odds of rapid growth. Given the high percent composition of oleic acid in our cohort as compared to the other MUFAs, variation within the IQR of this TGFA is associated with an almost fourfold increase in odds of rapid growth among infants. Our results replicate in a larger sample size those of an Australian study that found associations between oleic acid and cis-vaccenic acid and infant growth at 6 months among 18 mother–infant dyads [12]. Among adults, increased levels of circulating oleic acid have been found to be associated with metabolic syndrome and cardiovascular disease [10,67]. Further, in rats, a high MUFA/SFA ratio in maternal milk results in increased offspring adiposity, particularly when those MUFAs include oleic acid, palmitoleate, and cis-vaccenic acid [69].
MUFAs may play a role in mediating (rather than confounding) the relation between maternal obesity and infant obesity, although human milk protects against obesity compared with other infant feeding methods [70,71]. While controversial, some studies suggest that circulating palmitoleate may actively affect metabolism, acting as a “lipokine”, exerting a hormone-like influence [72,73]. Other studies have linked elevated levels of circulating palmitoleate to increased cell proliferation in adipose tissue in small-for-gestational-age mice [69]. Alternatively, high levels of oleic acid, palmitoleate, and cis-vaccenic acid may be markers for other pro-growth components highly represented in the milk of obese women [65,67]. Taken together, our results suggest that maternal obesity and metabolism may play a role in infant MUFA exposure and, therefore, growth. Clinical interventions targeting appropriate gestational weight gain may help to reduce risk factors for childhood obesity.
4.3. Polyunsaturated Fatty Acids
N-3 fatty acids have robust positive health effects, and the main dietary source is cold-water fish [74]. We found a positive association between fatty fish and cod liver oil intake and n-3 fatty acids levels in milk, replicating previous studies [2,3,19,24,30,31,75,76]. However, from a public-health standpoint, the positive role of dietary cod liver oil and fatty fish on n-3 fatty acid levels in human milk must be balanced with the risk of increased fat-soluble toxicant levels in human milk that may accompany high fish intake. Exposure to fat-soluble toxicants in human milk has been associated with obesity in children [52,53,77,78,79,80].
We found that maternal obesity had inverse associations with EPA, DHA, and total n-3 PUFAs. In Sweden, obese mothers also had lower levels of milk n-3 PUFAs than normal-weight mothers [81], and results from the French EDEN cohort suggest that obesity is associated with decreased DHA and ALA in colostrum [31]. Dietary intake is the major determinant of milk levels of EPA and DHA [27,82], while the majority of other PUFAs in milk derive from adipose tissue [68,83]. It is possible that in the milk of obese women, fats from maternal stores displace dietary fats, which may already be low due to diets with fewer n-3 PUFAs.
Parity was inversely associated with EPA and DHA. Dutch studies have documented maternal depletion of DHA over the course of pregnancy, and multiparity has been associated with decreased maternal serum DHA [84,85], although not at one year after pregnancy [48]. The EDEN cohort also found increased DHA in the colostrum of primiparous as compared to multiparous women [31]. Conversely, the CHILD cohort did not find any association between parity and TFGAs in milk [30]. Although there may be some depletion of EPA and DHA from maternal fat stores over the course of pregnancy and lactation, the relation between parity and EPA and DHA remains unclear. Future research could investigate the relation between inter-pregnancy interval and these TGFAs in milk and optimal n-3 PUFAs levels for multiparous mothers.
We found no association between n-3 PUFAs and infant growth, which supports the findings from a study of Australian women that found no significant association between EPA and DHA and infant growth [12]. While some studies suggest associations between these TGFAs and metabolic outcomes in adults and pregnant people [10,65], previous research among infants has suggested that these TGFAs may play a more substantial role in infant neurodevelopment [4,7,9].
