A Prognostic Model Based on Nutritional Risk Index in Operative Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection and Classification
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
3.1. The Optimal Cut-Off Value of NRI
3.2. Clinical Characteristics of Patients and Their Relationship with NRI
3.3. Prognostic Value of NRI for Overall Survival (OS)
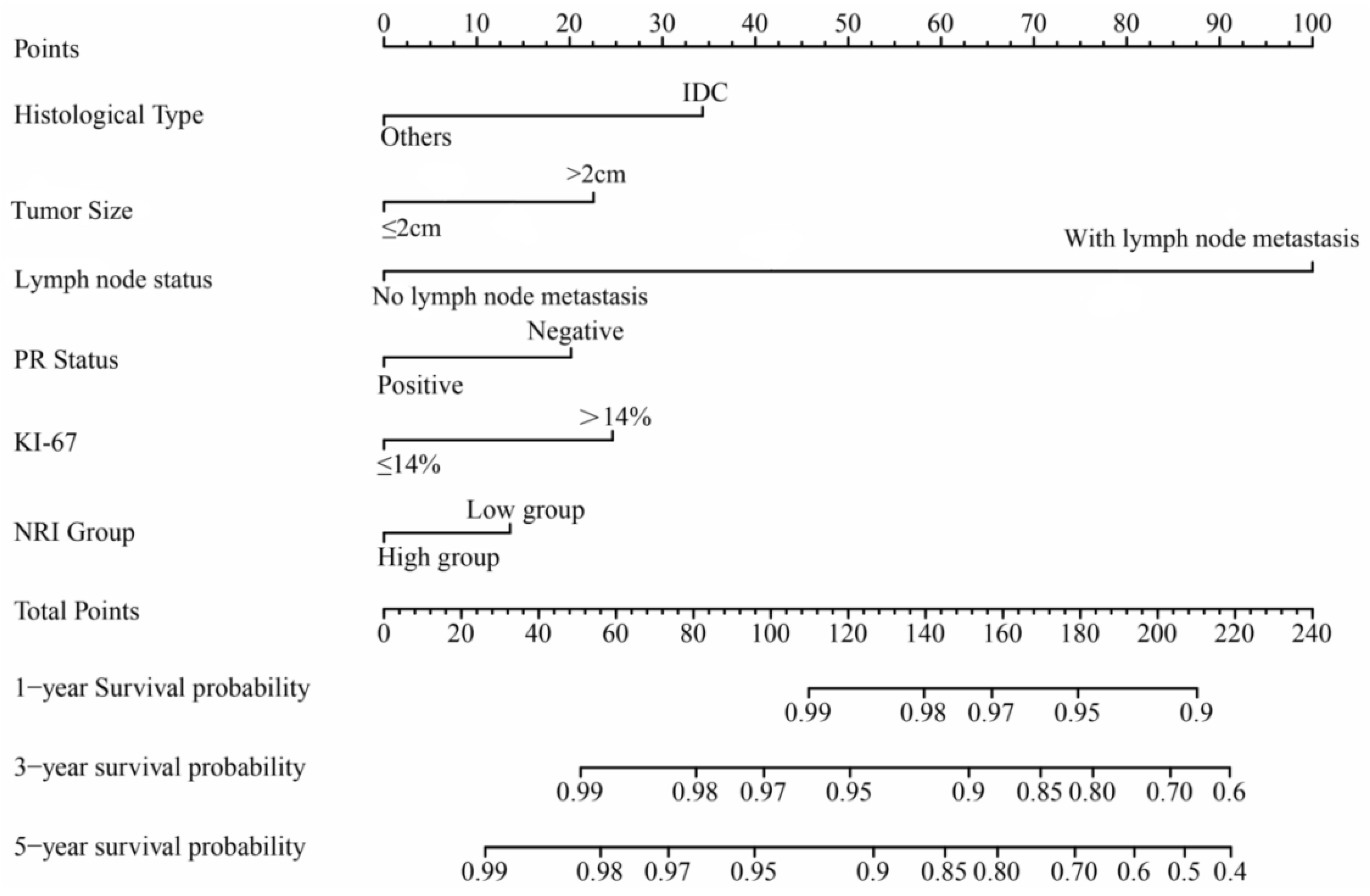
3.4. Univariate and Multivariate Cox Regression Analyses
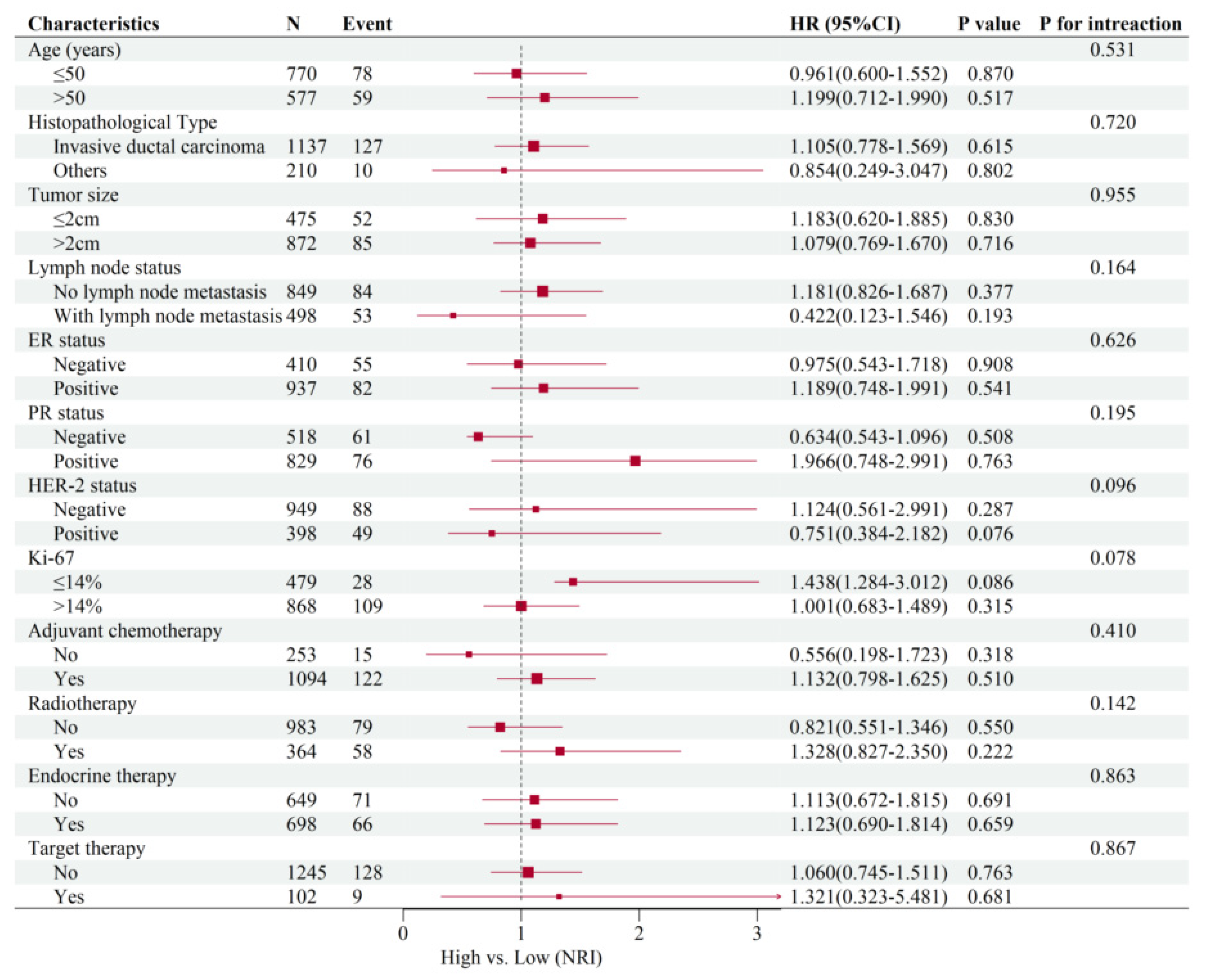
3.5. Subgroup Analysis of Common Clinical Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Trapani, D.; Ginsburg, O.; Fadelu, T.; Lin, N.U.; Hassett, M.; Ilbawi, A.M.; Anderson, B.O.; Curigliano, G. Global challenges and policy solutions in breast cancer control. Cancer Treat. Rev. 2022, 104, 102339. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Zhang, K.; Shi, Z.T.; Zhang, X.; Xie, J.; Liu, J.Y.; Chang, C. Triple-negative invasive breast carcinoma: The association between the sonographic appearances with clinicopathological feature. Sci. Rep. 2018, 8, 9040. [Google Scholar] [CrossRef]
- Frasier, L.L.; Holden, S.; Holden, T.; Schumacher, J.R.; Leverson, G.; Anderson, B.; Greenberg, C.C.; Neuman, H.B. Temporal Trends in Postmastectomy Radiation Therapy and Breast Reconstruction Associated with Changes in National Comprehensive Cancer Network Guidelines. JAMA Oncol. 2016, 2, 95–101. [Google Scholar] [CrossRef]
- Yeo, S.K.; Guan, J.L. Breast Cancer: Multiple Subtypes within a Tumor? Trends Cancer 2017, 3, 753–760. [Google Scholar] [CrossRef]
- McMillan, D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 223–226. [Google Scholar] [CrossRef]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef]
- Liu, X.; Meng, Q.H.; Ye, Y.; Hildebrandt, M.A.; Gu, J.; Wu, X. Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with non-metastatic breast cancer. Carcinogenesis 2015, 36, 243–248. [Google Scholar] [CrossRef]
- Mayne, S.T.; Playdon, M.C.; Rock, C.L. Diet, nutrition, and cancer: Past, present and future. Nat. Rev. Clin. Oncol. 2016, 13, 504–515. [Google Scholar] [CrossRef]
- Rothman, K.J. BMI-related errors in the measurement of obesity. Int. J. Obes. 2008, 32 (Suppl. 3), S56–S59. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, P.; Deurenberg-Yap, M.; Guricci, S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes. Rev. 2002, 3, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Spinella, R.; Sawhney, R.; Jalan, R. Albumin in chronic liver disease: Structure, functions and therapeutic implications. Hepatol. Int. 2016, 10, 124–132. [Google Scholar] [CrossRef]
