Acute Flaxseed Intake Reduces Postprandial Glycemia in Subjects with Type 2 Diabetes: A Randomized Crossover Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
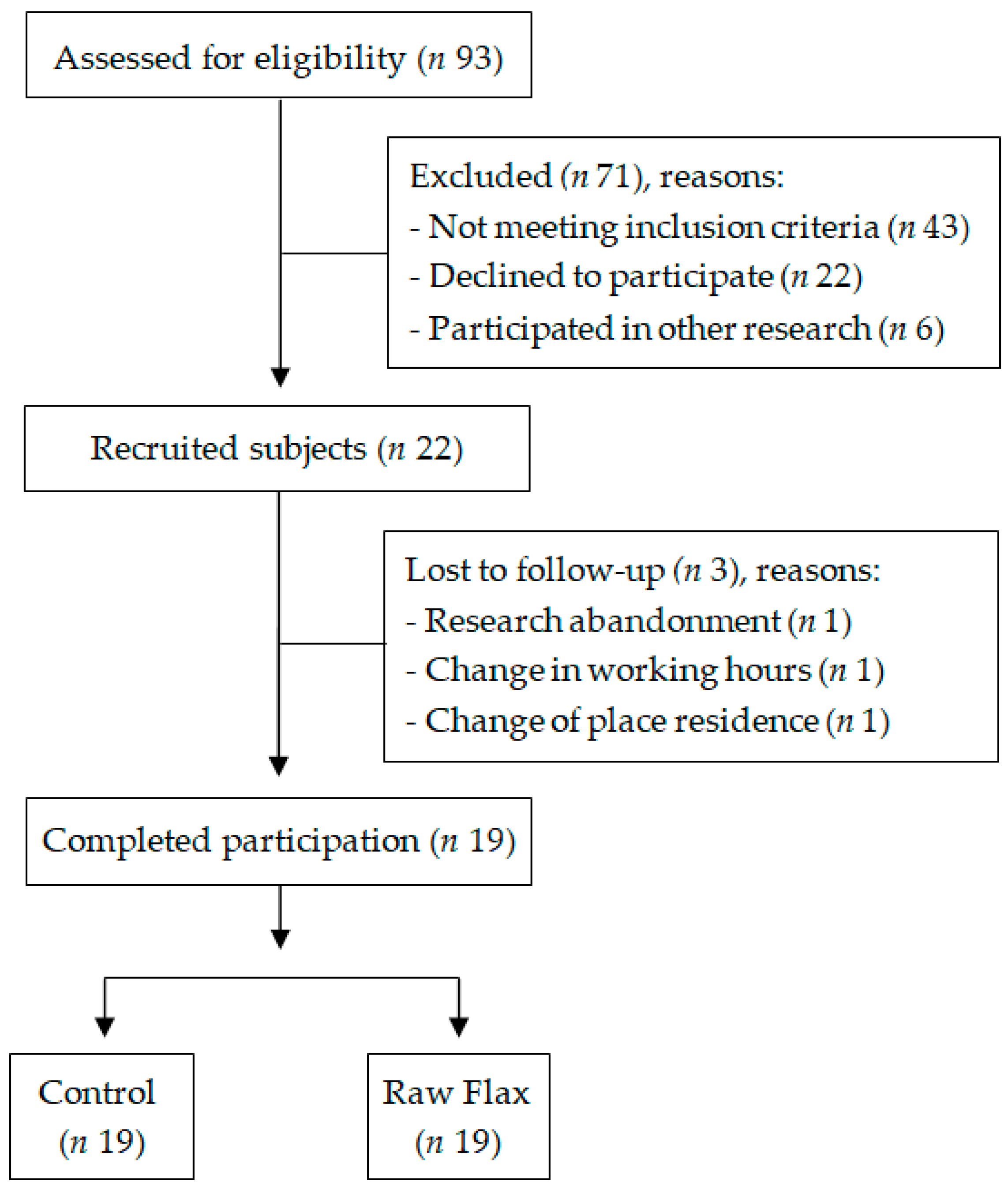
2.2. Study Design
2.3. Screening
2.4. Anthropometric and Body Composition Measurements
2.5. Experimental Protocol
2.6. Blood Glucose Assessment
2.7. Palatability Assessment
2.8. Statistical Analysis
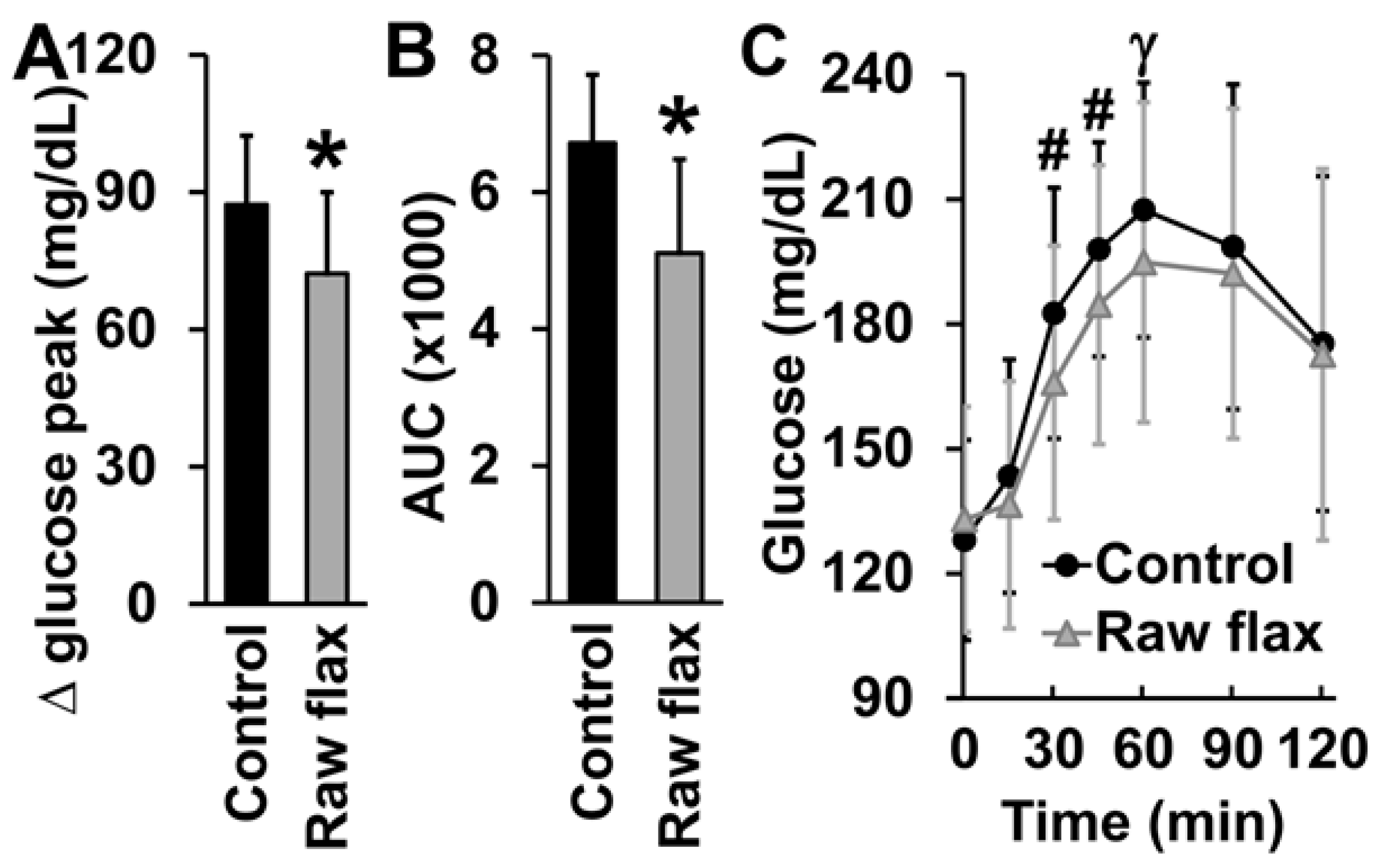
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirsch, I.B. Glycemic Variability and Diabetes Complications: Does It Matter? Of Course It Does! Diabetes Care 2015, 38, 1610–1614. [Google Scholar] [CrossRef]
- Nusca, A.; Tuccinardi, D.; Albano, M.; Cavallaro, C.; Ricottini, E.; Manfrini, S.; Pozzilli, P.; Di Sciascio, G. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes/Metab. Res. Rev. 2018, 34, e3047. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Ciotola, M.; Carleo, D.; Schisano, B.; Sardelli, L.; Di Tommaso, D.; Misso, L.; Saccomanno, F.; Ceriello, A.; Giugliano, D. Post-Meal Glucose Peaks at Home Associate with Carotid Intima-Media Thickness in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Ketema, E.B.; Kibret, K.T. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch. Public Health 2015, 73, 43. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.K.; Holman, R.R. Do glycoalbumin levels preferentially reflect changes in postprandial glucose excursions? Diabet. Med. 2017, 34, 1284–1290. [Google Scholar] [CrossRef]
- Kado, S.; Murakami, T.; Aoki, A.; Nagase, T.; Katsura, Y.; Noritake, M.; Matsuoka, T.; Nagata, N. Effect of acarbose on postprandial lipid metabolism in type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 1998, 41, 49–55. [Google Scholar] [CrossRef]
- Meza, C.A.; La Favor, J.D.; Kim, D.-H.; Hickner, R.C. Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Int. J. Mol. Sci. 2019, 20, 3775. [Google Scholar] [CrossRef]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef]
- Mani, U.V.; Mani, I.; Biswas, M.; Kumar, S.N. An open-label study on the effect of flax seed powder (Linum usitatissimum) sup-plementation in the management of diabetes mellitus. J. Diet. Suppl. 2011, 8, 257–265. [Google Scholar] [CrossRef]