4.4. Strengths and Limitations
To our knowledge, ours is among the first studies to assess such a broad array of TGFAs in association with maternal predictors and growth outcomes. Our study is strengthened by a large sample size. Due to linkage with the Norwegian Medical Birth Registry, variables that are often subject to recall bias may have been reported with more precision than in other studies. Finally, our study is generalizable to the Norwegian population, as our sample population had similar characteristics to the general population of women who had recently given birth (Supplemental Table S2). While Norway has lower obesity rates than many other nations [86], oversampling for obesity increased the power of our analysis.
Our study had some limitations. First, the timeframe for milk sampling was wide, ranging from 14 to 108 days. However, all but two subjects collected milk samples between 14 and 60 days. Furthermore, all samples represented mature milk, so this range should not negatively affect our accuracy in reporting concentrations and percent composition. The concentrations and patterns of composition in our study are consistent with European and global averages [27,30,44,87,88]. The inclusion of women and infants who had combined human milk and formula feeding at the time of milk collection could have affected our results, in that different infant feeding patterns may change milk composition, and the nutritional content of formula may affect infant growth. We addressed this in our predictor model by adjusting for formula introduction. Associations between TGFAs and growth with and without adjustment for formula were not materially different, so inclusion of combination feeders should not reduce the validity of our results. Furthermore, a huge majority of the mothers was exclusively breastfeeding at the time of milk sampling.
We included fatty fish and cod liver oil intake as potential predictors of n-3 fatty acid composition, but lacked more comprehensive dietary exposures, which can be important contributors for other TGFAs in milk. There is evidence of a relation between fatty acid desaturase (FADS) gene expression and milk TGFA composition [30], and we did not have FADS profiles. Additionally, we did not have data on milk volume, total energy, or other energy-generating nutrients, such as protein concentrations in our milk samples, which can be factors that affect infant growth. As a result, unmeasured dietary or genetic variables may confound some of our findings.
Finally, we found very wide confidence intervals when running logistic regressions of TGFAs’ association with rapid growth, suggesting that logistic regression may not optimally model the association between the exposure and outcome. However, our findings do suggest patterns in TGFA exposure and growth outcomes that are corroborated by the linear regression findings.
5. Conclusions
In conclusion, our study found that excess weight gain in pregnancy and parity are associated with increased MUFAs and decreased n-3 PUFA content in milk. Importantly, exposure to MUFAs in breast milk was associated with obesity outcomes in infants. Our results indicate that targeting excessive pregnancy weight gain could be an effective intervention to prevent childhood obesity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14183858/s1, Table S1: Adjustment sets for each predictor-triglyceride fatty acid composition model; Table S2: Comparison of study subset (n = 789) to remaining HUMIS-NoMIC cohort (n = 1817) and larger Norwegian population; Table S3: Associations of maternal predictors and triglyceride fatty acid percent composition; Table S4: Associations between select triglyceride fatty acids and failure to thrive; Figure S1: Directed acyclic graph representing association between triglyceide fatty acid composition in milk and infant growth between 0 and 6 months.
Author Contributions
Conceptualization, R.L.C., B.V.K. and M.Å.E.; Data curation, R.L.C., N.I. and V.C.L.; Formal analysis, R.L.C. and H.D.; Funding acquisition, M.Å.E.; Investigation, R.L.C.; Methodology, R.L.C., N.I., T.B.A., V.C.L. and M.Å.E.; Supervision, B.V.K. and M.Å.E. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results received funding from the project “Early Nutrition”, grant agreement no. 289346 under the European Union’s Seventh Framework Program (FP7/2007-2013) (R.L.C., M.A.E.) and by funds from the Norwegian Research Council’s MILPAAHEL program, project No. 213148.F20 (N.I.).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Norwegian Data Inspectorate (refs. 200200473 LT7RH and 2002/1398-2 CLU/), the Regional Committees for Medical and Health Research Ethics (ref. 2014/2351/REK sør-øst C), and the Columbia University Institutional Review Board (ref. AAAR0308).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical concerns in this ongoing birth cohort.