- Litton, J.K.; Gonzalez-Angulo, A.M.; Warneke, C.L.; Buzdar, A.U.; Kau, S.W.; Bondy, M.; Mahabir, S.; Hortobagyi, G.N.; Brewster, A.M. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J. Clin. Oncol. 2008, 26, 4072–4077. [Google Scholar] [CrossRef]
- Takahashi, M.; Sowa, T.; Tokumasu, H.; Gomyoda, T.; Okada, H.; Ota, S.; Terada, Y. Comparison of three nutritional scoring systems for outcomes after complete resection of non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2021, 162, 1257–1268 e3. [Google Scholar] [CrossRef] [PubMed]
- Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N. Engl. J. Med. 1991, 325, 525–532. [Google Scholar] [CrossRef]
- Oh, J.; Liu, A.; Tran, E.; Berthelet, E.; Wu, J.; Olson, R.A.; Chau, N.; Bowman, A.; Hamilton, S.N. Association between nutritional risk index and outcomes for head and neck cancer patients receiving concurrent chemo-radiotherapy. Head Neck 2020, 42, 2560–2570. [Google Scholar] [CrossRef]
- Dong, W.; Liu, X.; Zhu, S.; Lu, D.; Cai, K.; Cai, R.; Li, Q.; Zeng, J.; Li, M. Selection and optimization of nutritional risk screening tools for esophageal cancer patients in China. Nutr. Res. Pract. 2020, 14, 20–24. [Google Scholar] [CrossRef]
- Sagou, K.; Ozeki, K.; Ukai, S.; Adachi, Y.; Fukushima, N.; Kohno, A. Impact of a Nutritional Risk Index on Clinical Outcomes after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 2287–2296. [Google Scholar] [CrossRef]
- Pagano, A.P.; Sicchieri, J.M.F.; Schiavoni, I.L.; Barbeiro, D.; Manca, C.S.; da Silva, B.R.; Bezerra, A.E.; Pinto, L.C.M.; Araujo, R.C.; Teixeira, A.C.; et al. Phase angle as a severity indicator for liver diseases. Nutrition 2020, 70, 110607. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, R.; Wu, P.; Zhang, W.; Mao, S.; Wu, Y.; Liu, J.; Ma, W.; Zheng, Z.; Zhang, J.; et al. Preoperative Nutritional Risk Index Predicts Recurrence of Oligometastatic Prostate Cancer in Patients Undergoing Cytoreductive Radical Prostatectomy. Nutr. Cancer 2021, 73, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, B.; Jin, F. The assessment of 8th edition AJCC prognostic staging system and a simplified staging system for breast cancer: The analytic results from the SEER database. Breast J. 2019, 25, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.W.; Hudis, C.A.; Pritchard, K.I. Adjuvant endocrine therapy in hormone receptor-positive postmenopausal breast cancer: Evolution of NCCN, ASCO, and St Gallen recommendations. J. Natl. Compr. Cancer Netw. 2006, 4, 971–979. