The Effect of Dietary Carbohydrate and Fat Manipulation on the Metabolome and Markers of Glucose and Insulin Metabolism: A Randomised Parallel Trial
Abstract
:1. Introduction
2. Materials and Methods
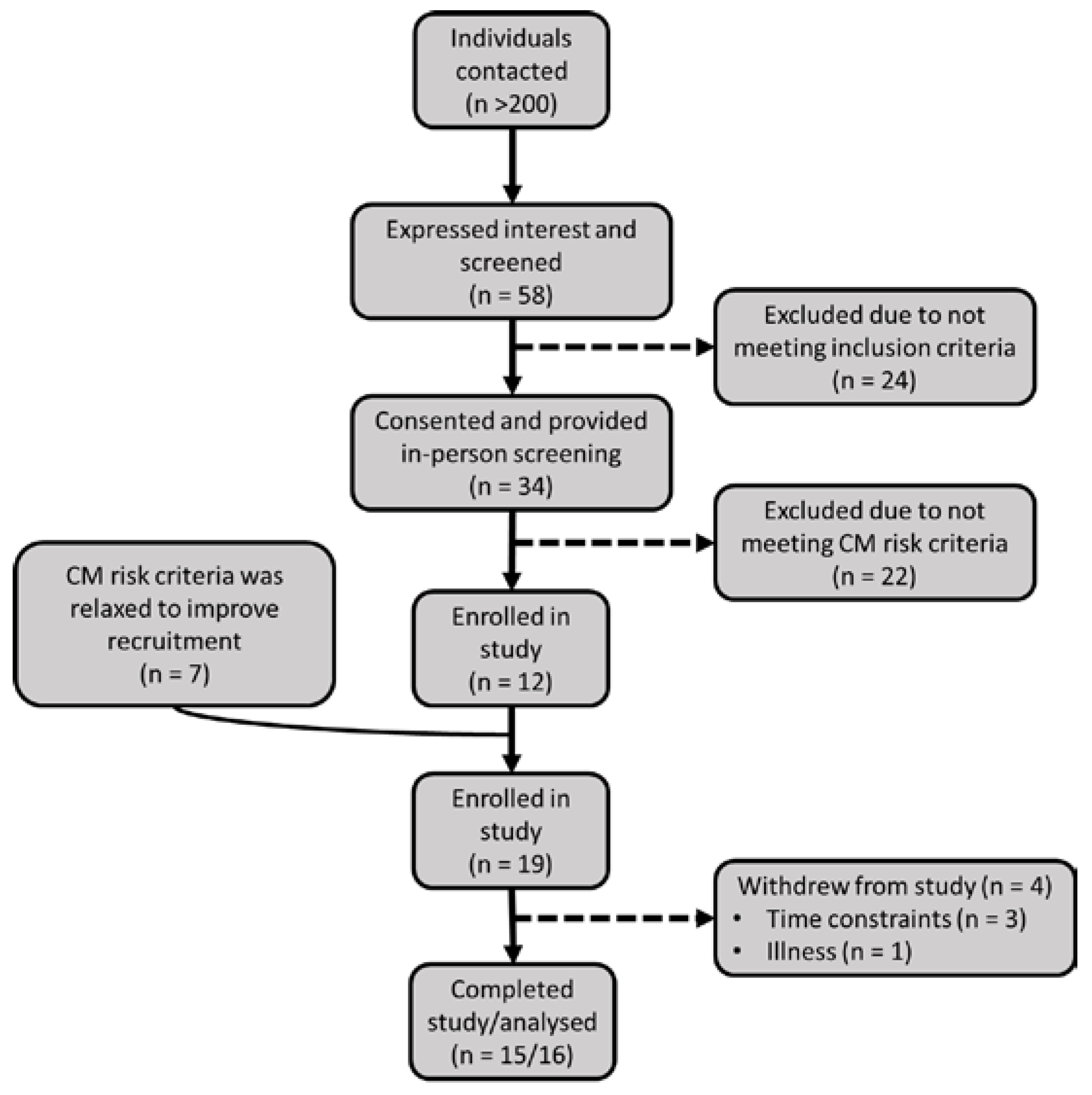
2.1. Participant Recruitment and Screening
2.2. Study Protocol and Diet
2.3. Biochemical Measurements
2.4. Measurement of Blood Pressure, Body Composition and Dietary Intake
2.5. Measurement of Metabolites
2.6. Statistics
3. Results
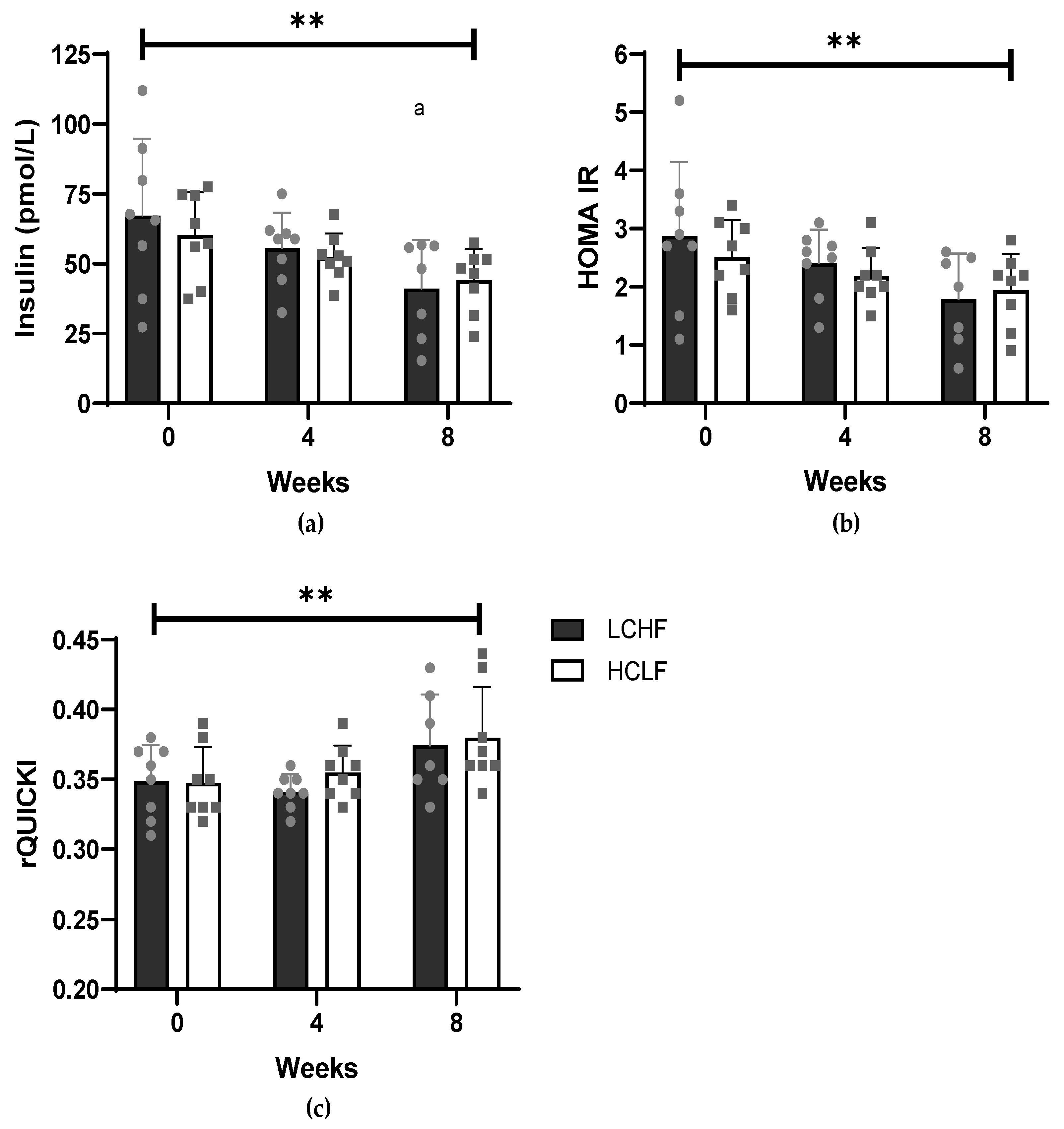
3.1. Cardiometabolic Risk Factors
3.2. Diet, Body Composition and Physical Activity Measures
3.3. Non-Targeted Metabolomic Profiling
3.4. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. The Eatwell Guide. Helping You Eat a Healthy Balance Diet; Public Health England: London, UK, 2016. [Google Scholar]
- DeSalvo, K.B.; Olson, R.; Casavale, K.O. Dietary Guidelines for Americans. JAMA 2016, 315, 457–458. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Healthy Diet; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Tay, J.; Thompson, C.H.; Luscombe-Marsh, N.D.; Wycherley, T.P.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S.; Brinkworth, G.D. Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: A 2-year randomized clinical trial. Diabetes Obes. Metab. 2018, 20, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Comparison of the long-term effects of high-fat v. low-fat diet consumption on cardiometabolic risk factors in subjects with abnormal glucose metabolism: A systematic review and meta-analysis. Br. J. Nutr. 2014, 111, 2047–2058. [Google Scholar] [CrossRef]
- Gjuladin-Hellon, T.; Davies, I.G.; Penson, P.; Baghbadorani, R.A. Effects of carbohydrate-restricted diets on low-density lipoprotein cholesterol levels in overweight and obese adults: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 161–180. [Google Scholar] [CrossRef]
- Mansoor, N.; Vinknes, K.J.; Veierod, M.B.; Retterstol, K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors a meta-analysis of randomised controlled trials. Br. J. Nutr. 2016, 115, 466–479. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Knapp, A.; Johnson, A.; Wong, J.M.W.; Greco, K.F.; Ma, C.; Mora, S.; Ludwig, D.S. Effects of a low-carbohydrate diet on insulin-resistant dyslipoproteinemia—a randomized controlled feeding trial. Am. J. Clin. Nutr. 2022, 115, 154–162. [Google Scholar] [CrossRef]
- Hyde, P.N.; Sapper, T.N.; Crabtree, C.D.; LaFountain, R.A.; Bowling, M.L.; Buga, A.; Fell, B.; McSwiney, F.T.; Dickerson, R.M.; Miller, V.J.; et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019, 4, e128308. [Google Scholar] [CrossRef]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef]