Conflicts of Interest
The authors have no conflict of interest to disclose.
References
- Prell, C.; Koletzko, B. Breastfeeding and complementary feeding. Dtsch. Arztebl. Int. 2016, 113, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B. Human milk lipids. Ann. Nutr. Metab. 2016, 69 (Suppl. 2), 28–40. [Google Scholar] [CrossRef] [PubMed]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Clandinin, M.T.; Larsen, B.M. Docosahexaenoic acid is essential to development of critical functions in infants. J. Pediatr. 2010, 157, 875–876. [Google Scholar] [CrossRef] [PubMed]
- Fleith, M.; Clandinin, M.T. Dietary pufa for preterm and term infants: Review of clinical studies. Crit. Rev. Food Sci. Nutr. 2005, 45, 205–229. [Google Scholar] [CrossRef] [PubMed]
- Prentice, P.; Ong, K.K.; Schoemaker, M.H.; van Tol, E.A.; Vervoort, J.; Hughes, I.A.; Acerini, C.L.; Dunger, D.B. Breast milk nutrient content and infancy growth. Acta Paediatr. 2016, 105, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Qawasmi, A.; Landeros-Weisenberger, A.; Bloch, M.H. Meta-analysis of lcpufa supplementation of infant formula and visual acuity. Pediatrics 2013, 131, e262–e272. [Google Scholar] [CrossRef]
- Steenweg-de Graaff, J.; Tiemeier, H.; Ghassabian, A.; Rijlaarsdam, J.; Jaddoe, V.W.; Verhulst, F.C.; Roza, S.J. Maternal fatty acid status during pregnancy and child autistic traits: The generation r study. Am. J. Epidemiol. 2016, 183, 792–799. [Google Scholar] [CrossRef]
- Steenweg-de Graaff, J.C.; Tiemeier, H.; Basten, M.G.; Rijlaarsdam, J.; Demmelmair, H.; Koletzko, B.; Hofman, A.; Jaddoe, V.W.; Verhulst, F.C.; Roza, S.J. Maternal lc-pufa status during pregnancy and child problem behavior: The generation r study. Pediatr. Res. 2015, 77, 489–497. [Google Scholar] [CrossRef]
- Vidakovic, A.; Gishti, O.; Graaff, J.S.-d.; Williams, M.; Duijts, L.; Felix, J.; Hodman, A.; Tiemeier, H.; Jaddoe, V.; Gaillard, R. Higher maternal plasma n-3 pufa and lower n-6 pufa concentrations in pregnancy are associated with lower childhood systolic blood pressure. J. Nutr. 2015, 145, 2362–2368. [Google Scholar] [CrossRef]
- Rucci, E.; den Dekker, H.T.; de Jongste, J.C.; Steenweg-de-Graaff, J.; Gaillard, R.; Pasmans, S.G.; Hofman, A.; Tiemeier, H.; Jaddoe, V.W.; Duijts, L. Maternal fatty acid levels during pregnancy, childhood lung function and atopic diseases. The generation r study. Clin. Exp. Allergy 2016, 46, 461–471. [Google Scholar] [CrossRef] [PubMed]
- George, A.D.; Gay, M.C.L.; Wlodek, M.E.; Murray, K.; Geddes, D.T. The fatty acid species and quantity consumed by the breastfed infant are important for growth and development. Nutrients 2021, 13, 4183. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.W. The first months of life: A critical period for development of obesity. Am. J. Clin. Nutr. 2008, 87, 1587–1589. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, N.; Gerard, P.D.; Baldwin, W.S. Perturbations in polar lipids, starvation survival and reproduction following exposure to unsaturated fatty acids or environmental toxicants in daphnia magna. Chemosphere 2016, 144, 2302–2311. [Google Scholar] [CrossRef]
