Dietary Patterns of Treatment–Resistant Depression Patients
Abstract
:1. Introduction
2. Materials and Methods
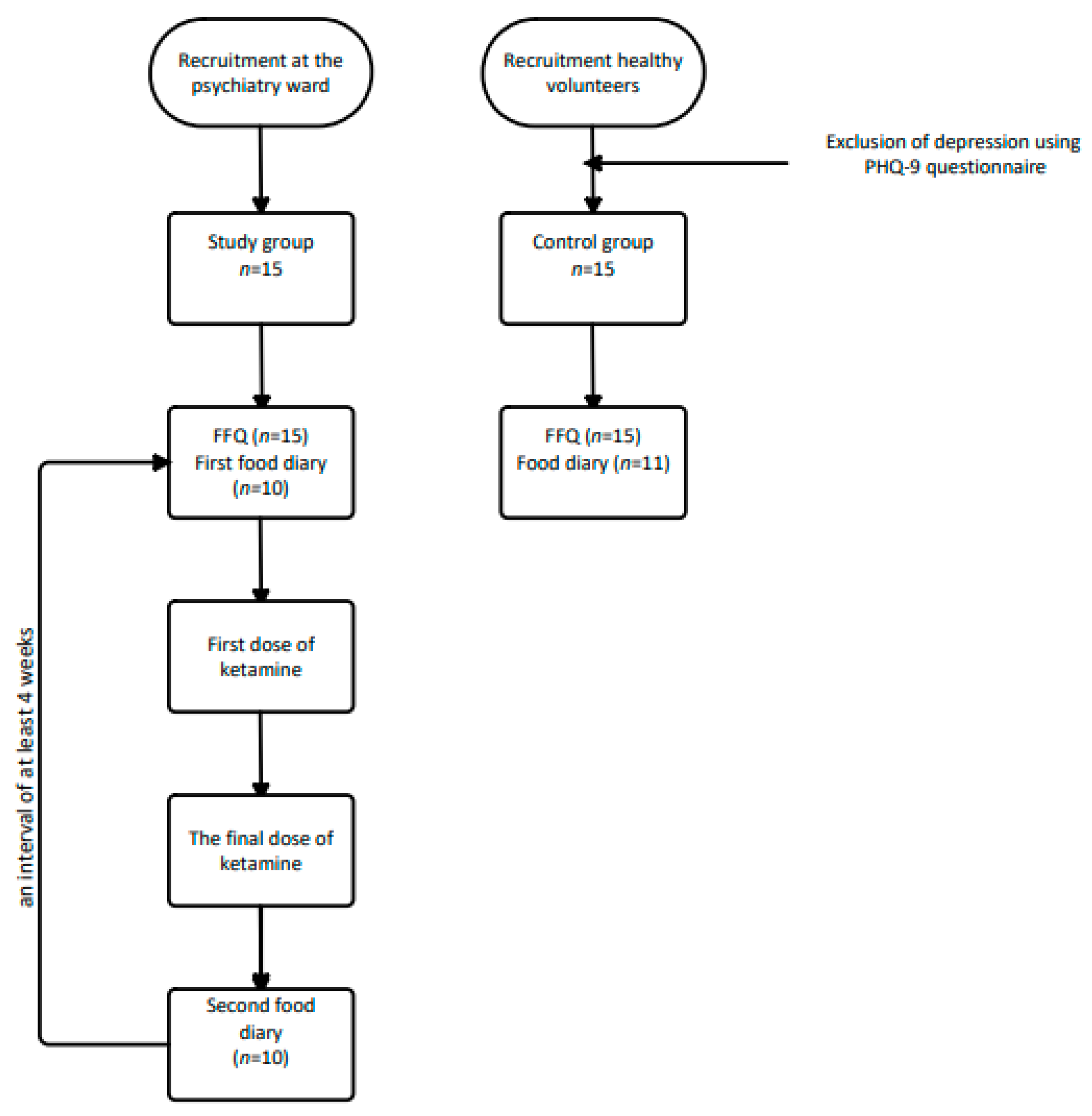
2.1. Study Procedure and Participants
2.2. Applied Questionaries
2.2.1. Personal and Medical Data
2.2.2. Exclusion of Depression
2.2.3. Diet
2.2.4. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Food Frequency Questionnaires Results
3.2.1. Consumption of Sweets and Snacks
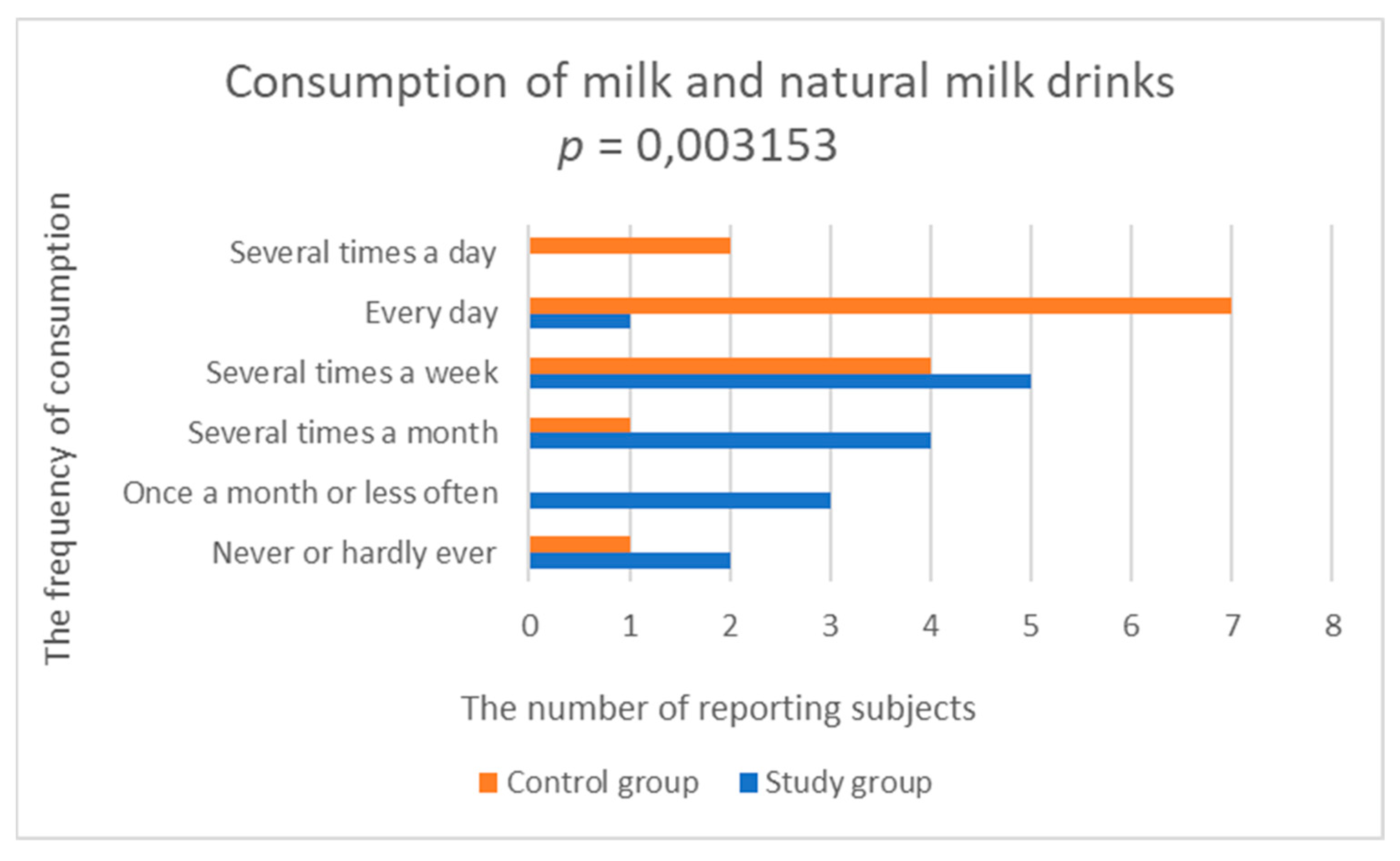
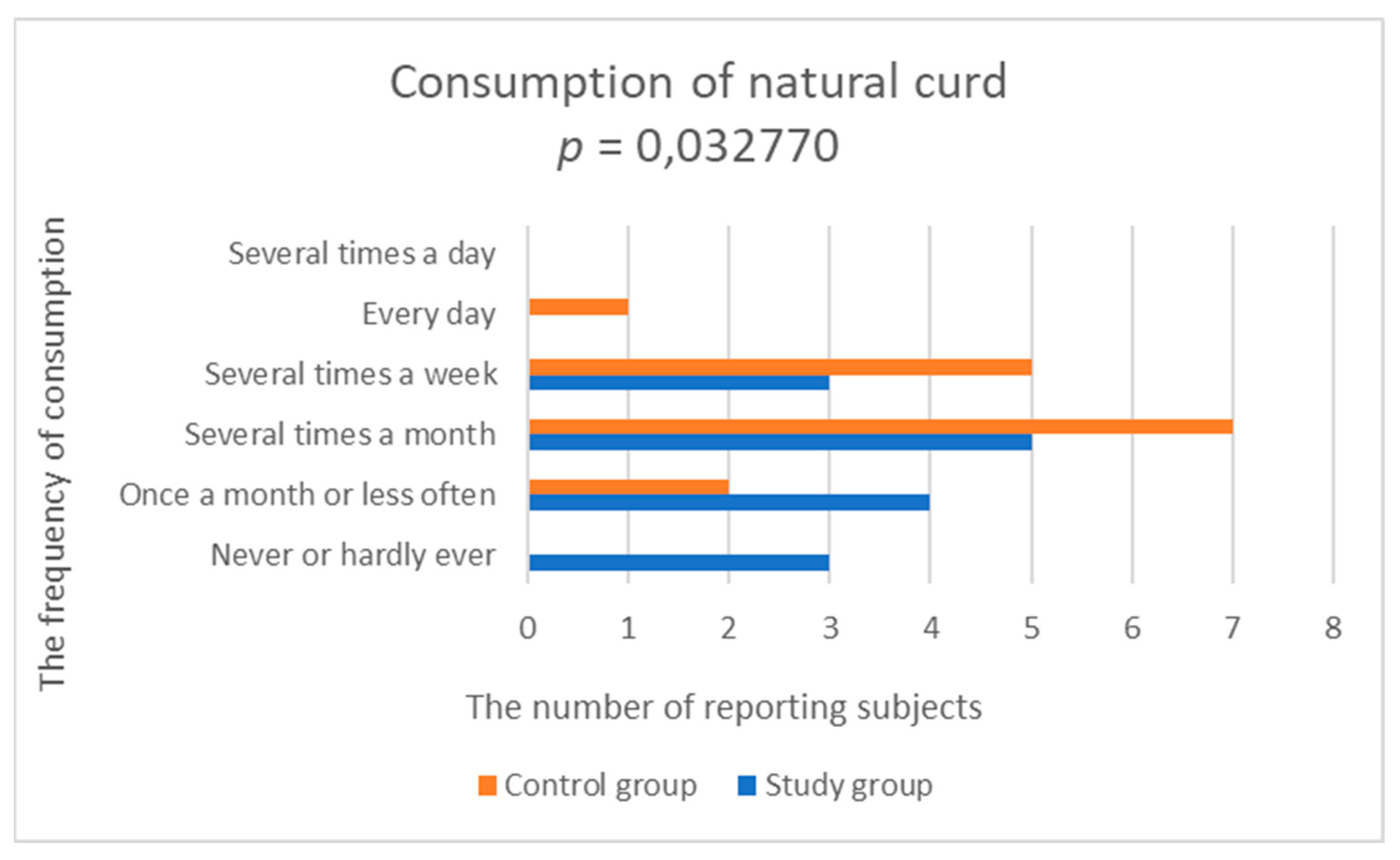
3.2.2. Consumption of Dairy Products and Eggs
