Abstract
Tube feeding is a therapeutic intervention that is aimed at providing nutritional support and is important in the nutritional and gastrointestinal management of children with neurological disability (ND) worldwide. Since the publication of the first European Society of Gastroenterology, Hepatology, and Nutrition (ESPGHAN) consensus paper in 2017, some aspects of tube-feeding modalities have attracted the interest of the scientific community more than others, including the type of enteral formulas, enteral access, and the challenging practice of tube weaning. The purpose of this review was to report on the most recent hot topics and new directions in tube-feeding strategies for children with ND.
1. Introduction
In up to 85% of cases, children with severe neurological disability (ND) have swallowing troubles and/or gastrointestinal symptoms and require prolonged tube feeding [1]. Tube-feeding indications encompass the optimization of nutritional status and growth; prevention of malnutrition; maintenance of fluid intake; support of distasteful diets (such as in metabolic diseases); improvement of drug compliance; reduction of aspiration and complications associated with gastroesophageal reflux disease (GERD); and improvement of children’s and caregivers’ health-related quality of life (HRQoL) [2]. The primary indication for tube feeding is nutritional rehabilitation, and the therapeutic efficacy is measured in terms of nutritional status normalization. Tube feeding has been demonstrated to be efficacious in preventing and/or reversing malnutrition in pediatric patients with ND. Many feeding techniques have been established and can be used in ND children. The nutritional care of pediatric patients with ND was addressed in the 2017 European Society of Gastroenterology, Hepatology, and Nutrition (ESPGHAN) consensus paper, which provided recommendations on the timing and modalities of tube feeding [3,4]. Among the different themes covered, some have undergone recent progress, while many open questions remain challenging. The purpose of this review was to synthesize the available literature on tube feeding in ND children, starting from the recommendations provided by international guidelines and experts [3,4,5], focus on the most recent hot topics in tube feeding strategies and surroundings, and update clinicians and healthcare providers with new directions in this challenging management area.
2. Materials and Methods
Relevant studies published over the last 10 years were identified via a PubMed/Medline (http://www.ncbi.nlm.nih.gov/pubmed/) search, using the following keywords or combination of keywords: “tube feeding”, “enteral nutrition”, “neurological disability”, “gastrostomy”, “enteral feed”, “tube weaning”, “gastroesophageal reflux disease”, and “pediatrics”. Additional papers were identified by reviewing reference lists of relevant publications. Emphasis was placed on original studies published from 2017 onward, reporting novel findings in management and highlights in knowledge gaps. Non-English-language publications were excluded. A systematic approach to study selection was not implemented. Instead, data were extracted based on its relevance to the topic.
3. Enteral Access
The type of enteral access is most of the time determined by the child’s nutritional and clinical health. Intragastric access is preferable because tube placement is easy and bolus feeding is feasible [3]. The gastrostomy can be surgically (ideally laparoscopically), radiologically, or more commonly, endoscopically (percutaneous endoscopic gastrostomy, PEG) performed. The ESPGHAN Endoscopy Special Interest Group has recently published a revised position paper for PEG in children [5]. Low weight (less than 10 kg) is no longer a restriction to the tube placement [6]. The standard pull-through approach is recommended with a shift to a low-profile balloon device once the stoma is stable. The external bolster of the PEG catheter should be tightly fixed to the skin’s surface to prevent gastroduodenal invagination and gastric outlet obstruction (known as “the ball valve syndrome”), while avoiding the best-known buried bumper syndrome [7,8]. Push one-step PEG insertion has grown in popularity in recent years [5]. The advantage of this technique over a standard PEG tube implant is that it enables the primary insertion of a balloon and avoids the need for a second general anesthesia for tube removal and substitution with a low-level device [9]. Because the large bumper is prevented from passing down the esophagus, the one-step device is preferred in patients with a significant anesthetic risk and a history of cardiac or esophageal surgery. Moreover, it may also be more cost-effective in centers where these facilities are expensive [10]. No studies have demonstrated that one approach is superior to another in terms of efficacy and safety, and the choice should be made based on the expertise of the team and expectations of the parents. When it is impossible to safely place the device, laparoscopic gastrostomy or laparoscopically assisted PEG can be safe alternatives [5]. Laparoscopic-assisted PEG is recommended in those cases that are at a high risk of bleeding, perforation, or unsuccessful procedure, such as ascites, kyphoscoliosis or spinal deformity, peritoneal dialysis, or the lack of transillumination of the abdomen [5].
Jejunal tube feeding is recommended as an alternative to fundoplication in all situations where gastric feeding is poorly tolerated, such as frequent vomiting, severe GERD with a risk of aspiration, and gastroparesis [11]. The feeding tube’s tip should be beyond the Treitz ligament to prevent retrograde tube dislodgement into the stomach [11]. The use of a jejunal extension in a previously placed PEG is the most-often-used method (Table 1). The experience of endoscopic gastrojejunal tube placement in children by using a preexisting gastrostomy tract or by the one-step push technique confirmed the feasibility and safety of this technique even in young infants [12].