- Veum, V.L.; Laupsa-Borge, J.; Eng, Ø.; Rostrup, E.; Larsen, T.H.; Nordrehaug, J.E.; Nygård, O.K.; Sagen, J.V.; Gudbrandsen, O.A.; Dankel, S.N.; et al. Visceral adiposity and metabolic syndrome after very high-fat and low-fat isocaloric diets: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Lakhani, I.; Gong, M.; Wong, W.T.; Bazoukis, G.; Lampropoulos, K.; Wong, S.H.; Wu, W.K.K.; Wong, M.C.S.; Ong, K.L.; Liu, T.; et al. Fibroblast growth factor 21 in cardio-metabolic disorders: A systematic review and meta-analysis. Metabolism 2018, 83, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ahl, S.; Guenther, M.; Zhao, S.; James, R.; Marks, J.; Szabo, A.; Kidambi, S. Adiponectin levels differentiate metabolically healthy vs unhealthy among obese and nonobese white individuals. J. Clin. Endocrinol. Metab. 2015, 100, 4172–4180. [Google Scholar] [CrossRef] [PubMed]
- Esteghamati, A.; Noshad, S.; Khalilzadeh, O.; Morteza, A.; Nazeri, A.; Meysamie, A.; Esteghamati, A.; Nakhjavani, M. Contribution of Serum Leptin to Metabolic Syndrome in Obese and Nonobese Subjects. Arch. Med. Res. 2011, 42, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Mertens, I.; Verrijken, A.; Michiels, J.J.; Van Der Planken, M.; Ruige, J.B.; Van Gaal, L.F. Among inflammation and coagulation markers, PAI-1 is a true component of the metabolic syndrome. Int. J. Obes. 2006, 30, 1308–1314. [Google Scholar] [CrossRef]
- Soto González, A.; Bellido Guerrero, D.; Buño Soto, M.; Pértega Díaz, S.; Martinez-Olmos, M.; Vidal, O. Metabolic syndrome, insulin resistance and the inflammation markers C-reactive protein and ferritin. Eur. J. Clin. Nutr. 2006, 60, 802–809. [Google Scholar] [CrossRef]
- Hung, J.; McQuillan, B.M.; Thompson, P.L.; Beilby, J.P. Circulating adiponectin levels associate with inflammatory markers, insulin resistance and metabolic syndrome independent of obesity. Int. J. Obes. 2008, 32, 772–779. [Google Scholar] [CrossRef]
- D’Elia, L.; Strazzullo, P.; Iacone, R.; Russo, O.; Galletti, F. Leptin levels predict the development of insulin resistance in a sample of adult men–The Olivetti Heart Study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 39–44. [Google Scholar] [CrossRef]
- Newgard, C.B. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 2017, 25, 43–56. [Google Scholar] [CrossRef]