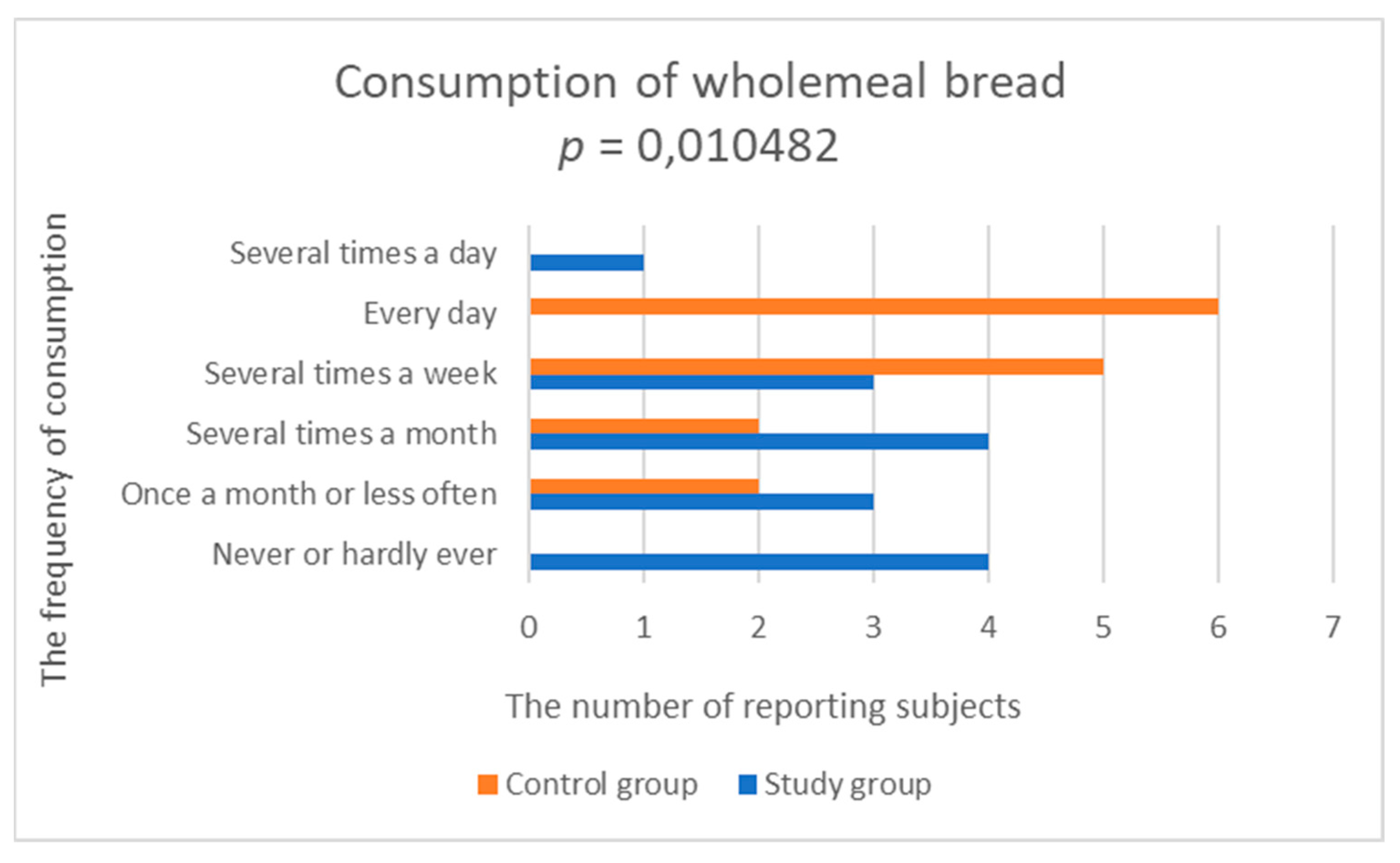
3.2.3. Consumption of Grain Products
3.2.4. Consumption of Fats
3.2.5. Consumption of Fruit
3.2.6. Consumption of Vegetables and Grains
3.2.7. Consumption of Meat and Fish Products
3.2.8. Consumption of Beverages
3.3. Statistical Analysis of Food Diaries. The Ketamine Treatment Correlated with Nutrients
3.3.1. Energy Consumption Analysis
3.3.2. Analysis of Macronutrient Intake: Proteins, Fats, and Carbohydrates
3.3.3. Analysis of Fatty Acid Consumption
3.3.4. Analysis of Dietary Fiber Intake