- Universidade Estadual de Campinas. Tabela Brasileira de Composição de Alimentos, 4th ed.; BookEditora: São Paulo, Brazil, 2020; pp. 1–164. [Google Scholar]
- Martinchik, A.N.; Baturin, A.K.; Zubtsov, V.V.; Molofeev, V.I. Nutritional value and functional properties of flaxseed. Vopr. Pitan. 2012, 81, 4–10. [Google Scholar]
- Shayan, M.; Kamalian, S.; Sahebkar, A.; Tayarani-Najaran, Z. Flaxseed for Health and Disease: Review of Clinical Trials. Comb. Chem. High Throughput Screen. 2020, 23, 699–722. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, A.M.; Brown, B.D.; Cunnane, S.C.; Domitrovich, S.G.; Adams, E.R.; Bobowiec, C.E. Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: A randomized study. Nutr. Res. 2013, 33, 367–375. [Google Scholar] [CrossRef]
- Yari, Z.; Cheraghpour, M.; Hekmatdoost, A. Flaxseed and/or hesperidin supplementation in metabolic syndrome: An open-labeled randomized controlled trial. Eur. J. Nutr. 2020, 60, 287–298. [Google Scholar] [CrossRef]
- Pan, A.; Sun, J.; Chen, Y.; Ye, X.; Li, H.; Yu, Z.; Wang, Y.; Gu, W.; Zhang, X.; Chen, X.; et al. Effects of a Flaxseed-Derived Lignan Supplement in Type 2 Diabetic Patients: A Randomized, Double-Blind, Cross-Over Trial. PLoS ONE 2007, 2, e1148. [Google Scholar] [CrossRef] [PubMed]
- Hasaniani, N.; Rahimlou, M.; Ahmadi, A.R.; Khalifani, A.M.; Alizadeh, M. The Effect of Flaxseed Enriched Yogurt on the Glycemic Status and Cardiovascular Risk Factors in Patients with Type 2 Diabetes Mellitus: Randomized, Open-labeled, Controlled Study. Clin. Nutr. Res. 2019, 8, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Lockert, E.A.; Cammer, A.L.; Whiting, S.J. Effects of Flax Fiber on Laxation and Glycemic Response in Healthy Volunteers. J. Med. Food 2005, 8, 508–511. [Google Scholar] [CrossRef]
- Vuksan, V.; Choleva, L.; Jovanovski, E.; Jenkins, A.L.; Au-Yeung, F.; Dias, A.G.; Ho, H.V.T.; Zurbau, A.; Duvnjak, L. Comparison of flax (Linum usitatissimum) and Salba-chia (Salvia hispanica L.) seeds on postprandial glycemia and satiety in healthy individuals: A randomized, controlled, crossover study. Eur. J. Clin. Nutr. 2017, 71, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Sartang, M.; Sohrabi, Z.; Boldaji, R.B.; Raeisi-Dehkordi, H.; Mazloom, Z. Flaxseed supplementation on glucose control and insulin sensitivity: A systematic review and meta-analysis of 25 randomized, placebo-controlled trials. Nutr. Rev. 2018, 76, 125–139. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- de Carvalho, C.M.; de Paula, T.P.; Viana, L.V.; Machado, V.M.; de Almeida, J.C.; Azevedo, M.J. Plasma glucose and insulin responses after consumption of breakfasts with different sources of soluble fiber in type 2 diabetes patients: A randomized crossover clinical trial. Am. J. Clin. Nutr. 2017, 106, 1238–1245. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, nutrition and the prevention of chronic diseases. World Health Organ. Tech. Rep. Ser. 2003, 916, 1–149. [Google Scholar]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; Office of Disease Prevention and Health Promotion: Washington, DC, USA, 2020; pp. 1–149. Available online: DietaryGuidelines.gov (accessed on 18 August 2022).
- Freckmann, G.; Schmid, C.; Baumstark, A.; Pleus, S.; Link, M.; Haug, C. System Accuracy Evaluation of 43 Blood Glucose Monitoring Systems for Self-Monitoring of Blood Glucose according to DIN EN ISO 15197. J. Diabetes Sci. Technol. 2012, 6, 1060–1075. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Cummings, J.H.; Englyst, H.N.; Key, T.; Liu, S.; Riccardi, G.; Summerbell, C.; Uauy, R.; van Dam, R.M.; Venn, B.; et al. FAO/WHO Scientific Update on carbohydrates in human nutrition: Conclusions. Eur. J. Clin. Nutr. 2007, 61, S132–S137. [Google Scholar] [CrossRef] [PubMed]
- Melson, C.E.; Nepocatych, S.; Madzima, T.A. The effects of whey and soy liquid breakfast on appetite response, energy metabolism, and subsequent energy intake. Nutrition 2019, 61, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mani, I.; Patel, J.J.; Mani, U.V. Studies on the Effect of Wheat Bran Fibre on Serum and Urinary Amino Acids and Imino Acids in Non Insulin Dependent Diabetic Patients. J. Clin. Biochem. Nutr. 1987, 3, 143–148. [Google Scholar] [CrossRef][Green Version]