Table 1.
Step-by-step protocol of the placement of a percutaneous endoscopic gastrostomy with jejunal extension.
Jejunal tube feeding is usually undertaken after an attempt of continuous gastric feeding with a hydrolyzed or aminoacidic formula and/or at least one prokinetic medication to promote gastric emptying [3,11].
3.1. Feeding Regimen
The feeding regimen should be selected depending on the type of enteral access, activity level, energy needs, and feed tolerance of the child [3]. Enteral tube feeding can be administered as boluses or continuously. Bolus feeding stimulates physiological endocrine responses to foods, allowing for more flexible feeding schedules and more independence and encouraging hunger before oral intake [13]. However, it may not be well tolerated in cases of GERD or delayed gastric emptying. Intermittent feeding enables adjustment of the feeding speed in relation to its tolerance [13,14]. Continuous feeding is advised in cases of poor feed-tolerance symptoms in jejunal feeding and can be used at any time of the day or night. Night enteral feeding has the advantages of less social burden and might preserve oral feeding. A combination of nightly continuous feedings and daytime boluses may be considered in cases of high energy requirements or poor tolerance to volume [3].
In contrast to continuous feeding, the bolus regimen appears to be more beneficial for children approaching tube weaning because of its facilitation of the physiological hunger–satiation cycle [15] or in the case of blenderized-diet use. However, there are no pediatric data on the effects of tube-feeding regimens (continuous, bolus, or combination) on hunger, oral skills, or overall health.
Feeding can be started as soon as 3 h after PEG implantation in children who are stable and have no contraindications [5]. There is no evidence to support the use of a clear fluid test or a dilute or hypotonic meal following the surgery. The feed volume should be progressively raised; excessive feeding may cause abdominal discomfort, distension, or dumping syndrome [5].
3.2. Anti-Reflux Surgery
GERD is one of the most observed gastrointestinal symptoms (up to 77%) in children with ND. It is also substantially more common in children with ND than in normally developing children [3,16]. Long-term proton pump inhibitors are the first-line treatment for GERD, combined with lifestyle changes such as thickening liquid formula and using whey-based enteral formula rather than casein-based [17]. According to the available evidence, GERD seems not to be worsened by PEG placement [18]. Therefore, antireflux surgery should not be considered routinely (during gastrostomy placement) but only in selected cases, such as severe GERD, recurrent respiratory disease, and aspiration pneumonia [3]. According to the ESPGHAN guidelines, laparoscopic Nissen fundoplication should be used as the primary surgical treatment for GERD [3]. The indications for Nissen fundoplication are summarized in Table 2. Radiological-position confirmation is not necessary after placement [3].

Table 2.
Indications for Nissen fundoplication.
In specific and difficult cases, such as repeated Nissen fundoplication, total esophagogastric disconnection (TEGD) is an option [3,19]. Both techniques (i.e., Nissen fundoplication and TEGD) appear to have similar efficacy and morbidity. A recent retrospective study reported the outcomes of 66 children with ND who had open TEGD between 1994 and 2015 for GERD as either a primary intervention or a rescue treatment [20]. Only 18.2% of the patients had complications, whereas nearly all of them (98%) did not have clinically significant reflux afterward [20]. Complications included postoperative pneumonia, subphrenic collection, leaks (early complications), esophageal stricture, gastric volvulus, and hiatus hernia (late complications). Recent evidence does not confirm that TEGD requires longer hospitalizations or more readmissions [20,21]. TEGD appears to be an effective procedure for children with severe ND needing repeated Nissen fundoplication, with a relatively low complication rate and a major improvement in overall health and HRQoL for patients and caregivers [22].
4. Real-Food-Based Enteral Nutrition
There are many nutritionally adequate, age-appropriate enteral formulas marketed for pediatric patients over the age of one year who require enteral nutrition, but there is no agreement on the optimal formula. The 2017 ESPGHAN guidelines provide enteral formula selection benchmarks, such as beginning with an isocaloric (1 kcal/mL) polymeric formula with fibers after the age of one year unless otherwise clinically indicated [3]. They recommend choosing the more appropriate formula based on clinical experience, the patient’s age, underlying condition, nutritional requirements, and type of enteral access [3,4]. Even if commercial formulas that are nutritionally complete are currently the most commonly used, real-food-based enteral nutrition, including blenderized tube feeds (BTFs) and real-food-containing formulas, is becoming more popular among parents and caregivers of patients who are on long-term enteral nutrition [23].