- Al-Tamer, Y.Y.; Mahmood, A.A. The influence of iraqi mothers’ socioeconomic status on their milk-lipid content. Eur. J. Clin. Nutr. 2006, 60, 1400–1405. [Google Scholar] [CrossRef][Green Version]
- Antonakou, A.; Skenderi, K.P.; Chiou, A.; Anastasiou, C.A.; Bakoula, C.; Matalas, A.L. Breast milk fat concentration and fatty acid pattern during the first six months in exclusively breastfeeding greek women. Eur. J. Nutr. 2013, 52, 963–973. [Google Scholar] [CrossRef]
- Argov-Argaman, N.; Mandel, D.; Lubetzky, R.; Hausman Kedem, M.; Cohen, B.C.; Berkovitz, Z.; Reifen, R. Human milk fatty acids composition is affected by maternal age. J. Matern. Fetal Neonatal Med. 2017, 30, 34–37. [Google Scholar] [CrossRef]
- Genzel-Boroviczényy, O.; Wahle, J.; Koletzko, B. Fatty acid composition of human milk during the first month after term and preterm delivery. Eur. J. Pediatr. 1997, 156, 142–147. [Google Scholar] [CrossRef]
- Krešić, G.; Dujmović, M.; Mandić, M.L.; Delaš, I. Relationship between mediterranean diet and breast milk fatty acid profile: A study in breastfeeding women in croatia. Dairy Sci. Technol. 2013, 93, 287–301. [Google Scholar] [CrossRef]
- Kumar, H.; du Toit, E.; Kulkarni, A.; Aakko, J.; Linderborg, K.M.; Zhang, Y.; Nicol, M.P.; Isolauri, E.; Yang, B.; Collado, M.C.; et al. Distinct patterns in human milk microbiota and fatty acid profiles across specific geographic locations. Front. Microbiol. 2016, 7, 1619. [Google Scholar] [CrossRef]
- Nasser, R.; Stephen, A.M.; Goh, Y.K.; Clandinin, M.T. The effect of a controlled manipulation of maternal dietary fat intake on medium and long chain fatty acids in human breast milk in saskatoon, canada. Int. Breastfeed. J. 2010, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Nayak, U.; Kanungo, S.; Zhang, D.; Ross Colgate, E.; Carmolli, M.P.; Dey, A.; Alam, M.; Manna, B.; Nandy, R.K.; Kim, D.R.; et al. Influence of maternal and socioeconomic factors on breast milk fatty acid composition in urban, low-income families. Matern. Child. Nutr. 2017, 13, e12423. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.; Jarjou, L.; Drury, P.; Dewit, O.; Crawford, M. Breast-milk fatty acids of rural gambian mothers: Effects of diet and maternal parity. J. Pediatric Gastroenterol. 1989, 8, 486–490. [Google Scholar] [CrossRef]
- Quinn, E.A.; Kuzawa, C.W. A dose-response relationship between fish consumption and human milk dha content among filipino women in cebu city, philippines. Acta Paediatr. 2012, 101, e439–e445. [Google Scholar] [CrossRef]
- Samur, G.; Topcu, A.; Turan, S. Trans fatty acids and fatty acid composition of mature breast milk in turkish women and their association with maternal diet’s. Lipids 2009, 44, 405–413. [Google Scholar] [CrossRef]
- Weseler, A.R.; Dirix, C.E.; Bruins, M.J.; Hornstra, G. Dietary arachidonic acid dose-dependently increases the arachidonic acid concentration in human milk. J. Nutr. 2008, 138, 2190–2197. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.; Varamini, B.; Jensen, R.; Diersen-Schade, D.; Boettcher, J.; Arterburn, L. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.A.; Hedderley, D.I.; Herath, T.D.; Paturi, G.; Glyn-Jones, S.; Wiens, F.; Stahl, B.; Gopal, P. Human milk composition and dietary intakes of breastfeeding women of different ethnicity from the manawatu-wanganui region of new zealand. Nutrients 2018, 10, 1231. [Google Scholar] [CrossRef]