- Papandreou, C.; Bulló, M.; Ruiz-Canela, M.; Dennis, C.; Deik, A.; Wang, D.; Guasch-Ferré, M.; Yu, E.; Razquin, C.; Corella, D.; et al. Plasma metabolites predict both insulin resistance and incident type 2 diabetes: A metabolomics approach within the Prevención con Dieta Mediterránea (PREDIMED) study. Am. J. Clin. Nutr. 2019, 109, 635–647. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 2019, 15, 93. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Alonso, P.; Giardina, S.; Cañueto, D.; Salas-Salvadó, J.; Cañellas, N.; Bulló, M. Changes in Plasma Metabolite Concentrations after a Low-Glycemic Index Diet Intervention. Mol. Nutr. Food Res. 2019, 63, e1700975. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L. Metabolomics in nutrition research—A powerful window into nutritional metabolism. Essays Biochem. 2016, 60, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Engeli, S.; Kaminski, J.; Witt, H.; Rein, D.; Kamlage, B.; Utz, W.; Fuhrmann, J.C.; Haas, V.; Mähler, A.; et al. Branched-chain amino acid catabolism rather than amino acids plasma concentrations is associated with diet-induced changes in insulin resistance in overweight to obese individuals. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, 698–702. [Google Scholar] [CrossRef]
- Jebb, S.A.; Lovegrove, J.A.; Griffin, B.A.; Frost, G.S.; Moore, C.S.; Chatfield, M.D.; Bluck, L.J.; Williams, C.M.; Sanders, T.A. Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: The RISCK trial. Am. J. Clin. Nutr. 2010, 92, 748–758. [Google Scholar] [CrossRef]
- Committee on Medical Aspects of Food Policy (COMA). Dietary Reference Values for Food Energy and Nutrients for the United Kingdom; The Stationery Office Ltd: Norwich, UK, 1991; ISBN 9780113213979. [Google Scholar]
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Low-carbohydrate nutrition and metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Perseghin, G.; Caumo, A.; Caloni, M.; Testolin, G.; Luzi, L. Incorporation of the fasting plasma FFA concentration into QUICKI improves its association with insulin sensitivity in nonobese individuals. J. Clin. Endocrinol. Metab. 2001, 86, 4776–4781. [Google Scholar] [CrossRef] [PubMed]
- Bonita, R.; Winkelmann, R.; Douglas, K.A.; de Courten, M. The WHO Stepwise Approach to Surveillance (Steps) of Non-Communicable Disease Risk Factors. In Global Behavioral Risk Factor Surveillance; Springer: Boston, MA, USA, 2003; pp. 9–22. ISBN 978-1-4615-0071-1. [Google Scholar]
- Dao, M.C.; Subar, A.F.; Warthon-Medina, M.; Cade, J.; Burrows, T.; Golley, R.K.; Forouhi, N.G.; Pearce, M.; Holmes, B.A. Dietary assessment toolkits: An overview. Public Health Nutr. 2019, 22, 404–418. [Google Scholar] [CrossRef]
- Cruickshank-Quinn, C.; Quinn, K.D.; Powell, R.; Yang, Y.; Armstrong, M.; Mahaffey, S.; Reisdorph, R.; Reisdorph, N. Multi-step preparation technique to recover multiple metabolite compound classes for in-depth and informative metabolomic analysis. J. Vis. Exp. 2014, 11, 51670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Cruickshank, C.; Armstrong, M.; Mahaffey, S.; Reisdorph, R.; Reisdorph, N. New sample preparation approach for mass spectrometry-based profiling of plasma results in improved coverage of metabolome. J. Chromatogr. A 2013, 1300, 217–226. [Google Scholar] [CrossRef]