3.3.5. Analysis of Cholesterol and Sugar Consumption
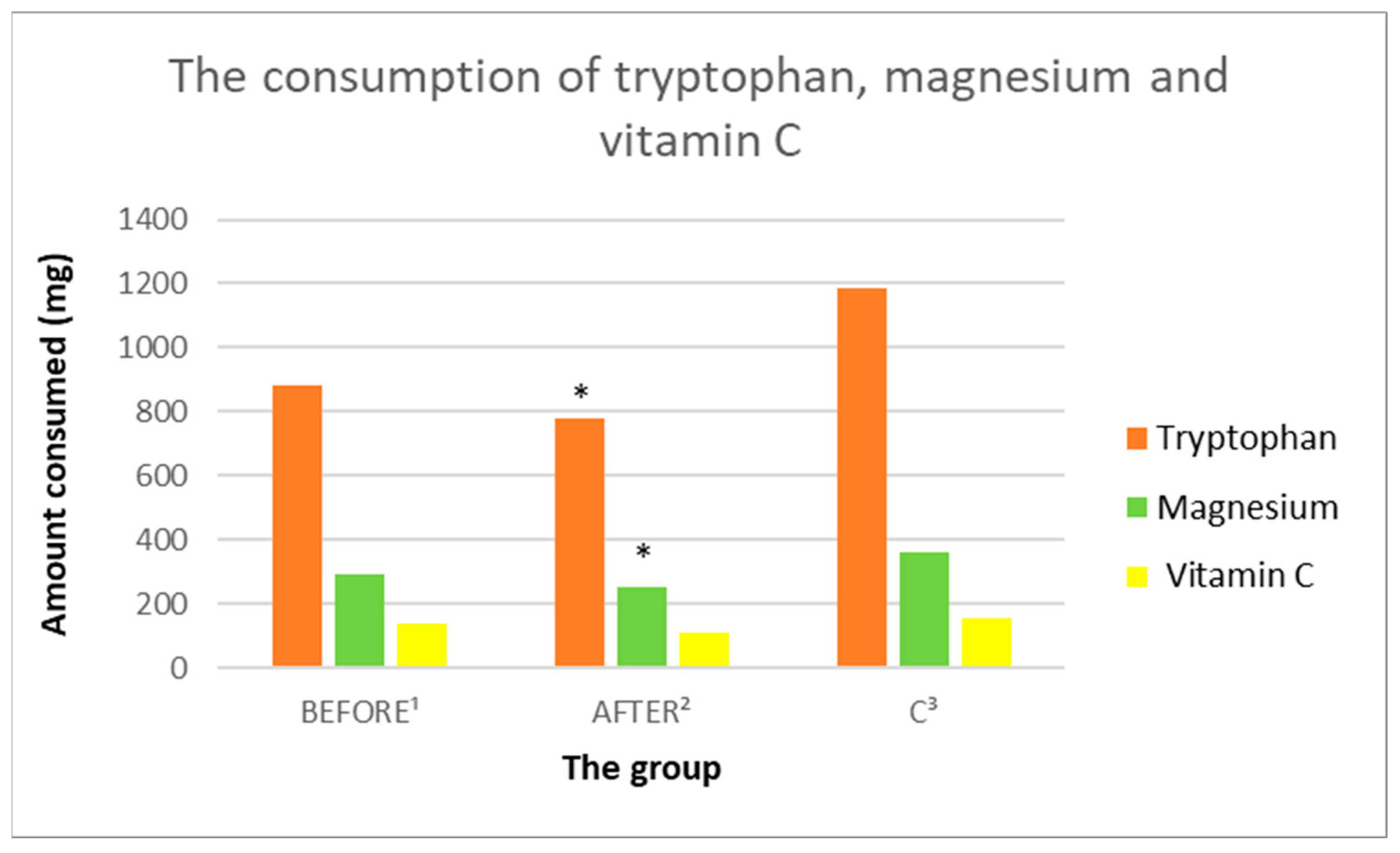
3.3.6. Tryptophan Intake Analysis
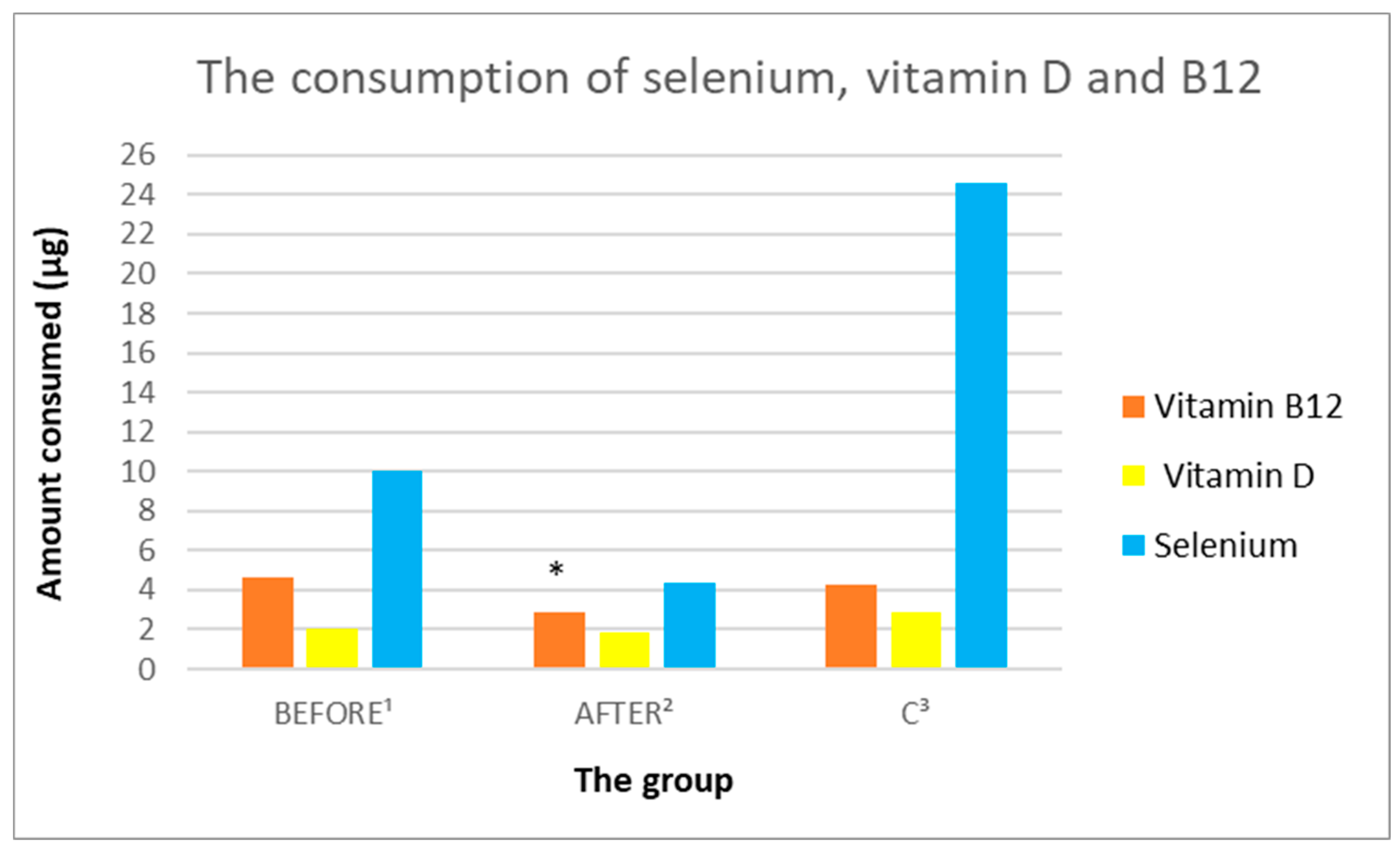
3.3.7. Analysis of Vitamin B9 and B12 Intake
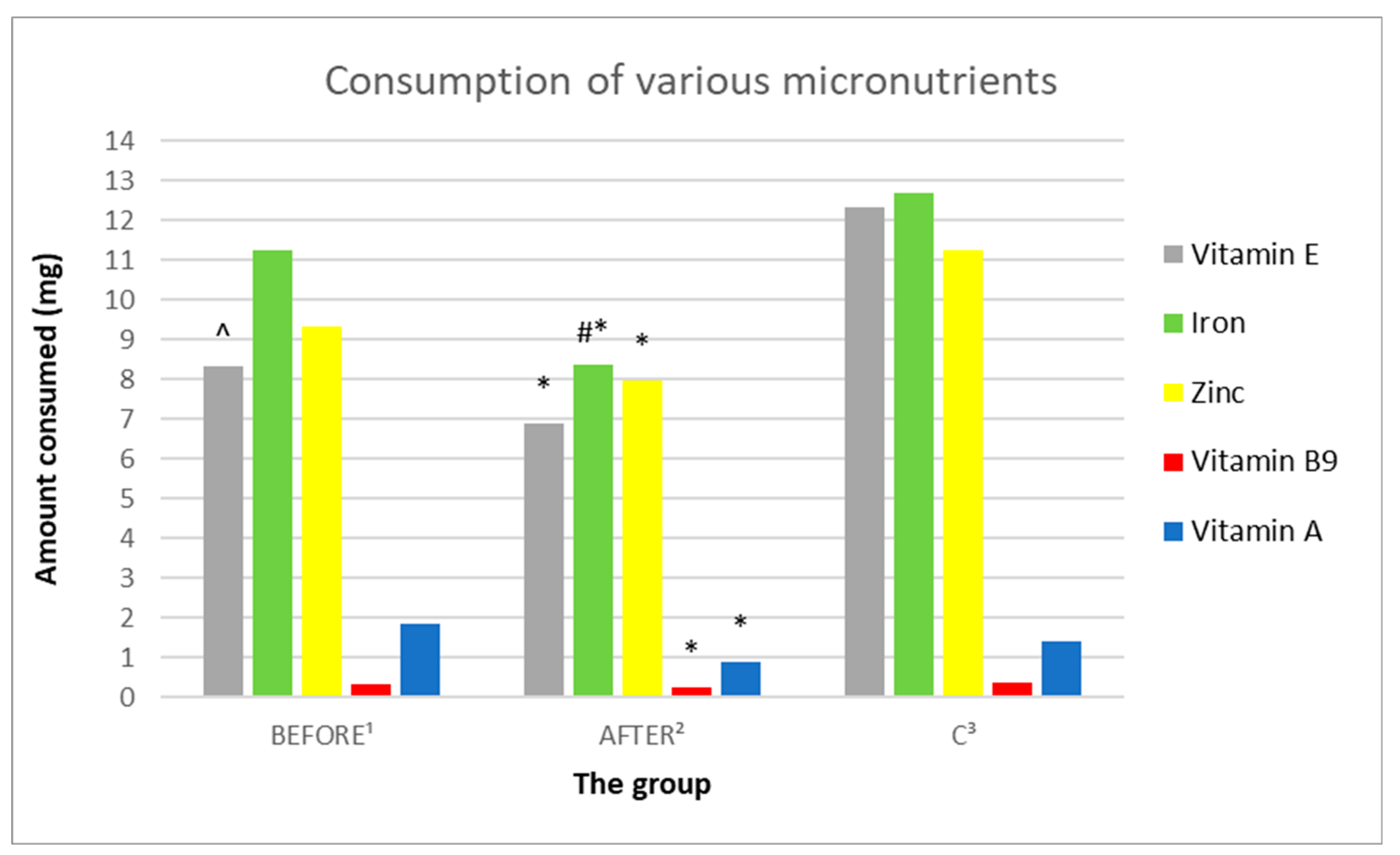
3.3.8. Analysis of the Consumption of Vitamins C, A, D, E
3.3.9. Analysis of the Consumption of Micronutrients; Selenium, Zinc, Magnesium, Iron
3.3.10. Assessment of Coverage of the Daily Requirements for Certain Nutrients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 20 May 2022).