4.1. Blenderized Tube Feeds
Blended food and liquids are delivered directly through the tube, using commercial real-food-containing formulas, handmade blended formulas, or puree [24,25]. BTFs can relate to homemade blended food, commercial formula mixed with pureed baby food, or ready-to-use real food products for tube feeding. When parents are willing to, transitioning to BTFs should be performed under the guidance of a medical team with experience who can check for fluids, deficits of micro- and macronutrients, and proper laboratory tests until the patient is stable. The criteria for starting BTFs include (i) age ≥6 months; (ii) >14-French tubes; (iii) medically stable patients on home enteral nutrition with a mature stoma; and, for home-made BTFs, (iv) patients stable on bolus feeding [25]. The use of BTFs may ameliorate the stooling pattern in this group of patients by (i) ingestion of complex whole-food nutrients, (ii) variation of fiber type and amount, (iii) alteration of fat type, and (iv) modification of the gut microbiome (via fibers and plant-based carbohydrates) [26,27]. Moreover, several teams have shown that real-food-based formulas could excite taste receptors in the gut and transfer sensory information to a variety of effector systems involved in immunological responses and gut motility [28,29]. Following activation by food, some receptors appear to engage a gut–brain–stomach route that generates a feedback inhibition of gastric motility to control the pulsatile rhythm of food entry into the gut [30]. The advantages and disadvantages of starting BTF through a gastrostomy are summarized in Table 3.

Table 3.
Blenderized-tube-feeding advantages and disadvantages [3,24,25].
The BLEND study [30] was the first to evaluate the possibility of transitioning pediatric patients with chronic diseases from enteral formulas to BTFs. BTFs were shown to be safe and well tolerated. However, as compared to commercial enteral formulas, BTFs were associated with increased calorie, protein, fiber, and salt supply, as well as increased bacterial variety and species diversity in the context of decreased Proteobacteria in stool. Because of worries about nutritional suitability and safety (such as microbial contamination of enteral feeds) [3,25,30], the 2017 ESPGHAN guidelines recommended being cautious with the use of BFTs through a gastrostomy. Furthermore, there is limited evidence of its efficacy in minimizing gagging and retching in children following Nissen fundoplication [3]. On the other hand, BTFs are considered “healthier” and “more natural” when compared to commercial standard enteral formulas [24,31].
4.2. Real-Food-Based Enteral Formulas
Tube-feeding formulas containing real food ingredients are a cost-effective and nutritionally adequate alternative to administering real-food-based enteral nutrition to children with ND [26,27,32,33]. In the United Kingdom and some European countries, a novel enteral formula made with real food ingredients is now available on the market. The formula contains 1.2 kcal/mL; 38% protein from chicken, peas, and green beans; and 53% fiber from vegetables and fruits. The advantage of commercial versus blenderized foods is that they are sterilized, aseptically packed, and hermetically sealed, which allows safe night-long feeding and storage. The literature on the use of tube-feeding formulas with real food components in patients with ND is scarce. A study on acceptability and tolerance was conducted on 19 children aged 1–14 with different diseases, including cerebral palsy [26]. Subjects received a seven-day-tube feeding regimen with an enteral formula containing real food components. The trial was completed by 16 patients. Some of them reported improved stool consistency. Two patients reported improvement of reflux symptoms, while one patient experienced improvement of mood, eye contact, and concentration [26]. Moreover, a retrospective real-world-evidence study has suggested that the same formula could improve gastrointestinal symptoms in clinical practice [27]. A recent report on four clinical cases of patients with various complex conditions, such as neurological and neuromuscular diseases, about the management of prolonged enteral nutrition with a tube-feeding formula with real food ingredients showed the feasibility and good tolerance in terms of intestinal motility and gastrointestinal symptoms (abdominal pain, bloating, and diarrhea), as well as satisfactory growth [33]. Larger-scale prospective studies are required to evaluate the nutritional, economic, and health advantages of these formulas.
5. Tube Weaning
Some tube-fed children can improve their oral skills and become able to eat by mouth as they mature, and their overall health improves. Weaning off tube feeding therefore becomes a therapeutic goal, increasing HRQoL in children who satisfy some criteria (Table 4).

Table 4.
Eligibility criteria for tube weaning [15].