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Caccialanza, R.; Goldwasser, F.; Marschal, O.; Ottery, F.; Schiefke, I.; Tilleul, P.; Zalcman, G.; Pedrazzoli, P. Unmet needs in clinical nutrition in oncology: A multinational analysis of real-world evidence. Ther. Adv. Med. Oncol. 2020, 12, 1758835919899852. [Google Scholar] [CrossRef]
- Senesse, P.; Assenat, E.; Schneider, S.; Chargari, C.; Magne, N.; Azria, D.; Hebuterne, X. Nutritional support during oncologic treatment of patients with gastrointestinal cancer: Who could benefit? Cancer Treat. Rev. 2008, 34, 568–575. [Google Scholar] [CrossRef]
- Barao, K.; Abe Vicente Cavagnari, M.; Silva Fucuta, P.; Manoukian Forones, N. Association between Nutrition Status and Survival in Elderly Patients with Colorectal Cancer. Nutr. Clin. Pract. 2017, 32, 658–663. [Google Scholar] [CrossRef]
- Norman, K.; Pichard, C.; Lochs, H.; Pirlich, M. Prognostic impact of disease-related malnutrition. Clin. Nutr. 2008, 27, 5–15. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, H.H.; Grote Beverborg, N.; Dickstein, K.; Anker, S.D.; Lang, C.C.; Ng, L.L.; van Veldhuisen, D.J.; Voors, A.A.; van der Meer, P. Iron deficiency in worsening heart failure is associated with reduced estimated protein intake, fluid retention, inflammation, and antiplatelet use. Eur. Heart J. 2019, 40, 3616–3625. [Google Scholar] [CrossRef] [PubMed]
- Kalli, S.; Semine, A.; Cohen, S.; Naber, S.P.; Makim, S.S.; Bahl, M. American Joint Committee on Cancer’s Staging System for Breast Cancer, Eighth Edition: What the Radiologist Needs to Know. Radiographics 2018, 38, 1921–1933. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J. Pathol. 2014, 232, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, J.; Zhu, Y.; Zhan, W.; Chen, X.; Shen, K. Association of sonographic features and molecular subtypes in predicting breast cancer disease outcomes. Cancer Med. 2020, 9, 6173–6185. [Google Scholar] [CrossRef] [PubMed]