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatr. 2017, 27, 101–111. [Google Scholar] [CrossRef]
- Saveanu, R.V.; Nemeroff, C.B. Etiology of Depression: Genetic and Environmental Factors. Psychiatr. Clin. N. Am. 2012, 35, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Perez-Caballero, L.; Torres-Sanchez, S.; Romero-López-Alberca, C.; González-Saiz, F.; Mico, J.A.; Berrocoso, E. Monoaminergic system and depression. Cell Tissue Res. 2019, 377, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Nikkheslat, N.; McLaughlin, A.P.; Hastings, C.; Zajkowska, Z.; Nettis, M.A.; Mariani, N.; Enache, D.; Lombardo, G.; Pointon, L.; Cowen, P.J.; et al. Childhood trauma, HPA axis activity and antidepressant response in patients with depression. Brain Behav. Immun. 2020, 87, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J. Cortisol and depression: Three questions for psychiatry. Psychol. Med. 2013, 43, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Daneshzad, E.; Darooghegi Mofrad, M.; Bellissimo, M.; Suitor, K.; Azadbakht, L. Vegetarian diet and the risk of depression, anxiety, and stress symptoms: A systematic review and meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2022, 1, 261–271. [Google Scholar] [CrossRef]
- Corriger, A.; Pickering, G. Ketamine and depression: A narrative review. Drug Des. Dev. Ther. 2019, 13, 3051–3067. [Google Scholar] [CrossRef]
- Gaynes, B.N.; Lux, L.; Gartlehner, G.; Asher, G.; Forman-Hoffman, V.; Green, J.; Boland, E.; Weber, R.P.; Randolph, C.; Bann, C.; et al. Defining treatment-resistant depression. Depress. Anxiety 2020, 37, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Mihaljević, S.; Pavlović, M.; Reiner, K.; Ćaćić, M. Therapeutic mechanisms of ketamine. Psychiatr. Danub. 2020, 32, 325–333. [Google Scholar] [CrossRef]
- Kennedy, S.H.; Lam, R.W.; McIntyre, R.S.; Tourjman, S.V.; Bhat, V.; Blier, P.; Hasnain, M.; Jollant, F.; Levitt, A.J.; MacQueen, G.M.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacologicals Treatments. Can. J. Psychiatr. 2016, 61, 540–560. [Google Scholar] [CrossRef]
- Caldiroli, A.; Capuzzi, E.; Tagliabue, I.; Capellazzi, M.; Marcatili, M.; Mucci, F.; Colmegna, F.; Clerici, M.; Buoli, M.; Dakanalis, A. Augmentative Pharmacological Strategies in Treatment-Resistant Major Depression: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 13070. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, D.F.; Rosenbaum, J.F.; Alpert, J.E. Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin. Neurosci. 2015, 17, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Mell, B.; Singh, V.; Yeoh, B.S. Treatment Resistant Depression: A Multi-Scale, Systems Biology Approach. Neurosci. Biobehav. Rev. 2017, 176, 139–148. [Google Scholar]
- Gosek, P.; Chojnacka, M.; Bieńkowski, P.; Świȩcicki, Ł. Zastosowanie antagonisty receptorów NMDA (N-metylo-D-asparaginianu)- ketaminy w leczeniu depresji lekoopornej (The use of NMDA receptor antagonist (N-methyl-D-aspartate)—Ketamine in the treatment of drug-resistant depression). Pol. Psychiatr. 2012, 46, 283–294. [Google Scholar]
- Szałach, Ł.P.; Lisowska, K.A.; Słupski, J.; Włodarczyk, A.; Górska, N.; Szarmach, J.; Jakuszkowiak-Wojten, K.; Gałuszko-Węgielnik, M.; Wiglusz, M.S.; Wilkowska, A.; et al. The immunomodulatory effect of ketamine in depression. Psychiatr. Danub. 2019, 31, 252–257. [Google Scholar]
- Dziugieł, R. Ketamine in the fight against depression, i.e. the old anesthetic in a new version. Probl. Med. Sci. Health Sci. 2019, 10, 25–32. [Google Scholar]
- Ruberto, V.L.; Jha, M.K.; Murrough, J.W. Pharmacological Treatments for Patients with Treatment-Resistant Depression. Pharmaceuticals 2020, 13, 116. [Google Scholar] [CrossRef]
- Drugbank Online Ketamine. Available online: https://go.drugbank.com/drugs/DB01221 (accessed on 23 May 2022).
- Jelen, L.A.; Stone, J.M. Ketamine for depression. Int. Rev. Psychiatr. 2021, 33, 207–228. [Google Scholar] [CrossRef]
- Majkutewicz, P.; Tyszko, P.; Okreglicka, K. Leczenie żywieniowe depresji. Fam. Med. Prim. Care Rev. 2014, 16, 48–50. [Google Scholar]
- Oh, J.; Yun, K.; Chae, J.-H.; Kim, T.-S. Association Between Macronutrients Intake and Depression in the United States and South Korea. Front. Psychiatr. 2020, 11, 207. [Google Scholar] [CrossRef]
- Gianfredi, V.; Koster, A.; Odone, A.; Amerio, A.; Sifnorelli, C.; Schapeer, N.C.; Bosma, H.; Köhler, S.; Dagnelie, P.C.; Stehouwer, C.D.A. Associations of Dietary Patterns with Incident Depression: The Maastricht Study. Nutrients 2021, 13, 1034. [Google Scholar] [CrossRef] [PubMed]
- Rudzińska, A.; Perera, I.; Gryglewska, B.; Gąsowski, J.; Piotrowicz, K. Can the Mediterranean diet decrease the risk of depression in older persons—A systematic review. Psychiatr. Pol. 2022, 265, 1–16. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Cubała, W.J.; Stawicki, M. Ketogenic diet for depression: A potential dietary regimen to maintain euthymia? Prog. Neuropsychopharmacol. Biol. Psychiatr. 2021, 109, 110257. [Google Scholar] [CrossRef] [PubMed]
- Sabet, J.A.; Ekman, M.S.; Lundvall, A.S.; Risérus, U.; Johansson, U.; Öström, Å.; Adamsson, V.; Cao, Y.; Msghina, M.; Brummer, R.J. Feasibility and Acceptability of a Healthy Nordic Diet Intervention for the Treatment of Depression: A Randomized Controlled Pilot Trial. Nutrients 2021, 13, 902. [Google Scholar] [CrossRef]
- Zhang, J. The Bidirectional Relationship between Body Weight and Depression across Gender: A Simultaneous Equation Approach. Int. J. Environ. Res. Public Health 2021, 18, 7673. [Google Scholar] [CrossRef]
- Jung, S.J.; Woo, H.; Cho, S.; Kyounghoon, P.; Jeong, S.; Lee, Y.J.; Kang, D.; Shind, A. Association between body size, weight change and depression: Systematic review and meta-analysis. Br. J. Psychiatr. 2017, 211, 14–21. [Google Scholar] [CrossRef]
- Cosgrovea, K.T.; Burrowsa, K.; Avery, J.A. Appetite change profiles in depression exhibit differential relationships between systemic inflammation and activity in reward and interoceptive neurocircuitry. Brain Behav. Immun. 2020, 83, 163–171. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 2008, pp. 222–223. Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 14 August 2022).
- Gałecki, P.; Pilecki, M.; Rymaszewska, J.; Szulc, A.; Sidorowicz, S.; Wciórka, J. Diagnostic and Statistical Manual of Mental Disorders DSM-5. 2018. Available online: https://ceer.com.pl/resources/html/article/details?id=229818&language=en (accessed on 14 August 2022).
- Gill, H.; Gill, B.; El-Halabi, S.; Chen-li, D.; Lipsitz, O.; Rosenblat, J.D.; van Rheenen, T.E.; Rodrigues, N.B.; Mansur, R.B.; Majeed, A.; et al. Antidepressant Medications and Weight Change: A Narrative Review. Obesity 2020, 28, 2064–2072. [Google Scholar] [CrossRef]
- World Health Organization. A Healthy Lifestyle—WHO Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle-who-recommendations (accessed on 20 May 2022).