Compared to other tube-fed pediatric patients, a small number of children with ND fulfill the weaning eligibility criteria [15]. Tube weaning is defined as “all the processes and interventions required to transition an individual from a nasogastric/gastrostomy tube dependency to oral feeding of solid or functionally appropriate food that would be considered age-appropriate in a typically developing cohort and meet all his or her nutritional needs without disproportionately affecting development, social environment, and family” [34]. Weaning off prolonged tube feeding may be challenging for a variety of reasons. Prolonged tube feeding may have a negative impact on the development of normal oral-feeding abilities, regardless of the underlying disease, due to the experience of undesirable oral triggers (tube placement, reflux, and vomiting), an absence of flavors and textures, and the impairment of parent–child interplay at feeding times [35,36,37]. Long-term tube feeding’s side effects include reduced appetite, disinterest in eating, or complete rejection of oral feeding. Many weaning methods for tube-dependent children have been reported in recent years [38,39,40,41]. Many of them have been based on a rapid decrease in caloric intake to induce hunger and wean patients within a few weeks during a therapeutic hospitalization under the control of a multidisciplinary team [38,39]. In 2021, the French Network of Rare Digestive Diseases (FIMATHO) and the French-speaking group of Pediatric Hepatology, Gastroenterology, and Nutrition (GFHGNP) published the first position paper outlining current evidence- and expert-opinion-based clinical-practice recommendations for weaning pediatric patients off tube feeding [15]. A multi-model weaning strategy that combines caloric restriction with psycho-behavioral and/or sensorimotor treatments is primarily recommended. Feeding-related psychological and behavioral characteristics of children and caregivers, as well as cultural expectations, mealtime habits, and concomitant mental disorders, are all addressed in psychological interventions. The sensorimotor intervention seeks to minimize tactile hypersensitivity, whether oral or of the whole body, which is considered a barrier to food. One of the earliest sensorimotor interventions described was based on oropharyngeal cavity afferentation or re-afferentation and the restoration of a normal circadian rhythm by using sensory oral stimulations during tube feeding at regular times [40]. The oral motor intervention focuses on the acquisition of abilities required for independent and advanced feeding (i.e., efficient sucking and chewing), such as good jaw stability, lateral tongue motions, helical mandibular movements, and effective lip tone. Tube weaning should be guided by a multidisciplinary team, ideally including a physician, dietitian, nurse, speech/language therapist, occupational therapist, and psychologist [15]. The outpatient clinic is the first option location for weaning, whereas inpatient weaning or intensive day hospital weaning may be considered as a second-line strategy if an outpatient program fails or if no progress is made despite a long-lasting weaning attempt [15]. In an outpatient setting, weaning from tube feeding is represented by a gradual reduction in caloric intake via tube (10–35% of total caloric intake), strict monitoring of clinical and anthropometric data, and guidance of the child and his/her family [40,42]. Outpatient programs can range in length from a few weeks to months or even a year. Inpatient or intensive day hospital weaning may be considered as a first-line strategy in the following situations: (i) high risk of malnutrition or dehydration (i.e., metabolic disease) and/or (ii) poor oral feeding abilities and/or (iii) inadequate social or family context and/or (iv) significant parental stress [15,43].
Inpatient weaning may consist of standard full-time hospitalization for 2 to 3 weeks (i.e., a pediatric ward) or up to 6 weeks (i.e., rehabilitation center or specific centers), or daily hospitalization one or more times a week for many weeks, depending on the center. If necessary, the reduced caloric intake via tube can be achieved quickly over a few days under strict medical control of weight, hydration status, daily oral intake, and glycemic state [15]. Tube feeding can be stopped as soon as the second week of intervention.
The reduction in calorie intake by tube feeding should always be customized, regardless of the program [42]. The faster the reduction, the tighter the surveillance.
6. Parental Decision-Making Process
Tube feeding is a therapeutic intervention that is subject to ethical guidelines [3]. The choice to begin the treatment is based on the expected balance of benefits and risks to support the best interests of the child. As with other therapies, informed and educated parental agreement and consent are crucial ethical principles. Parents must be provided with thorough information about the treatment’s advantages, risks, and alternatives, as well as adequate time to process the information to decide [3,42]. The decision-making process for parents is frequently hampered by adverse caregiver perceptions, and gastrostomy tube placement is often delayed [44]. Parents may accept this choice only as a last resort due to concerns about loss of regular eating, reliance on enteral feeding, and surgical complications [44]. When attempting to pick between opposing options, decisional conflict might arise, especially when the choice compromises or challenges personal values [45]. According to a systematic review, parents of children with ND on tube feeding frequently feel decisional conflict about this intervention [46]. The major causes of decisional conflict are represented in Figure 1.
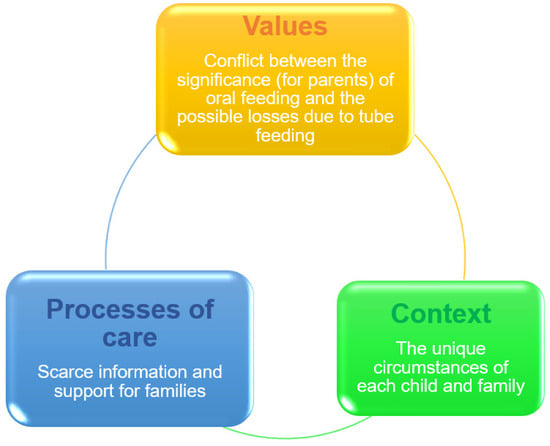
Figure 1.
Decisional conflict over tube feeding [44,46].