- Soltanian, N.; Janghorbani, M. A randomized trial of the effects of flaxseed to manage constipation, weight, glycemia, and lipids in constipated patients with type 2 diabetes. Nutr. Metab. 2018, 15, 36. [Google Scholar] [CrossRef]
- Soltanian, N.; Janghorbani, M. Effect of flaxseed or psyllium vs. placebo on management of constipation, weight, glycemia, and lipids: A randomized trial in constipated patients with type 2 diabetes. Clin. Nutr. ESPEN 2019, 29, 41–48. [Google Scholar] [CrossRef]
- Kay, B.A.; Trigatti, K.; MacNeil, M.B.; Klingel, S.L.; Repin, N.; Goff, H.D.; Wright, A.J.; Duncan, A.M. Pudding products enriched with yellow mustard mucilage, fenugreek gum or flaxseed mucilage and matched for simulated intestinal viscosity significantly reduce post-prandial peak glucose and insulin in adults at risk for type 2 diabetes. J. Funct. Foods 2017, 37, 603–611. [Google Scholar] [CrossRef]
- Almehmadi, A.; Lightowler, H.; Chohan, M.; Clegg, M.E. The effect of a split portion of flaxseed on 24-h blood glucose response. Eur. J. Nutr. 2020, 60, 1363–1373. [Google Scholar] [CrossRef]
- Lambeau, K.V.; McRorie, J.W. Fiber supplements and clinically proven health benefits: How to recognize and recommend an effective fiber therapy. J. Am. Assoc. Nurse Pract. 2017, 29, 216–223. [Google Scholar] [CrossRef] [PubMed]
- McRorie, J.W. Evidence-based approach to fiber supplements and clinically meaningful health benefits, Part 1: What to look for and how to recommend an effective fiber therapy. Nutr. Today 2015, 50, 82–89. [Google Scholar] [CrossRef] [PubMed]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Kabisch, S.; Honsek, C.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Birkenfeld, A.L.; Dambeck, U.; Osterhoff, M.A.; Weickert, M.O.; Pfeiffer, A.F.H. Dose-dependent effects of insoluble fibre on glucose metabolism: A stratified post hoc analysis of the Optimal Fibre Trial (OptiFiT). Acta Diabetol. 2021, 58, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, S.; Meyer, N.M.T.; Honsek, C.; Gerbracht, C.; Dambeck, U.; Kemper, M.; Osterhoff, M.A.; Birkenfeld, A.L.; Arafat, A.M.; Hjorth, M.F.; et al. Fasting Glucose State Determines Metabolic Response to Supplementation with Insoluble Cereal Fibre: A Secondary Analysis of the Optimal Fibre Trial (OptiFiT). Nutrients 2019, 11, 2385. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef]