- Kokoszka, A.; Jastrzębski, A.; Obrębski, M. Assessment of the psychometric properties of the Polish version of the Patient Health Questionnaire-9 for adults. Psychiatry 2016, 13, 187–193. [Google Scholar]
- Tomaszewski, K.; Zarychta, M.; Bieńkowska, A.; Chmurowicz, E.; Nowak, W.; Skalska, A. Validation of the Patient Health Questionnaire-9 Polish version in the hospitalised elderly population. Psychiatr. Pol. 2011, 45, 223–233. [Google Scholar]
- Elmadfa, I.; Meyer, A.L. Developing suitable methods of nutritional status assessment: A continuous challenge. Adv. Nutr. 2014, 5, 590S–598S. [Google Scholar] [CrossRef]
- Wądołowska, L. Validation of Food Frequency Questionnaire (FFQ). Reproducibility Assessment. Brom. Chem. Toksykol. 2005, 1, 27–33. [Google Scholar]
- FFQ-6 Food Frequency Questionnaire. Available online: http://www.uwm.edu.pl/edu/lidiawadolowska/ (accessed on 1 July 2021).
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Nutrition Standards for the Polish Population and Their Application. 2020. Available online: https://ncez.pzh.gov.pl/abc-zywienia/normy-zywienia-2020/ (accessed on 20 May 2022).
- Hoepner, C.T.; Mcintyre, R.S.; Papakostas, G.I. Impact of Supplementation and Nutritional Interventions on Pathogenic Processes of Mood Disorders: A Review of the Evidence. Nutrients 2021, 13, 767. [Google Scholar] [CrossRef] [PubMed]
- Romero-Blanco, C.; Hernández-Martínez, A.; Parra-Fernández, M.L.; Onieva-Zafra, M.D.; Prado-Laguna, M.d.C.; Rodríguez-Almagro, J. Food Addiction and Lifestyle Habits among University Students. Nutrients 2021, 13, 1352. [Google Scholar] [CrossRef]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatr. 2019, 24, 965–986. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kim, E.J.; Kim, A.; Lee, H.J.; Choi, H.J.; Yang, S.J. Nutritional factors affecting mental health. Clin. Nutr. Res. 2016, 5, 143–152. [Google Scholar] [CrossRef]
- Kyle Simmons, W.; Burrows, K.; Avery, J.A.; Lerr, K.L.; Taylor, A.; Bodurka, J.; Potter, W.; Teague, K.T.; Drevets, W.C. Appetite Changes Reveal Depression Subgroups with Distinct Endocrine, Metabolic, and Immune States. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Velázquez-Alva, M.C.; Irigoyen-Camacho, M.E.; Cabrer-Rosales, M.F.; Lazarevich, I.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R.; Zepeda-Zepeda, M.A. Prevalence of malnutrition and depression in older adults living in nursing homes in Mexico City. Nutrients 2020, 12, 2429. [Google Scholar] [CrossRef]
- Godos, J.; Currenti, W.; Angelino, D.; Mena, P.; Castellano, S.; Caraci, F.; Galvano, F.; del Rio, D.; Ferri, R.; Grosso, G. Diet and mental health: Review of the recent updates on molecular mechanisms. Antioxidants 2020, 9, 346. [Google Scholar] [CrossRef]
- Carochoa, M.; Morales, P.; Ciudad-Mulero, M.; Fernández-Ruiz, V.; Ferreira, E.; Heleno, S.; Rodrigues, P.; Barros, L.; Ferreira, I.C.F.R. Comparison of different bread types: Chemical and physical parameters. Food Chem. 2020, 310, 125954. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G. Nutritional Psychiatry: How Diet Affects Brain through Gut Microbiota. Nutrients 2021, 13, 1282. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Sanchez-Villegas, A. Food patterns and the prevention of depression. Proc. Nutr. Soc. 2016, 75, 139–146. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Fatahi, S.; Matin, S.S.; Sohouli, M.H.; Găman, M.-H.; Raee, P.; Olang, B.; Kathirgamathamby, V.; Santors, H.O.; Guimarães, N.S.; Shidfar, F. Association of dietary fiber and depression symptom: A systematic review and meta-analysis of observational studies. Complement. Ther. Med. 2021, 56, 102621. [Google Scholar] [CrossRef] [PubMed]
- Jacques, A.; Chaaya, N.; Beecher, K.; Ali, S.A.; Belmer, A.; Bartlett, S. The impact of sugar consumption on stress driven, emotional and addictive behaviors. Neurosci. Biobehav. Rev. 2019, 103, 178–199. [Google Scholar] [CrossRef] [PubMed]
- Danqing, H.; Lixiao, D.; Wenjie, J. Sugar-sweetened beverages consumption and the risk of depression: A meta-analysis of observational studies. J. Affect. Disord. 2019, 245, 348–355. [Google Scholar]
- Knüppel, A.; Shipley, M.J.; Llewellyn, C.H.; Brunner, E.J. Sugar intake from sweet food and beverages, common mental disorder and depression: Prospective fndings from the Whitehall II study. Sci. Rep. 2017, 7, 6287. [Google Scholar] [CrossRef]
- Lia, D.; Liangb, H.; Tonga, Y.; Zhenga, H.; Li, Y. Association between saturated fatty acid intake and depressive symptoms in midlife women: A prospective study. J. Affect. Disord. 2020, 267, 17–22. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Juárez, M.; Angel de la Fuente, M. Milk fatty acids and potential health benefits: An updated vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef]
- Da Silva, M.S.S.; Rudkowska, I. Dairy nutrients and their effect on inflammatory profilein molecular studies. Mol. Nutr. Food Res. 2015, 59, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Boulkrane, M.S.; Fedotova, J.; Kolodyaznaya, V.; Micale, V.; Drago, F.; van den Tol, A.J.; Baranenko, D. Vitamin D and Depression in Women: A Mini-review. Curr. Neuropharmacol. 2019, 18, 288–300. [Google Scholar] [CrossRef]
- Lindseth, G.; Helland, B.; Caspers, J. The Effects of Dietary Tryptophan on Affective Disorders. Arch. Psychiatr. Nurs. 2015, 29, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Berkins, S.; Schiöth, H.B.; Rukh, G. Depression and Vegetarians: Association between Dietary Vitamin B6, B12 and Folate Intake and Global and Subcortical Brain Volumes. Nutrients 2021, 13, 1790. [Google Scholar] [CrossRef] [PubMed]
- Household Budget Survey in 2020; Central Statistical Office: Warsaw, Poland, 2021. Available online: https://stat.gov.pl/obszary-tematyczne/warunki-zycia/dochody-wydatki-i-warunki-zycia-ludnosci/budzety-gospodarstw-domowych-w-2020-roku,9,15.html (accessed on 10 August 2022).