Physicians must develop successful family-centered and patient-centered compliance methods for tube-fed children with ND. The World Health Organization (WHO) established the International Classification of Functioning, Disability, and Health (ICF) classification system to categorize the dimensions of health [47]. The framework goes beyond illness to emphasize everyone’s ability to function and engage within their personal environment, with the goal of assisting physicians in guiding decision-making discussions with families. The tube-feeding option might be discussed for children with an ND as a safety or efficiency concern (i.e., focusing on impairment) and/or as a therapeutic strategy (i.e., focusing on malnutrition). The ICF’s biopsychosocial paradigm, on the other hand, enables a debate on how tube feeding might increase a child’s engagement in family life by shortening feeding periods and opening up opportunities for other community activities [47]. The ICF encourages physicians to address tube feeding’s influence on the child’s function, activity, and involvement, in addition to its role as a medical intervention [44,47]. Moreover, both in the hospital and in the community, education and training on gastrostomy tube feeding assist the caregivers of patients in coping with the transition from oral to gastrostomy tube feeding, while ongoing social support is crucial to enhance their HRQoL. The ESPGHAN guidelines recommend collaboration with a professional ethicist in all circumstances when invasive tests or treatments (i.e., gastrostomy, fundoplication, and parenteral supplementation) raise ethical problems [3].
Quality of Life
Feeding difficulties and gastrointestinal complaints are frequent in pediatric patients with ND, leading to malnutrition and lower HRQoL. Generally, quality of life refers to an individual’s perceived quality of everyday life, including all emotional, social, and physical aspects. In medicine, HRQoL is an evaluation of how an illness, disability, or dysfunction may impair an individual’s overall health [48]. For chronic illnesses such as ND, HRQoL is seen as the most significant long-term objective of care. Caregivers of children with ND have been shown to experience very low QoL, poorer mental health, and increased degrees of burnout [49]. Because children with severe ND are unable to self-report their HRQoL, parent-proxy reports are the only accessible metrics. Sullivan et al. [50] discovered a substantial increase in caregiver QoL six and twelve months after initiating enteral nutrition in children with cerebral palsy. Caregivers reported substantial increases in social functioning, mental health, energy, and overall health perception after beginning tube feeding. Similarly, Grzybowska-Chlebowczyk et al. [51] observed that the insertion of a gastrostomy tube improved the lives of more than 70% of 302 caregivers. Almost all caregivers reported a satisfactory HRQoL in a recent cross-sectional investigation of HRQoL in neurologically disabled children on home tube feeding [52]. Notably, children with cerebral palsy had higher HRQoL ratings than those with genetic or metabolic diseases, most likely due to the nonprogressive character of cerebral-palsy-related ND. The variables impacting HRQoL are also being studied. Because of factors such as the effect of finances and/or level of education on healthcare access, unscheduled inpatient hospitalization, the difficulty of following prescriptions, and post-discharge survival, the family’s social status is regarded as a key factor affecting an individual’s neurocognitive growth [53]. The first study to investigate the relationship between caregiver social status and quality of life associated with home enteral feeding in children with ND reported that higher education levels may have a detrimental influence on caregivers’ quality of life [54]. The authors suggested some possible explanations, such as the commitment to long-term care for these children, which often requires them to forego some of their own life projects and expectations in order to dedicate significant amounts of time to look after their child; and/or greater awareness of the child’s chronic and, quite often, progressive disorder. More research is needed to identify the factors influencing QoL and, from there, the at-risk families that need closer psychological support.
Author Contributions
All authors have equally contributed to the study. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no specific funding for this work. Nestlé Health Science S.A. provided funding support for open-access publication fees.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. F.G. reports serving on advisory boards for Nestlé and Danone.
References