- van Hees, V.T.; Gorzelniak, L.; Dean León, E.C.; Eder, M.; Pias, M.; Taherian, S.; Ekelund, U.; Renström, F.; Franks, P.W.; Horsch, A.; et al. Separating Movement and Gravity Components in an Acceleration Signal and Implications for the Assessment of Human Daily Physical Activity. PLoS ONE 2013, 8, e61691. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Shih, C.W.; Hauser, M.E.; Aronica, L.; Rigdon, J.; Gardner, C.D. Changes in blood lipid concentrations associated with changes in intake of dietary saturated fat in the context of a healthy low-carbohydrate weight-loss diet: A secondary analysis of the Diet Intervention Examining the Factors Interacting with Treatment. Am. J. Clin. Nutr. 2019, 109, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; Abegaz, K.H.; Abolhassani, H.; Aboyans, V.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Soeters, M.R.; Serlie, M.J.; Sauerwein, H.P.; Duran, M.; Ruiter, J.P.; Kulik, W.; Ackermans, M.T.; Minkler, P.E.; Hoppel, C.L.; Wanders, R.J.A.; et al. Characterization of D-3-hydroxybutyrylcarnitine (ketocarnitine): An identified ketosis-induced metabolite. Metabolism 2012, 61, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes 2013, 62, 1–8. [Google Scholar] [CrossRef]
- Zhong, H.; Fang, C.; Fan, Y.; Lu, Y.; Wen, B.; Ren, H.; Hou, G.; Yang, F.; Xie, H.; Jie, Z.; et al. Lipidomic profiling reveals distinct differences in plasma lipid composition in healthy, prediabetic, and type 2 diabetic individuals. Gigascience 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Mai, M.; Tönjes, A.; Kovacs, P.; Stumvoll, M.; Fiedler, G.M.; Leichtle, A.B. Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS ONE 2013, 8, e82459. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, R.R.; Gandour, R.D.; Van Der Leij, F.R. Molecular enzymology of carnitine transfer and transport. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 2001, 1546, 21–43. [Google Scholar] [CrossRef]
- Bruls, Y.M.; de Ligt, M.; Lindeboom, L.; Phielix, E.; Havekes, B.; Schaart, G.; Kornips, E.; Wildberger, J.E.; Hesselink, M.K.; Muoio, D.; et al. Carnitine supplementation improves metabolic flexibility and skeletal muscle acetylcarnitine formation in volunteers with impaired glucose tolerance: A randomised controlled trial. EBioMedicine 2019, 49, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P.; Cattaneo, D.; Loriga, G.; Ledda, F.; Motterlini, N.; Gherardi, G.; Orisio, S.; Remuzzi, G. Ameliorating hypertension and insulin resistance in subjects at increased cardiovascular risk: Effects of acetyl-l-carnitine therapy. Hypertension 2009, 54, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Koulman, A.; Griffin, J.L. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: Progress from the metabolome. Lancet Diabetes Endocrinol. 2014, 2, 65–75. [Google Scholar] [CrossRef]
- Kaikkonen, J.E.; Jula, A.; Viikari, J.S.A.; Juonala, M.; Hutri-Kähönen, N.; Kähönen, M.; Lehtimäki, T.; Raitakari, O.T. Associations of Serum Fatty Acid Proportions with Obesity, Insulin Resistance, Blood Pressure, and Fatty Liver: The Cardiovascular Risk in Young Finns Study. J. Nutr. 2021, 151, 970–978. [Google Scholar] [CrossRef]
- Garaulet, M.; Pérez-Llamas, F.; Pérez-Ayala, M.; Martínez, P.; de Medina, F.S.; Tebar, F.J.; Zamora, S. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: Relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am. J. Clin. Nutr. 2001, 74, 585–591. [Google Scholar] [CrossRef]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Ahn, D.; Hwang, J.Y.; Kang, M.J.; Chung, S.J. Linoleic acid exerts antidiabetic effects by inhibiting protein tyrosine phosphatases associated with insulin resistance. J. Funct. Foods 2021, 83, 104532. [Google Scholar] [CrossRef]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids 2021, 8848161. [Google Scholar] [CrossRef]
- Wong, C.K.; Botta, A.; Pither, J.; Dai, C.; Gibson, W.T.; Ghosh, S. A high-fat diet rich in corn oil reduces spontaneous locomotor activity and induces insulin resistance in mice. J. Nutr. Biochem. 2015, 26, 319–326. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.C.; Wheelock, C.E.; Haeggström, J.Z. The Eicosanoids, Redox-Regulated Lipid Mediators in Immunometabolic Disorders. Antioxid. Redox Signal. 2018, 29, 275–296. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yue, H.; Jia, M.; Liu, W.; Qiu, B.; Hou, H.; Huang, F.; Xu, T. Effect of low-ratio n-6/n-3 PUFA on blood glucose: A meta-analysis. Food Funct. 2019, 10, 4557–4565. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins, Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]
- Calvani, R.; Rodriguez-Mañas, L.; Picca, A.; Marini, F.; Biancolillo, A.; Laosa, O.; Pedraza, L.; Gervasoni, J.; Primiano, A.; Conta, G.; et al. Identification of a circulating amino acid signature in frail older persons with type 2 diabetes mellitus: Results from the metabofrail study. Nutrients 2020, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Legry, V.; Francque, S.; Haas, J.T.; Verrijken, A.; Caron, S.; Chávez-Talavera, O.; Vallez, E.; Vonghia, L.; Dirinck, E.; Verhaegen, A.; et al. Bile acid alterations are associated with insulin resistance, but not with NASH, in obese subjects. J. Clin. Endocrinol. Metab. 