- Khosravi, M.; Sotoudeh, G.; Amini, M.; Raisi, F.; Mansoori, A.; and Hosseinzadeh, M. The relationship between dietary patterns and depression mediated by serum levels of Folate and vitamin B12. BMC Psychiatr. 2020, 20, 4–11. [Google Scholar] [CrossRef]
- Markun, S.; Gravestock, I.; Jäger, L.; Rosemann, T.; Pichierri, G.; Burgstaller, J.M. Effects of Vitamin B12 Supplementation on Cognitive Function, Depressive Symptoms, and Fatigue: A Systematic Review, Meta-Analysis, and Meta-Regression. Nutrients 2021, 13, 923. [Google Scholar] [CrossRef]
- Styczeń, K.; Sowa-Kućma, M.; Siwek, M.; Dudek, D.; Reczyński, W.; Szewczyk, B.; Misztak, P.; Topór-Mądry, R.; Opoka, W.; Nowak, G. The serum zinc concentration as a potential biological marker in patients with major depressive disorder. Metab. Brain Dis. 2017, 32, 97–103. [Google Scholar] [CrossRef]
- Tarleton, E.K.; Littenberg, B. Magnesium intake and depression in adults. J. Am. Board Family Med. 2015, 28, 249–256. [Google Scholar] [CrossRef]
- Issn, P.L.; Czad, K. Risk factors for depression. New evidence on selenium deficiency and depressive disorders. Psychiatr. Pol. 2020, 54, 1109–1121. [Google Scholar]
- Hameed, S.; Naser, I.A.; Ghussein, M.A.; Ellulu, M.S. Is Iron Deficiency a Risk Factor for Postpartum Depression? A Case-Control Study in the Gaza Strip, Palestine. Public Health Nutr. 2021, 25, 1631–1638. [Google Scholar] [CrossRef]
- Upton, N. Developing our understanding of nutrition in depression. Br. J. Nutr. 2022, 127, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef] [PubMed]

| S n = 15 | C n = 15 | |
|---|---|---|
| AGE (SD) | 35.6 (11.5) | 35.6 (11.6) |
| SEX (Woman) | 8 (53.3%) | 8 (53.3%) |
| SEX (Man) | 7 (46.6%) | 7 (46.6%) |
| BMI (SD) | 27.8 (5.8) | 25.3 (4.4) |
| S 1 (SD) | C 1 (SD) | p-Value 2 | |
|---|---|---|---|
| Sugar | 3.067 (2.017) | 2.857 (1.956) | 0.846694 |
| Honey | 2.400 (1.682) | 1.929 (1.072) | 0.813179 |
| Chocolates and chocolate products | 3.667 (0.900) | 3.786 (0.975) | 0.714830 |
| Non-chocolate candies | 3.067 (1.624) | 2.714 (1.139) | 0.620867 |
| Biscuits | 2.533 (1.356) | 3.071 (0.730) | 0.093216 |
| Ice cream and pudding | 2.067 (0.961) | 2.571 (0.852) | 0.171789 |
| Salty snacks | 2.667 (1.113) | 2.214 (0.802) | 0.251712 |
| Milk and natural milk drinks | 3.000 (1.195) | 4.429 (1.284) | 0.003153 |
| Sweetened milk drinks | 2.733 (1.033) | 2.714 (1.069) | 0.982865 |
| Natural curd | 2.533 (1.060) | 3.429 (0.756) | 0.032770 |
| Flavored curds | 2.067 (1.100) | 2.071 (1.141) | 1.000000 |
| Cheeses | 3.333 (0.724) | 4.071 (0.997) | 0.076919 |
| Eggs | 3.000 (1.000) | 3.571 (0.756) | 0.171789 |
| Wholemeal bread | 2.667 (1.447) | 4.000 (1.109) | 0.010482 |
| White bread | 3.533 (1.407) | 3.357 (1.277) | 0.747192 |
| Coarse-grained groats | 2.400 (0.986) | 2.857 (0.949) | 0.234007 |
| Fine grain groats | 2.467 (0.834) | 2.929 (0.616) | 0.171789 |
| Ready-made breakfast products | 2.200 (1.320) | 2.714 (0.994) | 0.251712 |
| Oils | 3.733 (0.961) | 3.500 (1.019) | 0.504539 |
| Butter | 3.933 (1.580) | 3.929 (1.269) | 0.880478 |
| Margarine | 1.333 (0.900) | 1.571 (0.756) | 0.270299 |
| Cream | 2.600 (1.298) | 2.214 (0.975) | 0.400494 |