- Huysentruyt, K.; Geeraert, F.; Allemon, H.; Prinzie, P.; Roelants, M.; Ortibus, E.; Vandenplas, Y.; De Schepper, J. Nutritional red flags in children with cerebral palsy. Clin. Nutr. 2020, 39, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Lezo, A.; Capriati, T.; Spagnuolo, M.I.; Lacitignola, L.; Goreva, I.; Di Leo, G.; Cecchi, N.; Gandullia, P.; Amarri, S.; Forchielli, M.L.; et al. Paediatric home artificial nutrition in Italy: Report from 2016 survey on behalf of artificial nutrition network of Italian society for gastroenterology, hepatology and nutrition (SIGENP). Nutrients 2018, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; van Wynckel, M.; Hulst, J.; Broekaert, I.; Bronsky, J.; Dall’Oglio, L.; Mis, N.F.; Hojsak, I.; Orel, R.; Papadopoulou, A.; et al. European Society for Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for the Evaluation and Treatment of Gastrointestinal and Nutritional Complications in Children with Neurological Impairment. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 242–264. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Dipasquale, V.; Gottrand, F.; Sullivan, P.B. Gastrointestinal and nutritional issues in children with neurological disability. Dev. Med. Child Neurol. 2018, 60, 892–896. [Google Scholar] [CrossRef]
- Homan, M.; Hauser, B.; Romano, C.; Tzivinikos, C.; Torroni, F.; Gottrand, F.; Hojsak, I.; Dall’Oglio, L.; Thomson, M.; Bontems, P.; et al. Percutaneous endoscopic gastrostomy in children: An update to the ESPGHAN position paper. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 415–426. [Google Scholar] [CrossRef]
- Bawazir, O.A. Percutaneous endoscopic gastrostomy in children less than 10 kilograms: A comparative study. Saudi J. Gastroenterol. 2020, 26, 105–110. [Google Scholar] [CrossRef]
- Toh Yoon, E.W.; Nishihara, K. Ball valve syndrome caused by the migration of gastrostomy catheter tip. Clin. Gastroenterol. Hepatol. 2017, 15, e133–e134. [Google Scholar] [CrossRef][Green Version]
- Stewart, C.E.; Mutalib, M.; Pradhan, A.; Bassett, C.; Drake, D.; Upadhyaya, M. Short article: Buried bumper syndrome in children: Incidence and risk factors. Eur. J. Gastroenterol. Hepatol. 2017, 29, 181–184. [Google Scholar] [CrossRef]
- Gothberg, G.; Bjornsson, S. One-step insertion of low-profile gastrostomy in pediatric patients vs pull percutaneous endoscopic gastrostomy: Retrospective analysis of outcomes. JPEN J. Parenter. Enteral. Nutr. 2016, 40, 423–430. [Google Scholar] [CrossRef]
- Jacob, A.; Delesalle, D.; Coopman, S.; Bridenne, M.; Guimber, D.; Turck, D.; Gottrand, F.; Michaud, L. Safety of the one-step percutaneous endoscopic gastrostomy button in children. J. Pediatr. 2015, 166, 1526–1528. [Google Scholar] [CrossRef]
- Broekaert, I.J.; Falconer, J.; Bronsky, J.; Gottrand, F.; Dall’Oglio, L.; Goto, E.; Hojsak, I.; Hulst, J.; Kochavi, B.; Papadopoulou, A.; et al. The use of jejunal tube feeding in children: A position paper by the gastroenterology and nutrition committees of the European Society for Paediatric Gastroenterology, Hepatology, and nutrition 2019. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Elmehdi, S.; Ley, D.; Aumar, M.; Coopman, S.; Guimber, D.; Nicolas, A.; Antoine, M.; Turck, D.; Kyheng, M.; Gottrand, F. Endoscopic gastrojejunostomy in infants and children. J. Pediatr. 2022, 244, 115–119.e1. [Google Scholar] [CrossRef] [PubMed]
- Krom, H.; de Winter, J.P.; Kindermann, A. Development, prevention, and treatment of feeding tube dependency. Eur. J. Pediatr. 2017, 176, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, J.; Sun, S.; Huang, J.; Zhang, A.; Tang, X. Effects of intermittent feeding versus continuous feeding on enteral nutrition tolerance in critically ill patients: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e23528. [Google Scholar] [CrossRef] [PubMed]
- Clouzeau, H.; Dipasquale, V.; Rivard, L.; Lecoeur, K.; Lecoufle, A.; Le Ru-Raguénès, V.; Guimber, D.; Leblanc, V.; Malécot-Le Meur, G.; Baeckeroot, S.; et al. Weaning children from prolonged enteral nutrition: A position Paper. Eur. J. Clin. Nutr. 2022, 76, 505–515. [Google Scholar] [CrossRef]
- Fernando, T.; Goldman, R.D. Management of gastroesophageal reflux disease in pediatric patients with cerebral palsy. Can. Fam. Physician 2019, 65, 796–798. [Google Scholar]
- Rosen, R.; Vandenplas, Y.; Singendonk, M.; Cabana, M.; DiLorenzo, C.; Gottrand, F.; Gupta, S.; Langendam, M.; Staiano, A.; Thapar, N.; et al. Pediatric gastroesophageal reflux clinical practice guidelines: Joint recommendations of the north American Society for Pediatric Gastroenterology, Hepatology, and nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 516–554. [Google Scholar] [CrossRef]