- Garziera, M.; Cecchin, E.; Giorda, G.; Sorio, R.; Scalone, S.; De Mattia, E.; Roncato, R.; Gagno, S.; Poletto, E.; Romanato, L.; et al. Clonal Evolution of TP53 c.375+1G>A Mutation in Pre- and Post- Neo-Adjuvant Chemotherapy (NACT) Tumor Samples in High-Grade Serous Ovarian Cancer (HGSOC). Cells. 2019, 8, 1186. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, J.Z.; Chen, S.; Lin, S.Z.; Pan, W.; Meng, Z.W.; Cai, X.R.; Chen, Y.L. Development and validation of novel nomograms for predicting the survival of patients after surgical resection of pancreatic ductal adenocarcinoma. Cancer Med. 2020, 9, 3353–3370. [Google Scholar] [CrossRef]
- Miao, D.L.; Song, W.; Qian, J.; Zhu, Z.G.; Wu, Q.; Lv, C.G.; Chen, L. Development and Validation of a Nomogram for Predicting Overall Survival in Pancreatic NeuroendocrineTumors. Transl. Oncol. 2018, 11, 1097–1103. [Google Scholar] [CrossRef]
- Plichta, J.K.; Ren, Y.; Thomas, S.M.; Greenup, R.A.; Fayanju, O.M.; Rosenberger, L.H.; Hyslop, T.; Hwang, E.S. Implications for Breast Cancer Restaging Based on the 8th Edition AJCC Staging Manual. Ann. Surg. 2020, 271, 169–176. [Google Scholar] [CrossRef]
- Bao, X.; Liu, F.; Lin, J.; Chen, Q.; Chen, L.; Chen, F.; Wang, J.; Qiu, Y.; Shi, B.; Pan, L.; et al. Nutritional assessment and prognosis of oral cancer patients: A large-scale prospective study. BMC Cancer 2020, 20, 146. [Google Scholar] [CrossRef]
- Gittleman, H.; Lim, D.; Kattan, M.W.; Chakravarti, A.; Gilbert, M.R.; Lassman, A.B.; Lo, S.S.; Machtay, M.; Sloan, A.E.; Sulman, E.P.; et al. An independently validated nomogram for individualized estimation of survival among patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. Neuro Oncol. 2017, 19, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Chen, L.; Xu, A.M. A Simple Model Established by Blood Markers Predicting Overall Survival after Radical Resection of Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2020, 10, 583. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Nadal, E.; Peiro, I.; Masuet-Aumatell, C.; Macia, I.; Rivas, F.; Rosado, G.; Rodriguez, P.; Urena, A.; Padrones, S.; et al. Preoperative nutritional status assessment predicts postoperative outcomes in patients with surgically resected non-small cell lung cancer. Eur. J. Surg. Oncol. 2018, 44, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Kim, S.Y.; Song, J.H.; Kim, Y.S.; Jeong, S.J.; Lee, J.G.; Paik, H.C.; Park, M.S. Usefulness of the preoperative prognostic nutritional index score as a predictor of the outcomes of lung transplantation: A single-institution experience. Clin. Nutr. 2019, 38, 2423–2429. [Google Scholar] [CrossRef]