- Barrera, C.; Valenzuela, R.; Chamorro, R.; Bascunan, K.; Sandoval, J.; Sabag, N.; Valenzuela, F.; Valencia, M.-P.; Puigrredon, C.; Valenzuela, A. The impact of maternal diet during pregnancy and lactation on the fatty acid composition of erythrocytes and breast milk of chilean women. Nutrients 2018, 10, 839. [Google Scholar] [CrossRef]
- Miliku, K.; Duan, Q.L.; Moraes, T.J.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; Subbarao, P.; Field, C.J.; et al. Human milk fatty acid composition is associated with dietary, genetic, sociodemographic, and environmental factors in the child cohort study. Am. J. Clin. Nutr. 2019, 110, 1370–1383. [Google Scholar] [CrossRef]
- Armand, M.; Bernard, J.Y.; Forhan, A.; Heude, B.; Charles, M.A.; Annesi-Maesano, I.; Botton, J.; Dargent-Molina, P.; de Lauzon-Guillain, B.; Ducimetière, P.; et al. Maternal nutritonal determinants of colostrum fatty acids in the eden mother-child cohort. Clin. Nutr. 2018, 37, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- de la Garza Puentes, A.; Martí Alemany, A.; Chisaguano, A.M.; Montes Goyanes, R.; Castellote, A.I.; Torres-Espínola, F.J.; García-Valdés, L.; Escudero-Marín, M.; Segura, M.T.; Campoy, C.; et al. The effect of maternal obesity on breast milk fatty acids and its association with infant growth and cognition-the preobe follow-up. Nutrients 2019, 11, 2154. [Google Scholar] [CrossRef] [PubMed]
- Siziba, L.P.; Lorenz, L.; Brenner, H.; Carr, P.; Stahl, B.; Mank, M.; Marosvölgyi, T.; Decsi, T.; Szabó, É.; Rothenbacher, D.; et al. Changes in human milk fatty acid composition and maternal lifestyle-related factors over a decade: A comparison between the two ulm birth cohort studies. Br. J. Nutr. 2021, 126, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Eggesbø, M.; Stigum, H.; Longnecker, M.P.; Polder, A.; Aldrin, M.; Basso, O.; Thomsen, C.; Skaare, J.U.; Becher, G.; Magnus, P. Levels of hexachlorobenzene (hcb) in breast milk in relation to birth weight in a norwegian cohort. Environ. Res. 2009, 109, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Eggesbo, M.; Thomsen, C.; Jorgensen, J.V.; Becher, G.; Odland, J.O.; Longnecker, M.P. Associations between brominated flame retardants in human milk and thyroid-stimulating hormone (tsh) in neonates. Environ. Res. 2011, 111, 737–743. [Google Scholar] [CrossRef]
- Lenters, V.; Iszatt, N.; Forns, J.; Čechová, E.; Kočan, A.; Legler, J.; Leonards, P.; Stigum, H.; Eggesbø, M. Early-life exposure to persistent organic pollutants (ocps, pbdes, pcbs, pfass) and attention-deficit/hyperactivity disorder: A multi-pollutant analysis of a norwegian birth cohort. Environ. Int. 2019, 125, 33–42. [Google Scholar] [CrossRef]
- Levels of Pcbs, Pcdds, and Pcdfs in Breast Milk; WHO Regional Office for Europe: Copenhagen, Denmark, 1989.
- World Health Organization. Global Database on Child Growth and Malnutrition: Description: Cut off Points and Summary Statistics. Available online: http://www.who.int/nutgrowthdb/about/introduction/en/index5.html (accessed on 30 July 2022).