- Aumar, M.; Lalanne, A.; Guimber, D.; Coopman, S.; Turck, D.; Michaud, L.; Gottrand, F. Influence of percutaneous endoscopic gastrostomy on gastroesophageal reflux disease in children. J. Pediatr. 2018, 197, 116–120. [Google Scholar] [CrossRef]
- Lansdale, N.; McNiff, M.; Morecroft, J.; Kauffmann, L.; Morabito, A. Long-term and ‘patient-reported’ outcomes of total esophagogastric dissociation versus laparoscopic fundoplication for gastroesophageal reflux disease in the severely neurodisabled child. J. Pediatr. Surg. 2015, 50, 1828–1832. [Google Scholar] [CrossRef]
- Coletta, R.; Aldeiri, B.; Jackson, R.; Morabito, A. Total esophagogastric dissociation (TEGD): Lessons from two decades of experience. J. Pediatr. Surg. 2019, 54, 1214–1219. [Google Scholar] [CrossRef]
- DeAntonio, J.H.; Parrish, D.W.; Rosati, S.F.; Oiticica, C.; Lanning, D.A. Laparoscopic gastroesophageal dissociation in neurologically impaired children with gastroesophageal reflux disease. J. Pediatr. Surg. 2017, 53, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Orizio, P.; Boroni, G.; Cheli, M.; Colusso, M.M.; Parolini, F.; Bianchi, A.; Alberti, D. Total oesophagogastric dissociation in neurologically impaired children: 18 years’ experience and long-term follow-up. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, É.P.C.R.; Silva, E.C.D.; Lins Filho, O.L.; Antunes, M.M.C.; Brandt, K.G. Blenderized tube feeding for children: An integrative review. Rev. Paul. Pediatr. 2021, 40, e2020419. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.W.; Spurlock, A.; Pierce, L. Survey study assessing attitudes and experiences of pediatric registered dietitians regarding blended food by gastrostomy tube feeding. Nutr. Clin. Pract. 2015, 30, 402–405. [Google Scholar] [CrossRef]
- Oparaji, J.A.; Sferra, T.; Sankararaman, S. Basics of blenderized tube feeds: A primer for pediatric primary care clinicians. Gastroenterol. Res. 2019, 12, 111–114. [Google Scholar] [CrossRef]
- Thornton-Wood, C.; Saduera, S. Tolerance and acceptability of a new paediatric enteral tube feeding formula containing ingredients derived from food: A multicentre trial (in the United Kingdom). J. Neonatol. Clin. Pediatr. 2020, 7, 50. [Google Scholar] [CrossRef]
- O’Connor, G.; Watson, M.; Van Der Linde, M.; Bonner, R.S.; Hopkins, J.; Saduera, S. Monitor gastrointestinal tolerance in children who have switched to an “enteral formula with food-derived ingredients”: A national, multicenter retrospective chart review (RICIMIX study). Nutr. Clin. Pract. 2022, 37, 929–934. [Google Scholar] [CrossRef]
- Steensels, S.; Depoortere, I. Chemoreceptors in the gut. Annu. Rev. Physiol. 2018, 80, 117–141. [Google Scholar] [CrossRef]
- Williams, E.K.; Chang, R.B.; Strochlic, D.E.; Umans, B.D.; Lowell, B.B.; Liberles, S.D. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 2016, 166, 209–221. [Google Scholar] [CrossRef]
- Gallagher, K.; Flint, A.; Mouzaki, M.; Carpenter, A.; Haliburton, B.; Bannister, L.; Norgrove, H.; Hoffman, L.; Mack, D.; Stintzi, A.; et al. Blenderized enteral nutrition diet study: Feasibility, clinical, and microbiome outcomes of providing blenderized feeds through a gastric tube in a medically complex pediatric population. JPEN J. Parenter. Enteral. Nutr. 2018, 42, 1046–1060. [Google Scholar] [CrossRef]
- Boston, M.; Wile, H. Caregivers’ perceptions of real-food containing tube feeding: A Canadian survey. Can. J. Diet Pract. Res. 2020, 81, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Samela, K.; Mokha, J.; Emerick, K.; Davidovics, Z.H. Transition to a tube feeding formula with real food ingredients in pediatric patients with intestinal failure. Nutr. Clin. Pract. 2017, 32, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Dipasquale, V.; Diamanti, A.; Trovato, C.M.; Elia, D.; Romano, C. Real food in enteral nutrition for chronically ill children: Overview and practical clinical cases. Curr. Med. Res. Opin. 2022, 38, 831–835. [Google Scholar] [CrossRef]
- Dovey, T.M.; Wilken, M.; Martin, C.I.; Meyer, C. Definitions and clinical guidance on the enteral dependence component of the avoidant/restrictive food intake disorder diagnostic criteria in children. JPEN J. Parenter. Enter. Nutr. 2018, 42, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.J.; Harris, G.; Blissett, J. Tube feeding in infancy: Implications for the development of normal eating and drinking skills. Dysphagia 2005, 20, 46–61. [Google Scholar] [CrossRef]
- Edwards, S.; Davis, A.M.; Bruce, A.; Mousa, H.; Lyman, B.; Cocjin, J.; Dean, K.; Ernst, L.; Almadhoun, O.; Hyman, P. Caring for tube-fed children: A review of management, tube weaning, and emotional considerations. JPEN J. Parenter. Enteral. Nutr. 2016, 40, 616–622. [Google Scholar] [CrossRef]
- Wilken, M.; Bartmann, P.; Dovey, T.M.; Bagci, S. Characteristics of feeding tube dependency with respect to food aversive behaviour and growth. Appetite 2018, 123, 1–6. [Google Scholar] [CrossRef]