- Sasahara, M.; Kanda, M.; Ito, S.; Mochizuki, Y.; Teramoto, H.; Ishigure, K.; Murai, T.; Asada, T.; Ishiyama, A.; Matsushita, H.; et al. The Preoperative Prognostic Nutritional Index Predicts Short-Term and Long-Term Outcomes of Patients with Stage II/III Gastric Cancer: Analysis of a Multi-Institution Dataset. Dig. Surg. 2020, 37, 135–144. [Google Scholar] [CrossRef]
- Sasaki, M.; Miyoshi, N.; Fujino, S.; Ogino, T.; Takahashi, H.; Uemura, M.; Matsuda, C.; Yamamoto, H.; Mizushima, T.; Mori, M.; et al. The Geriatric Nutritional Risk Index predicts postoperative complications and prognosis in elderly patients with colorectal cancer after curative surgery. Sci. Rep. 2020, 10, 10744. [Google Scholar] [CrossRef]
- Fujiya, K.; Kawamura, T.; Omae, K.; Makuuchi, R.; Irino, T.; Tokunaga, M.; Tanizawa, Y.; Bando, E.; Terashima, M. Impact of Malnutrition After Gastrectomy for Gastric Cancer on Long-Term Survival. Ann. Surg. Oncol. 2018, 25, 974–983. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Y.; Kong, X.; Su, Z.; Wang, Z.; Wang, X.; Du, Y.; Fang, Y.; Li, X.; Wang, J. Nutritional Risk Index Predicts Survival in Patients with Breast Cancer Treated with Neoadjuvant Chemotherapy. Front. Nutr. 2021, 8, 786742. [Google Scholar] [CrossRef]
- Hothorn, T.; Zeileis, A. Generalized maximally selected statistics. Biometrics 2008, 64, 1263–1269. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, H.; Zhang, H.; Liu, Q.; Ye, Y.; Hao, J.; Zhao, Q.; Qi, X.; Liu, S.; Zhang, E.; et al. Prognostic Value of N-Terminal Pro-B-Type Natriuretic Peptide in Elderly Patients with Valvular Heart Disease. J. Am. Coll. Cardiol. 2020, 75, 1659–1672. [Google Scholar] [CrossRef]
- Jawitz, N.G.; Raman, V.; Jawitz, O.K.; Shimpi, R.A.; Wood, R.K.; Hartwig, M.G.; D’Amico, T.A. Utilization Trends and Volume-Outcomes Relationship of Endoscopic Resection for Early Stage Esophageal Cancer. Ann. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Corti, F.; Lonardi, S.; Intini, R.; Salati, M.; Fenocchio, E.; Belli, C.; Borelli, B.; Brambilla, M.; Prete, A.A.; Quara, V.; et al. The Pan-Immune-Inflammation Value in microsatellite instability-high metastatic colorectal cancer patients treated with immune checkpoint inhibitors. Eur. J. Cancer 2021, 150, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Shao, W.; Yin, W.; Xu, X.; Chen, H.; Qiu, Y.; He, J. Video-assisted thoracoscopic surgery for stage I non-small cell lung cancer: Long-term survival and prognostic