| Other animal fats | 1.333 (0.724) | 1.357 (0.633) | 0.779995 |
| Mayonnaise and dressings | 2.333 (0.900) | 2.643 (0.929) | 0.376588 |
| All fruit | 4.133 (1.125) | 4.286 (0.914) | 0.714830 |
| Stone fruit | 2.733 (0.704) | 2.714 (0.914) | 0.714830 |
| Kiwi and citrus | 3.000 (1.363) | 3.571 (0.938) | 0.234007 |
| Tropical fruit | 2.067 (1.100) | 2.286 (0.726) | 0.376588 |
| Berries | 2.333 (0.900) | 2.786 (0.975) | 0.353554 |
| Bananas | 3.067 (1.033) | 3.286 (1.069) | 0.747192 |
| Apples and pears | 3.200 (1.474) | 3.714 (1.139) | 0.310143 |
| Avocado | 1.467 (0.743) | 2.071 (0.917) | 0.076919 |
| Olives | 1.733 (1.033) | 2.214 (0.893) | 0.158309 |
| Dried fruit | 2.267 (1.335) | 2.357 (1.151) | 0.779995 |
| Sweet fruit preserves | 2.400 (0.986) | 2.429 (0.938) | 0.982865 |
| All vegetables | 4.200 (1.373) | 4.857 (1.027) | 0.201205 |
| Cruciferous vegetables | 2.267 (0.961) | 3.000 (0.679) | 0.051090 |
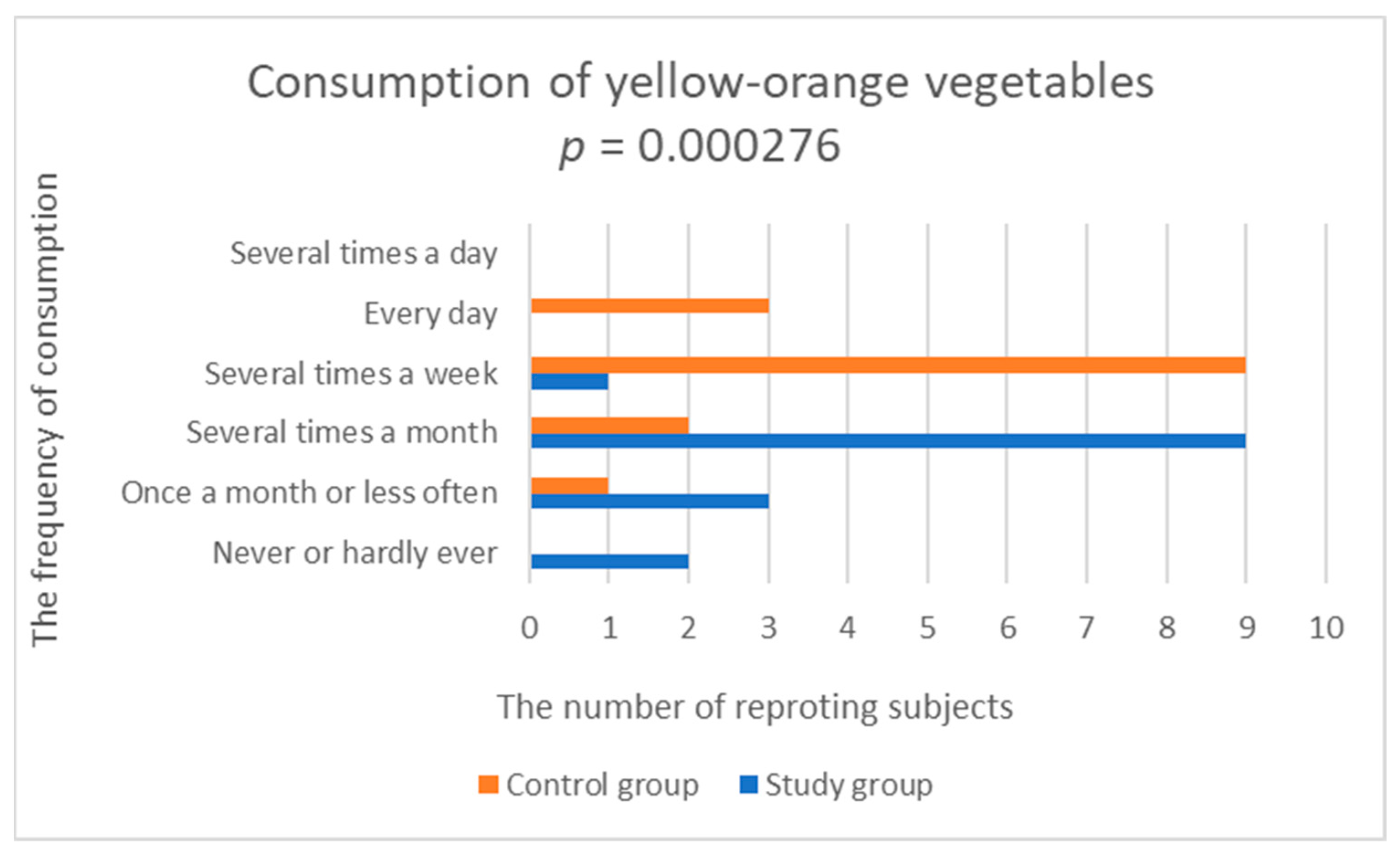
| Yellow-orange vegetables | 2.533 (0.915) | 3.929 (0.829) | 0.000276 |
| Green leafy vegetables | 2.933 (1.163) | 3.786 (0.802) | 0.093216 |
| Tomatoes | 3.400 (1.352) | 4.357 (0.745) | 0.045875 |
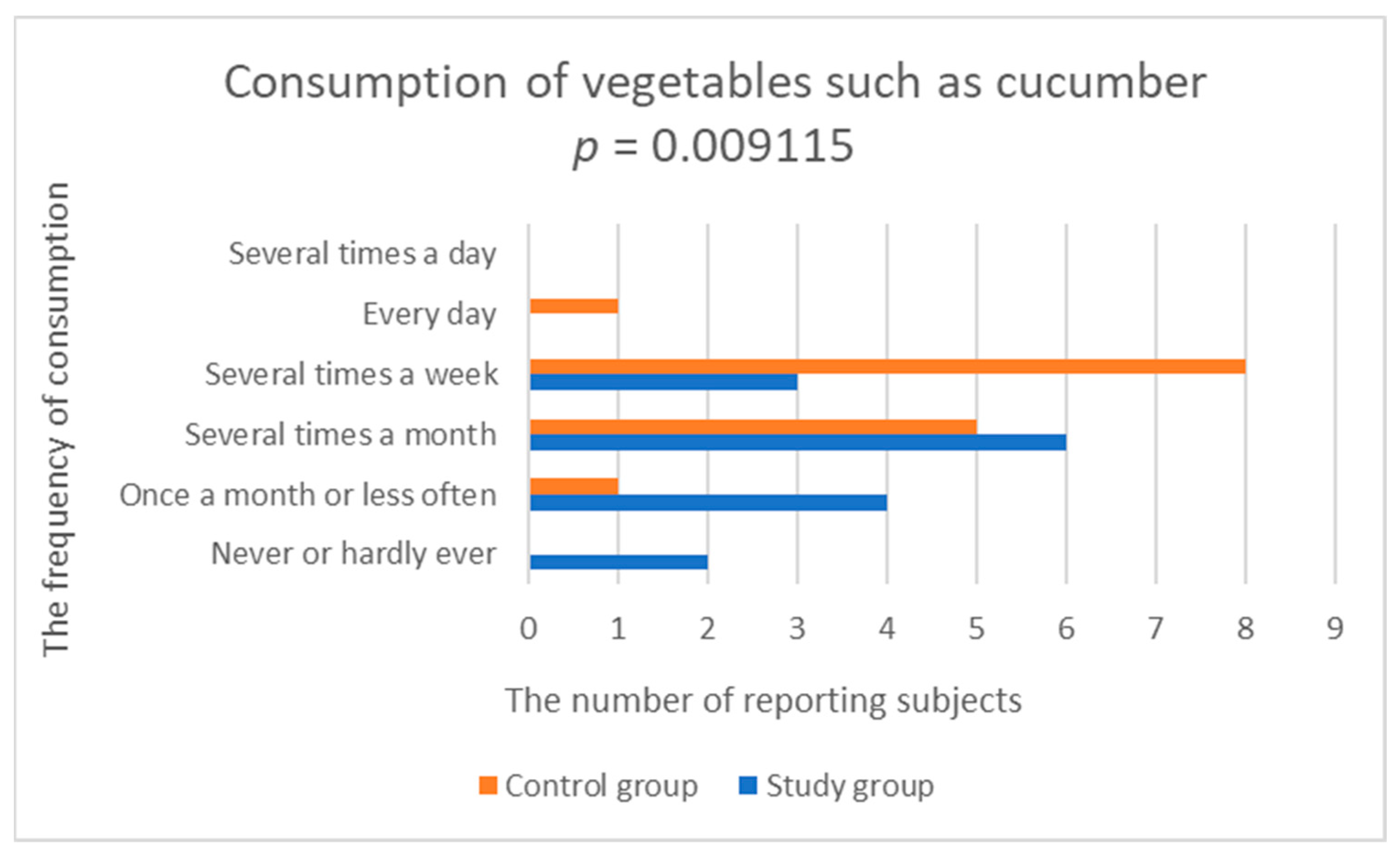
| Vegetables such as cucumber | 2.600 (1.056) | 3.643 (0.745) | 0.009115 |
| Root vegetables | 2.933 (1.100) | 3.786 (0.975) | 0.051090 |
| Potatoes | 3.667 (0.900) | 3.500 (0.650) | 0.400494 |
| Fresh legume seeds | 2.200 (0.941) | 2.571 (0.938) | 0.331405 |
| Dry legume seeds | 1.867 (0.834) | 2.286 (0.994) | 0.310143 |
| Nuts | 2.800 (1.320) | 3.214 (1.051) | 0.251712 |
| Seeds | 1.800 (0.941) | 2.286 (0.914) | 0.158309 |
| Sausages | 2.467 (1.187) | 3.000 (1.038) | 0.270299 |
| Cured meats | 2.867 (1.356) | 3.500 (1.019) | 0.217176 |
| Sausage products and organ meat | 1.733 (0.961) | 1.857 (0.949) | 0.747192 |
| Red meat | 2.667 (1.047) | 2.500 (1.092) | 0.682954 |
| Poultry and rabbit meat | 3.133 (0.915) | 3.429 (0.938) | 0.310143 |
| Venison | 1.133 (0.352) | 1.071 (0.267) | 0.779995 |
| Lean fish | 2.267 (1.100) | 2.143 (0.663) | 0.813179 |
| Oily fish | 2.200 (0.941) | 2.286 (0.825) | 0.846694 |
| Fruit juices and nectars | 3.200 (1.207) | 3.143 (0.535) | 0.779995 |
| Vegetable and vegetable and fruit juices | 1.600 (0.828) | 2.143 (1.099) | 0.201205 |
| Energy drinks | 1.933 (1.223) | 1.857 (1.099) | 0.880478 |
| Sweetened beverages | 2.667 (1.496) | 2.429 (1.453) | 0.651619 |
| Beer | 1.800 (1.082) | 2.000 (1.038) | 0.590745 |
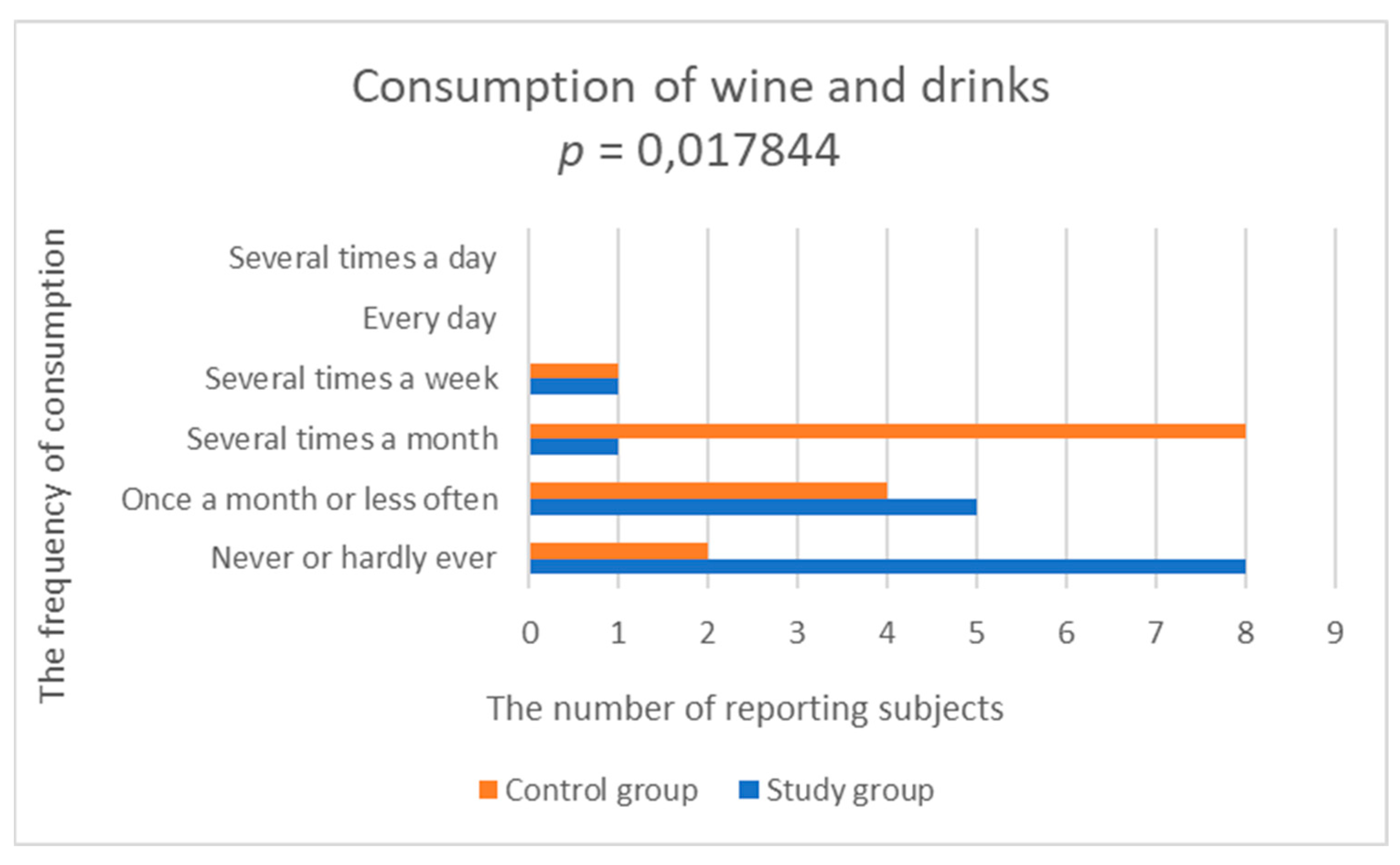
| Wine and drinks | 1.667 (0.900) | 2.500 (0.855) | 0.017844 |
| Vodka and hard alcohol | 1.333 (0.488) | 1.929 (0.829) | 0.062953 |
| S (n = 10) | C (n = 11) | p-Value 1 | p-Value 2 | p-Value 3 | ||
|---|---|---|---|---|---|---|
| A | ||||||
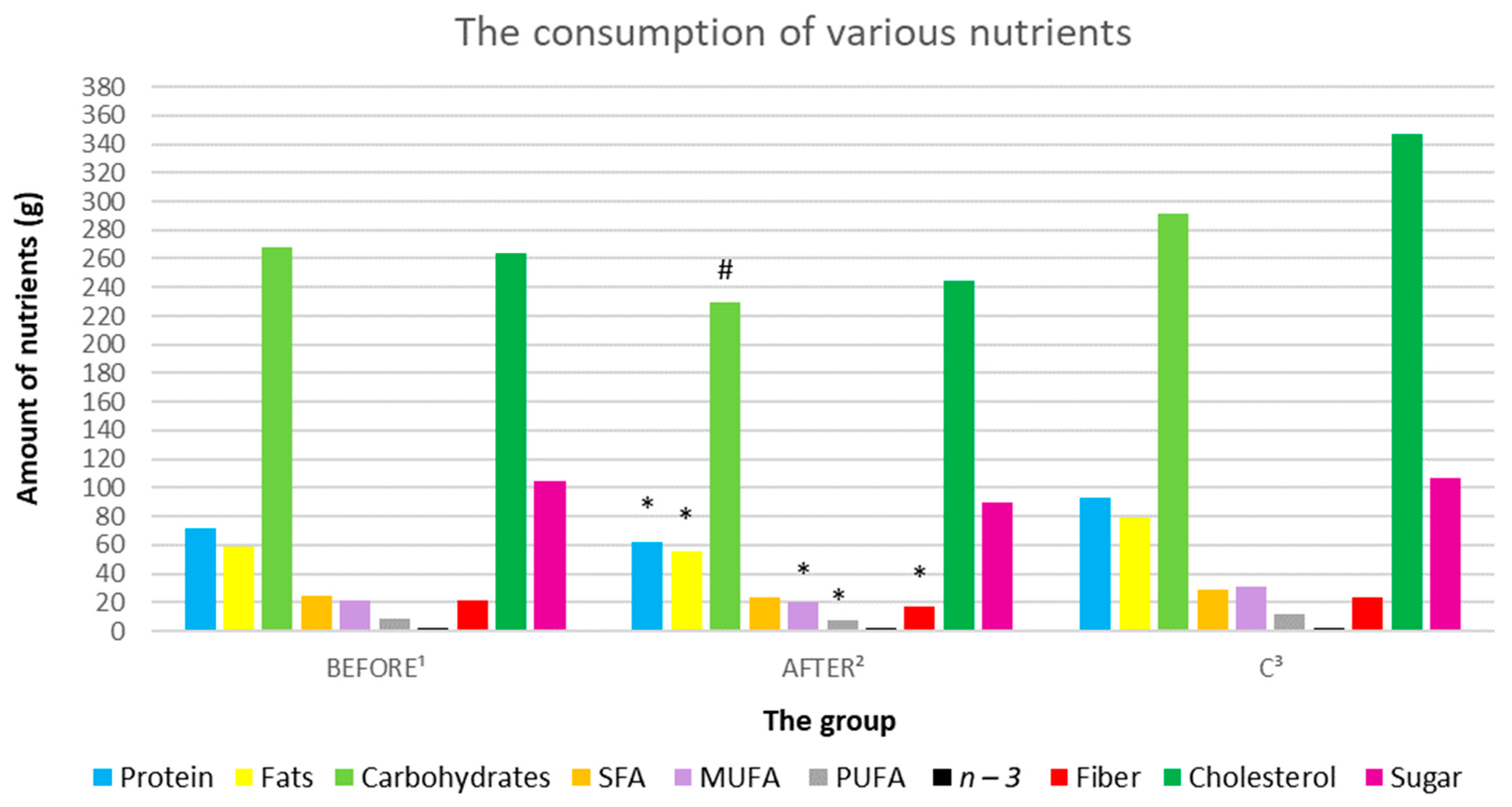
| Energy (kcal) | 1848.34 (885.49) | 1629.68 (829.14) | 2216.08 (596.84) | 0.129617 | 0.274265 | 0.076456 |
| Protein (g) | 71.93 (36.52) | 62.23 (24.83) | 93.34 (20.53) | 0.334764 | 0.109819 | 0.005365 |
| Fats (g) | 58.91 (37.18) | 55.06 (26.43) | 79.13 (25.50) | 0.597186 | 0.159118 | 0.04707 |
| Carbohydrates (g) | 268.18 (122.92) | 229.68 (142.76) | 291.98 (93.97) | 0.037566 | 0.621827 | 0.247834 |