- Bingham, S.A.; Day, N.E.; Luben, R.; Ferrari, P.; Slimani, N.; Norat, T.; Clavel-Chapelon, F.; Kesse, E.; Nieters, A.; Boeing, H.; et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): An observational study. Lancet 2003, 361, 1496–1501. [Google Scholar] [CrossRef]
- Arun, K.B.; Dhanya, R.; Chandran, J.; Abraham, B.; Satyan, S.; Nisha, P. A comparative study to elucidate the biological activities of crude extracts from rice bran and wheat bran in cell line models. J. Food Sci. Technol. 2020, 57, 3221–3231. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef]
- Gatti, E.; Noè, D.; Pazzucconi, F.; Gianfranceschi, G.; Porrini, M.; Testolin, G.; Sirtori, C.R. Differential effect of unsaturated oils and butter on blood glucose and insulin response to carbohydrate in normal volunteers. Eur. J. Clin. Nutr. 1992, 46, 161–166. [Google Scholar]
- Normand, S.; Khalfallah, Y.; Louche-Pelissier, C.; Pachiaudi, C.; Antoine, J.-M.; Blanc, S.; Desage, M.; Riou, J.P.; Laville, M. Influence of dietary fat on postprandial glucose metabolism (exogenous and endogenous) using intrinsically13C-enriched durum wheat. Br. J. Nutr. 2001, 86, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Owen, B.; Wolever, T.M. Effect of fat on glycaemic responses in normal subjects: A dose-response study. Nutr. Res. 2003, 23, 1341–1347. [Google Scholar] [CrossRef]
- Müller, M.; Canfora, E.E.; Blaak, E.E. Gastrointestinal Transit Time, Glucose Homeostasis and Metabolic Health: Modulation by Dietary Fibers. Nutrients 2018, 10, 275. [Google Scholar] [CrossRef]
- Taylor, C.G.; Noto, A.D.; Stringer, D.M.; Froese, S.; Malcolmson, L. Dietary Milled Flaxseed and Flaxseed Oil Improve N-3 Fatty Acid Status and Do Not Affect Glycemic Control in Individuals with Well-Controlled Type 2 Diabetes. J. Am. Coll. Nutr. 2010, 29, 72–80. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42 (Suppl. S1), S13–S28. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Manson, J.E.; Stevenson, J.C.; Fonseca, V.A. Menopausal Hormone Therapy and Type 2 Diabetes Prevention: Evidence, Mechanisms, and Clinical Implications. Endocr. Rev. 2017, 38, 173–188. [Google Scholar] [CrossRef]