factors. Tumour Biol. 2013, 34, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Yotsukura, M.; Ohtsuka, T.; Kaseda, K.; Kamiyama, I.; Hayashi, Y.; Asamura, H. Value of the Glasgow Prognostic Score as a Prognostic Factor in Resectable Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2016, 11, 1311–1318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takagi, K.; Yagi, T.; Umeda, Y.; Shinoura, S.; Yoshida, R.; Nobuoka, D.; Kuise, T.; Araki, H.; Fujiwara, T. Preoperative Controlling Nutritional Status (CONUT) Score for Assessment of Prognosis Following Hepatectomy for Hepatocellular Carcinoma. World J. Surg. 2017, 41, 2353–2360. [Google Scholar] [CrossRef]
- Yoshida, N.; Harada, K.; Baba, Y.; Kosumi, K.; Iwatsuki, M.; Kinoshita, K.; Nakamura, K.; Sakamoto, Y.; Miyamoto, Y.; Karashima, R.; et al. Preoperative controlling nutritional status (CONUT) is useful to estimate the prognosis after esophagectomy for esophageal cancer. Langenbecks Arch. Surg. 2017, 402, 333–341. [Google Scholar] [CrossRef]
- Hua, X.; Long, Z.Q.; Huang, X.; Deng, J.P.; He, Z.Y.; Guo, L.; Zhang, W.W.; Lin, H.X. The Value of Prognostic Nutritional Index (PNI) in Predicting Survival and Guiding Radiotherapy of Patients with T1-2N1 Breast Cancer. Front. Oncol. 2019, 9, 1562. [Google Scholar] [CrossRef]


| Characteristic Total No. (%) (n = 1347) | Low-NRI Group (n = 813) | High-NRI Group (n = 534) | p | |
|---|---|---|---|---|
| Age (years) median (IQR) | 47 (40–58) | 48 (41–59) | 47(40–59) | 0.006 |
| Histological type | ||||
| Invasive ductal carcinoma | 1137 (84.4%) | 687 (51.0%) | 450 (33.4%) | 0.908 |
| Others | 210 (15.6%) | 126 (9.4%) | 84 (6.2%) | |
| Tumor size | ||||
| ≤2 cm | 475 (35.3%) | 300 (22.3%) | 175 (13.0%) | 0.624 |
| >2 cm | 872 (64.7%) | 513 (38.1%) | 359 (26.6%) | |
| Lymph node status | ||||
| No lymph node metastasis | 849 (63.1%) | 487 (36.2%) | 362 (26.9%) | 0.810 |
| With lymph node metastasis | 498 (36.9%) | 294 (21.8%) | 204 (15.1%) | |
| Clinical stage | ||||
| I | 379 (28.2%) | 253 (18.8%) | 126 (9.4%) | 0.753 |
| II | 624 (46.3%) | 315 (23.4%) | 309 (22.9%) | |
| III | 344 (25.5%) | 245 (18.2%) | 99 (7.3%) | |
| BMI kg/m2, median (IQR) | 22 (19.1–23) | 21 (19.7–22.0) | 21 (20.0–22.0) | 0.189 |
| ER status | ||||
| Positive | 937 (69.6%) | 561 (41.7%) | 376 (27.9%) | 0.583 |
| Negative | 410 (30.4%) | 252 (18.7%) | 158 (11.7%) | |
| PR status | ||||
| Positive | 829 (61.5%) | 505 (37.5%) | 324 (24.0%) | 0.595 |