| SFA (g) | 24.1 (16.64) | 23.04 (12.28) | 29.28 (12.37) | 0.708677 | 0.425038 | 0.261382 |
| MUFA (g) | 21.12 (12.93) | 20.19 (9.56) | 31.28 (10.95) | 0.748712 | 0.066247 | 0.0236 |
| PUFA (g) | 8.83 (5.09) | 7.29 (3.31) | 12.13 (4.21) | 0.275192 | 0.120986 | 0.009078 |
| n – 3 (g) | 1.68 (0.84) | 1.62 (1.05) | 2.49 (1.2) | 0.802552 | 0.092528 | 0.095492 |
| Fiber (g) | 21.02 (9.43) | 16.58 (6.92) | 23.79 (4.71) | 0.06617 | 0.397802 | 0.01101 |
| Cholesterol (g) | 263.43 (183.82) | 244.95 (132.66) | 346.6 (156.96) | 0.649039 | 0.277337 | 0.12742 |
| Sugar (g) | 105.13 (86.78) | 89.79 (105.37) | 106.58 (74.84) | 0.297065 | 0.967556 | 0.67605 |
| Tryptophan (mg) | 884.78 (475.61) | 776.2 (306.24) | 1185.53 (297.63) | 0.430469 | 0.09532 | 0.005833 |
| B9 (µg) | 320.11 (182.43) | 255.92 (126.25) | 382.97 (126.77) | 0.181217 | 0.36685 | 0.033071 |
| B12 (µg) | 4.63 (4.27) | 2.88 (1.8) | 4.3 (1.27) | 0.1372 | 0.807196 | 0.049312 |
| C (mg) | 139.66 (80.08) | 110.19 (69.04) | 154.02 (87.88) | 0.21693 | 0.701004 | 0.222366 |
| A (µg) | 1846.53 (1931.33) | 904.84 (329.47) | 1416.43 (504.93) | 0.137721 | 0.483948 | 0.013656 |
| D (µg) | 2.01 (1.61) | 1.9 (1.28) | 2.83 (2.7) | 0.764501 | 0.41533 | 0.332884 |
| E (mg) | 8.31 (3.95) | 6.9 (3.21) | 12.33 (2.56) | 0.204891 | 0.011688 | 0.000378 |
| Selenium (µg) | 9.98 (12.9) | 4.39 (4.49) | 24.56 (43.11) | 0.225044 | 0.317598 | 0.158082 |
| Zinc (mg) | 9.33 (4.58) | 7.98 (3.81) | 11.23 (1.81) | 0.240153 | 0.21886 | 0.020072 |
| Magnesium (mg) | 292.96 (139.06) | 253.84 (120.38) | 362.29 (54.33) | 0.248926 | 0.141767 | 0.014033 |
| Iron (mg) | 11.26 (5.91) | 8.35 (4.24) | 12.68 (2.35) | 0.029401 | 0.471301 | 0.008538 |
| Coverage of the Daily Requirement | Before (n = 10) | After (n=10) | C (n = 11) |
|---|---|---|---|
| n − 3 | 20% | 30% | 54% |
| Fiber | 40% | 20% | 36% |
| Tryptophan | 90% | 70% | 100% |
| Selenium | 0% | 0% | 9% |
| Zinc | 70% | 40% | 100% |
| Magnesium | 40% | 30% | 73% |
| Iron | 40% | 40% | 45% |
| B12 | 70% | 60% | 91% |
| B9 | 40% | 30% | 36% |
| Vitamin C | 70% | 60% | 73% |
| Vitamin A | 60% | 50% | 100% |
| Vitamin D | 0% | 0% | 0% |
| Vitamin E | 60% | 30% | 91% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechlińska, A.; Włodarczyk, A.; Gruchała-Niedoszytko, M.; Małgorzewicz, S.; Cubała, W.J. Dietary Patterns of Treatment–Resistant Depression Patients. Nutrients 2022, 14, 3766. https://doi.org/10.3390/nu14183766
Mechlińska A, Włodarczyk A, Gruchała-Niedoszytko M, Małgorzewicz S, Cubała WJ. Dietary Patterns of Treatment–Resistant Depression Patients. Nutrients. 2022; 14(18):3766. https://doi.org/10.3390/nu14183766
Chicago/Turabian StyleMechlińska, Agnieszka, Adam Włodarczyk, Marta Gruchała-Niedoszytko, Sylwia Małgorzewicz, and Wiesław Jerzy Cubała. 2022. "Dietary Patterns of Treatment–Resistant Depression Patients" Nutrients 14, no. 18: 3766. https://doi.org/10.3390/nu14183766
APA StyleMechlińska, A., Włodarczyk, A., Gruchała-Niedoszytko, M., Małgorzewicz, S., & Cubała, W. J. (2022). Dietary Patterns of Treatment–Resistant Depression Patients. Nutrients, 14(18), 3766. https://doi.org/10.3390/nu14183766