| Variables | Mean ± SD | Range |
|---|---|---|
| Age | 52.1 ± 6.7 | 38–59 |
| Body weight (kg) | 94.0 ± 17.5 | 67.6–128.4 |
| Height (cm) | 172.5 ± 7.9 | 154–192 |
| BMI (kg/m²) | 31.7 ± 5.5 | 23–41 |
| Waist circumference (cm) | 109.1 ± 12.7 | 85–132 |
| Body fat (%) | 31.3 ± 9.1 | 15.6–46.6 |
| Diabetes duration (months) | 76.5 ± 72.6 | 6–300 |
| Fasting blood glucose (mg/dL) | 124.7 ± 26.0 | 81–191 |
| Insulin (µUI/mL) | 15.6 ± 8.1 | 4.0–33.7 |
| HbA1c (%) | 6.9 ± 0.8 | 5.6–8.3 |
| HOMA-IR | 4.8 ± 2.5 | 1.2–9.7 |
| Palatability | Control | Flax | p-Value |
|---|---|---|---|
| Visual appeal | 7.7 ± 2.0 | 7.8 ± 1.7 | 0.89 |
| Smell | 7.6 ± 1.7 | 7.6 ± 1.7 | 0.96 |
| Pleasantness of taste | 7.6 ± 1.7 | 7.2 ± 1.8 | 0.35 |
| Sweetness | 5.3 ± 2.3 | 4.6 ± 2.5 | 0.48 |
| Saltiness | 3.1 ± 2.3 | 3.1 ± 2.2 | 0.91 |
| Bitterness | 2.3 ± 2.2 | 2.4 ± 2.0 | 0.27 |
| Sourness | 2.4 ± 2.1 | 2.4 ± 1.9 | 0.42 |
| Creaminess | 7.5 ± 2.1 | 7.4 ± 2.0 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, F.D.; Reis, C.E.G.; Welker, A.F.; Gallassi, A.D. Acute Flaxseed Intake Reduces Postprandial Glycemia in Subjects with Type 2 Diabetes: A Randomized Crossover Clinical Trial. Nutrients 2022, 14, 3736. https://doi.org/10.3390/nu14183736
Moreira FD, Reis CEG, Welker AF, Gallassi AD. Acute Flaxseed Intake Reduces Postprandial Glycemia in Subjects with Type 2 Diabetes: A Randomized Crossover Clinical Trial. Nutrients. 2022; 14(18):3736. https://doi.org/10.3390/nu14183736
Chicago/Turabian StyleMoreira, Fernanda Duarte, Caio Eduardo Gonçalves Reis, Alexis Fonseca Welker, and Andrea Donatti Gallassi. 2022. "Acute Flaxseed Intake Reduces Postprandial Glycemia in Subjects with Type 2 Diabetes: A Randomized Crossover Clinical Trial" Nutrients 14, no. 18: 3736. https://doi.org/10.3390/nu14183736
APA StyleMoreira, F. D., Reis, C. E. G., Welker, A. F., & Gallassi, A. D. (2022). Acute Flaxseed Intake Reduces Postprandial Glycemia in Subjects with Type 2 Diabetes: A Randomized Crossover Clinical Trial. Nutrients, 14(18), 3736. https://doi.org/10.3390/nu14183736