| Negative | 518 (38.5%) | 308 (22.9%) | 210 (15.6%) | |
| HER-2 status | ||||
| Positive | 398(29.5%) | 234 (17.4%) | 164 (12.1%) | 0.357 |
| Negative | 949 (70.5%) | 580 (43.1%) | 369 (27.4%) | |
| Ki-67 | ||||
| >14% | 868 (64.4%) | 499 (37.0%) | 369 (27.4%) | 0.300 |
| ≤14% | 479 (35.6%) | 314 (23.3%) | 165 (12.3%) | |
| Type of Surgery | ||||
| Modified radical mastectomy | 1078 (80.0%) | 645 (47.9%) | 433 (32.1%) | 0.432 |
| Others | 269 (20.0%) | 168 (12.5%) | 101 (7.5%) | |
| Radiotherapy | ||||
| Yes | 364 (27.0%) | 218 (16.2%) | 146 (10.8%) | 0.831 |
| No | 983 (73.0%) | 595 (44.2%) | 388 (28.8%) | |
| Adjuvant chemotherapy | ||||
| Yes | 1094 (81.2%) | 654 (48.5%) | 440 (32.7%) | 0.369 |
| No | 253 (18.8%) | 159 (11.8%) | 94 (7.0%) | |
| Endocrine therapy | ||||
| Yes | 698 (51.9%) | 421 (31.3%) | 277 (20.6%) | 0.955 |
| No | 649 (48.1%) | 392 (29.1%) | 257 (19.0%) | |
| Target therapy | ||||
| Yes | 102 (7.6%) | 78 (5.8%) | 24 (1.8%) | 0.561 |
| No | 1245 (92.4%) | 590(43.8%) | 655 (48.6%) | |
| Characteristics | Training Set (n = 943) | Validation Set (n = 404) |
|---|---|---|
| Age (Years) Median (IQR) | 47 (41–56) | 48 (42–57) |
| Tumor size | ||
| ≤2 cm | 340 (36.0%) | 145 (35.9%) |
| >2 cm | 603 (63.9%) | 259 (64.1%) |
| Lymph node status | ||
| No lymph node metastasis | 481 (51.0%) | 210 (52.0%) |
| With lymph node metastasis | 462 (49.0%) | 194 (48.0%) |
| Clinical stage | ||
| I | 210 (22.2%) | 86 (21.2%) |
| II | 516 (54.7%) | 223 (55.3%) |
| III | 217 (23.1%) | 95 (23.5%) |
| Histological type | ||
| Invasive ductal carcinoma | 760 (80.6%) | 316 (78.2%) |
| Others | 183 (19.4%) | 88 (21.8%) |
| ER status | ||
| Positive | 658 (69.8%) | 284 (70.3%) |
| Negative | 285 (30.2%) | 120 (29.7%) |
| PR status | ||
| Positive | 636 (67.4%) | 272 (67.3%) |
| Negative | 307 (32.6%) | 132 (32.7%) |
| HER-2 status | ||
| Positive | 249 (26.4%) | 75 (18.6%) |
| Negative | 694 (73.6%) | 329 (81.4%) |
| Ki-67 | ||
| >14% | 386 (41.0%) | 151 (37.4%) |
| ≤14% | 557 (59.0%) | 253 (62.6%) |
| NRI | ||
| >110.59 | 367 (38.9%) | 169 (41.8%) |
| ≤110.59 | 576 (61.1%) | 235 (58.2%) |
| Characteristics | Univariate Analysis Hazard Ratio (95% CI) | Multivariate Analysis Hazard Ratio (95% CI) | ||
|---|---|---|---|---|
| p | p | |||
| Age (years) | ||||
| ≤50 | 1 | - | - | |
| >50 | 1.134 (0.810–1.587) | 0.465 | - | - |
| Histopathological Type | ||||