- Hartdorff, C.M.; Kneepkens, C.M.F.; Stok-Akerboom, A.M.; van Dijk-Lokkart, E.M.; Engels, M.A.; Kindermann, A. Clinical tube weaning supported by hunger provocation in fully-tube-fed children. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 538–543. [Google Scholar] [CrossRef]
- Krom, H.; de Meij, T.G.J.; Benninga, M.A.; van Dijk-Lokkart, E.M.; Engels, M.; Kneepkens, C.M.F.; Kuiper-Cramer, L.; Otten, M.G.M.; van der Sluijs Veer, L.; Stok-Akerboom, A.M.; et al. Long-term efficacy of clinical hunger provocation to wean feeding tube dependent children. Clin. Nutr. 2020, 39, 2863–2871. [Google Scholar] [CrossRef]
- Dipasquale, V.; Lecoeur, K.; Aumar, M.; Guimber, D.; Coopman, S.; Nicolas, A.; Lecoufle, A.; Van Malleghem, A.; Turck, D.; Ley, D.; et al. Weaning children from prolonged enteral nutrition: A survey of practice on behalf of the French Society of Paediatric Gastroenterology, Hepatology, and Nutrition. JPEN J. Parenter. Enteral. Nutr. 2022, 46, 215–221. [Google Scholar] [CrossRef]
- Dipasquale, V.; Lecoeur, K.; Aumar, M.; Guimber, D.; Coopman, S.; Nicolas, A.; Turck, D.; Gottrand, F.; Ley, D. Factors associated with success and failure of weaning children from prolonged enteral nutrition: A retrospective cohort study. J. Pediatr. Gastroenterol Nutr 2021, 72, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Senez, C.; Guys, J.M.; Mancini, J.; Paz Paredes, A.; Lena, G.; Choux, M. Weaning children from tube to oral feeding. Childs Nerv Syst. 1996, 12, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, A.Y.; Vuillermin, P.J.; Fuller, D.G. A descriptive comparison of approaches to paediatric tube weaning across five countries. Int. J. Speech Lang. Pathol. 2017, 19, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Mahant, S.; Cohen, E.; Nelson, K.E.; Rosenbaum, P. Decision-making around gastrostomy tube feeding in children with neurologic impairment: Engaging effectively with families. Paediatr. Child Health 2018, 23, 209–213. [Google Scholar] [CrossRef]
- Craig, G.M.; Scambler, G. Negotiating mothering against the odds: Gastrostomy tube feeding, stigma, governmentality and disabled children. Soc. Sci. Med. 2006, 62, 1115–1125. [Google Scholar] [CrossRef]
- Mahant, S.; Jovcevska, V.; Cohen, E. Decision-making around gastrostomy-feeding in children with neurologic disabilities. Pediatrics 2011, 127, e1471–e1481. [Google Scholar] [CrossRef]
- WHO. International Classification of Functioning, Disability, and Health: Children and Youth Version ICF-CY; WHO: Geneva, Switzerland, 2007; Available online: http://apps.who.int/iris/bitstream/10665/43737/1/9789241547321_eng.pdf (accessed on 30 June 2022).
- Centers for Disease Control and Prevention. Measuring Healthy Days: Population Assessment of Health-Related Quality of Life; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2000. [Google Scholar]
- Basaran, A.; Karadavut, K.I.; Uneri, S.O.; Balbaloglu, O.; Atasoy, N. The effect of having a children with cerebral palsy on quality of life, burn-out, depression and anxiety scores: A comparative study. Eur. J. Phys. Rehabil. Med. 2013, 49, 815–822. [Google Scholar]
- Sullivan, P.B.; Juszczak, E.; Bachlet, A.M.; Thomas, A.G.; Lambert, B.; Vernon-Roberts, A.; Grant, H.W.; Eltumi, M.; Alder, N.; Jenkinson, C. Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy. Dev. Med. Child Neurol. 2004, 46, 796–800. [Google Scholar] [CrossRef]
- Grzybowska-Chlebowczyk, U.; Wiecek, S.; Popi’nska, K.; Szlagatys-Sidorkiewicz, A.; Toporowska-Kowalska, E.; Hapyn, E.; Wernicka, A.; Sibilska, M.; Gebora-Kowalska, B.; Borkowska, A.; et al. The evaluation of life quality of families of children after percutaneous endoscopic gastrostomy. Pediatr. Pol. 2015, 90, 103–107. [Google Scholar] [CrossRef]
- Dipasquale, V.; Ventimiglia, M.; Gramaglia, S.M.C.; Parma, B.; Funari, C.; Selicorni, A.; Armano, C.; Salvatore, S.; Romano, C. Health-related quality of life and home enteral nutrition in children with neurological impairment: Report from a multicenter survey. Nutrients 2019, 11, 2968. [Google Scholar] [CrossRef]
- Takeuchi, H.; Taki, Y.; Nouchi, R.; Yokoyama, R.; Kotozaki, Y.; Nakagawa, S.; Sekiguchi, A.; Iizuka, K.; Yamamoto, Y.; Hanawa, S.; et al. The effects of family socioeconomic status on psychological and neural mechanisms as well as their sex differences. Front. Hum. Neurosci. 2019, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Dipasquale, V.; Ventimiglia, M.; Gramaglia, S.M.C.; Parma, B.; Funari, C.; Selicorni, A.; Armano, C.; Salvatore, S.; Romano, C. Caregiver social status and health-related quality of life in neurologically impaired children on home enteral nutrition. Nutrients 2021, 13, 1928. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).