- Ahmed, T.B.; Eggesbo, M.; Criswell, R.; Uhl, O.; Demmelmair, H.; Koletzko, B. Total fatty acid and polar lipid species composition of human milk. Nutrients 2021, 14, 158. [Google Scholar] [CrossRef]
- Stimming, M.; Mesch, C.M.; Kersting, M.; Kalhoff, H.; Demmelmair, H.; Koletzko, B.; Schmidt, A.; Bohm, V.; Libuda, L. Vitamin e content and estimated need in german infant and follow-on formulas with and without long-chain polyunsaturated fatty acids (lc-pufa) enrichment. J. Agric. Food Chem. 2014, 62, 10153–10161. [Google Scholar] [CrossRef]
- Gianni, M.L.; Roggero, P.; Baudry, C.; Fressange-Mazda, C.; Galli, C.; Agostoni, C.; Ruyet, P.l.; Mosca, F. An infant formula containing dairy lipids increased red blood cell membrane omega 3 fatty acids in 4 month-old healthy newborns: A randomized controlled trial. BMC Pediatrics 2018, 18, 53. [Google Scholar] [CrossRef]
- Suburu, J.; Shi, L.; Wu, J.; Wang, S.; Samuel, M.; Thomas, M.J.; Kock, N.D.; Yang, G.; Kridel, S.; Chen, Y.Q. Fatty acid synthase is required for mammary gland development and milk production during lactation. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1132–E1143. [Google Scholar] [CrossRef]
- Bachour, P.; Yafawi, R.; Jaber, F.; Choueiri, E.; Abdel-Razzak, Z. Effects of smoking, mother’s age, body mass index, and parity number on lipid, protein, and secretory immunoglobulin a concentrations of human milk. Breastfeed. Med. 2012, 7, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Grote, V.; Verducci, E.; Scaglioni, S.; Vecchi, F.; Contarini, G.; Giovannini, M.; Koletzko, B.; Agostoni, C. Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur. J. Clin. Nutr. 2016, 70, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.H.; Song, J.H.; Song, S.; Kang, N.M. Do gender and birth height of infant affect calorie of human milk? An association study between human milk macronutrient and various birth factors. J. Matern. Fetal Neonatal Med. 2016, 30, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, K.F. Nutrition and growth during infancy: The copenhagen study. Acta Paediatr. 1997, 420, 1–36. [Google Scholar] [CrossRef]
- Nommsen, L.A.; Lovelady, C.A.; Heinig, M.J.; Lonnerdal, B.; Dewey, K.G. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 months of lactation: The darling study. Am. J. Clin. Nutr. 1991, 53, 457–465. [Google Scholar] [CrossRef]
- van den Ham, E.C.; van Houwelingen, A.C.; Hornstra, G. Evaluation of the relation between n-3 and n-6 fattya cid status and parity in nonpregnant women from the netherlands. Am. J. Clin. Nutr. 2001, 73, 622–627. [Google Scholar] [CrossRef]
- World Health Organization. Available online: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (accessed on 30 July 2022).
- American College of Obstetricians and Gynecologists. Committee Opinion: Weight Gain during Pregnancy. Available online: http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Weight-Gain-During-Pregnancy (accessed on 30 July 2022).