| Invasive ductal carcinoma | 1 | 1 | ||
| Others | 0.422 (0.220–0.800) | 0.009 * | 0.414 (0.182–0.775) | 0.006 * |
| Tumor size | ||||
| ≤2 cm | 1 | 1 | ||
| >2 cm | 2.419 (1.551–3.788) | <0.001 * | 2.576 (1.231–2.742) | 0.035 * |
| Lymph node status | ||||
| No lymph node metastasis | 1 | 1 | ||
| With lymph node metastasis | 5.527 (3.803–8.060) | <0.001 * | 5.102 (3.598–6.350) | <0.001 * |
| ER status | ||||
| Negative | 1 | - | - | |
| Positive | 0.603 (0.426–1.844) | 0.600 | - | - |
| PR status | ||||
| Negative | 1 | 1 | ||
| Positive | 0.547 (0.391–0.764) | <0.001 * | 0.687 (0.398–0.812) | 0.009 * |
| HER-2 status | ||||
| Negative | 1 | 1 | ||
| Positive | 1.717 (1.219–2.419) | 0.002 * | 1.230 (0.687–1.701) | 0.463 |
| Ki-67 | ||||
| ≤14% | 1 | 1 | ||
| >14% | 2.197 (1.451–3.329) | <0.001 * | 1.820 (1.231–2.664) | 0.014 * |
| Adjuvant chemotherapy | ||||
| No | 1 | 1 | ||
| Yes | 1.770 (1.035–3.027) | 0.037 * | 2.386(0.552–2.798) | 0.082 |
| Radiotherapy | ||||
| No | 1 | 1 | ||
| Yes | 1.900 (1.355–2.669) | <0.001 * | 1.701 (0.582–1.909) | 0.148 |
| Endocrine therapy | ||||
| No | 1 | - | - | |
| Yes | 0.762 (0.545–1.064) | 0.111 | - | - |
| Target therapy | ||||
| No | 1 | - | - | |
| Yes | 1.015 (0.497–2.073) | 0.968 | - | - |
| NRI | ||||
| ≤110.59 | 1 | 1 | ||
| >110.59 | 0.684 (0.478–0.980) | 0.037 * | 0.620(0.505–0.890) | 0.042 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, F.; Xia, W.; Chen, M.; Jiang, T.; Guo, J.; Ouyang, Y.; Sun, H.; Chen, X.; Deng, W.; Guo, L.; et al. A Prognostic Model Based on Nutritional Risk Index in Operative Breast Cancer. Nutrients 2022, 14, 3783. https://doi.org/10.3390/nu14183783
Lin F, Xia W, Chen M, Jiang T, Guo J, Ouyang Y, Sun H, Chen X, Deng W, Guo L, et al. A Prognostic Model Based on Nutritional Risk Index in Operative Breast Cancer. Nutrients. 2022; 14(18):3783. https://doi.org/10.3390/nu14183783
Chicago/Turabian StyleLin, Fei, Wen Xia, Miao Chen, Tongchao Jiang, Jia Guo, Yi Ouyang, Haohui Sun, Xiaoyu Chen, Wuguo Deng, Ling Guo, and et al. 2022. "A Prognostic Model Based on Nutritional Risk Index in Operative Breast Cancer" Nutrients 14, no. 18: 3783. https://doi.org/10.3390/nu14183783
APA StyleLin, F., Xia, W., Chen, M., Jiang, T., Guo, J., Ouyang, Y., Sun, H., Chen, X., Deng, W., Guo, L., & Lin, H. (2022). A Prognostic Model Based on Nutritional Risk Index in Operative Breast Cancer. Nutrients, 14(18), 3783. https://doi.org/10.3390/nu14183783