- Textor, J.; Hardt, J.; Knüppel, S. Dagitty: A graphical tool for analyzing causal diagrams. Epidemiology 2011, 22, 745. [Google Scholar] [CrossRef]
- Iszatt, N.; Stigum, H.; Verner, M.A.; White, R.A.; Govarts, E.; Murinova, L.P.; Schoeters, G.; Trnovec, T.; Legler, J.; Pele, F.; et al. Prenatal and postnatal exposure to persistent organic pollutants and infant growth: A pooled analysis of seven european birth cohorts. Environ. Health Perspect. 2015, 123, 730–736. [Google Scholar] [CrossRef]
- Iszatt, N.; Stigum, H.; Govarts, E.; Murinova, L.P.; Schoeters, G.; Trnovec, T.; Legler, J.; Thomsen, C.; Koppen, G.; Eggesbo, M. Perinatal exposure to dioxins and dioxin-like compounds and infant growth and body mass index at seven years: A pooled analysis of three european birth cohorts. Environ. Int. 2016, 94, 399–407. [Google Scholar] [CrossRef]
- Koletzko, B.; Chourdakis, M.; Grote, V.; Hellmuth, C.; Prell, C.; Rzehak, P.; Uhl, O.; Weber, M. Regulation of early human growth: Impact on long-term health. Ann. Nutr. Metab. 2014, 65, 101–109. [Google Scholar] [CrossRef]
- Monteiro, P.; Victoria, C. Rapid growth in infancy and childhood and obesity in later life-a systematic review. Obes. Rev. 2005, 6, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons, Inc.: New York, NY, USA, 1987. [Google Scholar]
- Lampl, M.; Mummert, A.; Schoen, M. Promoting healthy growth or feeding obesity? The need for evidence-based oversight of infant nutritional supplement claims. Healthcare 2016, 4, 84. [Google Scholar] [CrossRef] [PubMed]
- Rioux, V.; Pedrono, F.; Legrande, P. Regulation of mammalian desaturases by myristic acid: N-terminal myristoylation and other modulations. Biochim. Et Biophys. Acta 2010, 1811, 1–8. [Google Scholar] [CrossRef]
- Temme, E.H.M.; Mensink, R.P.; Hornstra, G. Effects of medium chain fatty acids (mcfa), myristic acid, and oleic acid on serum lipoproteins in healthy subjects. J. Lipid Res. 1997, 38, 1746–1754. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, L.; Jin, J.; Mi, L.; Pang, J.; Liu, Z.; Gong, J.; Sun, C.; Li, J.; Wei, W.; et al. Role medium-chain fatty acids in the lipid metabolism of infants. Front. Nutr. 2022, 9, 804880. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Visentin, S.; Crotti, S.; Donazzolo, E.; S’Arocono, S.; Nitti, D.; Cosmi, E.; Agostini, M. Medium chain fatty acids in intrauterine growth restricted and small for gestational age pregnancies. Metabolomics 2017, 13, 54. [Google Scholar] [CrossRef]
- Mumme, K.; Stonehouse, W. Effects of medium-chain triglycerides on weight loss and body composition: A meta-analysis of randomized controlled trials. J. Acad. Nutr. Diet. 2014, 115, 249–263. [Google Scholar] [CrossRef]
- Gunasekaran, R.; Shaker, M.R.; Mohd-Zin, S.W.; Abdullah, A.; Annuar, A.; Abdul-Aziz, N.M. Maternal intake of dietary virgin coconut oil modifies essential fatty acids and causes low body weight and spiky fur in mice. BMC Complementary Altern. Med. 2017, 17, 79. [Google Scholar] [CrossRef]
- Warensjo, E.; Ohrvall, M.; Vessby, B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 128–136. [Google Scholar] [CrossRef]
- Rauschert, S.; Uhl, O.; Koletzko, B.; Kirchberg, F.; Mori, T.A.; Huang, R.C.; Beilin, L.J.; Hellmuth, C.; Oddy, W.H. Lipidomics reveals associations of phospholipids with obesity and insulin resistance in young adults. J. Clin. Endocrinol. Metab. 2016, 101, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Warensjo, E.; Riserus, U.; Vessby, B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia 2005, 48, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- Sauerwald, T.; Demmelmair, H.; Koletzko, B. Polyunsaturated fatty acid supply with human milk. Lipids 2001, 36, 991–996. [Google Scholar] [CrossRef]
- Yee, J.K.; Lee, W.-N.P.; Han, G.; Ross, M.G.; Desai, M. Organ-specific alterations in fatty acid de novo synthesis and desaturation in a rat model of programmed obesity. Lipids Health Dis. 2011, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Ruager-Martin, R.; Hyde, M.J.; Modi, N. Maternal obesity and infant outcomes. Early Hum. Dev. 2010, 86, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fein, S.; Grummer-Strawn, L. Do infants fed from bottles lack self-regulation of milk intake compared with directly breastfed infants? Pediatrics 2010, 125, e1386–e1393. [Google Scholar] [CrossRef]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Watkins, S.M.; Hotamisligil, G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cerll 2008, 134, 933–944. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Gutierrez-Aguilar, R. The role of the novel lipokine palmitoleic acid in health and disease. Adv. Nutr. 2017, 8, 173S–181S. [Google Scholar] [CrossRef]
- eAcademy, E.N. Lc-pufas in pregnancy and lactation. In Chemistry, Metabolism, and Biological Action; Ludwig-Maximillian University of Munich: Munich, Germany, 2016. [Google Scholar]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatric Clin. North Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Helland, I.B.; Saugstad, O.D.; Smith, L.; Saarem, K.; Solvoll, K.; Ganes, T.; Drevon, C.A. Similar effects on infants of n-3 and n-6 fatty acid supplementation to pregnant and lactating women. Pediatrics 2001, 108, e82. [Google Scholar] [CrossRef]
- La Merrill, M.; Birnbaum, L.S. Childhood obesity and environmental chemicals. Mt. Sinai J. Med. 2011, 78, 22–48. [Google Scholar] [CrossRef]
- Tang-Peronard, J.L.; Andersen, H.R.; Jensen, T.K.; Heitmann, B.L. Endocrine-disrupting chemicals and obesity development in humans: A review. Obes. Rev. 2011, 12, 622–636. [Google Scholar] [CrossRef]
- Criswell, R.; Lenters, V.; Mandal, S.; Stigum, H.; Iszatt, N.; Eggesbø, M. Persistent environmental toxicants in breast milk and rapid infant growth. Ann. Nutr. Metab. 2017, 70, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Landrigan, P. Neurobehavioral effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Lindholm, E.S.; Strandvik, B.; Altman, D.; Moller, A.; Kilander, C.P. Different fatty acid pattern in bresat milk of obese compared to normal weight mothers. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Ng, Y.-F.; Chan, S.-M.; Su, Y.-X.; Kwok, K.W.-H.; Chan, H.-M.; Cheung, C.-L.; Lee, H.-W.; Pak, W.-Y.; Li, S.-Y.; et al. Positive relationship between consumption of specific fish type and n-3 pufa in milk of hong kong lactating mothers. Br. J. Nutr. 2019, 121, 1431–1440. [Google Scholar] [CrossRef]
- Giuffrida, F.; Fleith, M.; Goyer, A.; Samuel, T.M.; Elmelegy-Masserey, I.; Fontannaz, P.; Cruz-Hernandez, C.; Thakkar, S.K.; Monnard, C.; De Castro, C.A.; et al. Human milk fatty acid composition and its association with maternal blood and adipose tissue fatty acid content in a cohort of women from europe. Eur. J. Nutr. 2022, 61, 2167–2182. [Google Scholar] [CrossRef]
- Al, M.D.M.; Houwelingen, A.V.; Hornstra, G. Relation between birth order and the maternal and neonatal docohexaenoic acid status. Eur. J. Clin. Nutr. 1997, 51, 548–553. [Google Scholar] [CrossRef]
- Al, M.D.; Van Houwelingen, A.C.; Kester, A.D.; Hasaart, T.H.; De Jong, A.E.; Hornstra, G. Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. Br. J. Nutr. 1995, 74, 55–68. [Google Scholar] [CrossRef]
- Obesity Update; OECD: Paris, France, 2017; pp. 1–12. Available online: https://www.oecd.org/health/health-systems/Obesity-Update-2017.pdf (accessed on 30 July 2022).
- Yuhas, R.; Pramuk, K.; Lien, E.L. Human milk fatty acid composition from nine countries varies most in dha. Lipids 2009, 41, 851–859. [Google Scholar] [CrossRef]
- Koletzko, B.; Thiel, I.; Abiodun, P. The fatty acid composition of human milk in europe and africa. J. Pediatr. 1992, 120, S62–S70. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).