2017, 102, 3783–3794. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin Receptor Signaling in Normal and Insulin-Resistant States. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Liu, C.; Feng, X.; Li, Q.; Wang, Y.; Li, Q.; Hua, M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine 2016, 86, 100–109. [Google Scholar] [CrossRef]
- Steckhan, N.; Hohmann, C.D.; Kessler, C.; Dobos, G.; Michalsen, A.; Cramer, H. Effects of different dietary approaches on inflammatory markers in patients with metabolic syndrome: A systematic review and meta-analysis. Nutrition 2016, 32, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.Y.; Lam, K.S.L.; Wang, B.; Xu, A. Signaling mechanisms underlying the insulin-sensitizing effects of adiponectin. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Koch, C.; Augustine, R.A.; Steger, J.; Ganjam, G.K.; Benzler, J.; Pracht, C.; Lowe, C.; Schwartz, M.W.; Shepherd, P.R.; Anderson, G.M.; et al. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J. Neurosci. 2010, 30, 16180–16187. [Google Scholar] [CrossRef]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients 2019, 11, 454. [Google Scholar] [CrossRef]
- Norata, G.D.; Raselli, S.; Grigore, L.; Garlaschelli, K.; Dozio, E.; Magni, P.; Catapano, A.L. Leptin:Adiponectin Ratio Is an Independent Predictor of Intima Media Thickness of the Common Carotid Artery. Stroke 2007, 38, 2844–2846. [Google Scholar] [CrossRef]
- Lasa, A.; Miranda, J.; Bulló, M.; Casas, R.; Salas-Salvadó, J.; Larretxi, I.; Estruch, R.; Ruiz-Gutiérrez, V.; Portillo, M.P. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. Eur. J. Clin. Nutr. 2014, 68, 767–772. [Google Scholar] [CrossRef]
- Oberhauser, F.; Schulte, D.M.; Faust, M.; Güdelhöfer, H.; Hahn, M.; Müller, N.; Neumann, K.; Krone, W.; Laudes, M. Weight loss due to a very low calorie diet differentially affects insulin sensitivity and interleukin-6 serum levels in nondiabetic obese human subjects. Horm. Metab. Res. 2012, 44, 465–470. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Ambrosi, J.; Gallego-Escuredo, J.M.; Catalán, V.; Rodríguez, A.; Domingo, P.; Moncada, R.; Valentí, V.; Salvador, J.; Giralt, M.; Villarroya, F.; et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin. Nutr. 2017, 36, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, C.; Dyson, P.; Sprecher, D.; Tsintzas, K.; Karpe, F. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. J. Clin. Endocrinol. Metab. 2009, 94, 3594–3601. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| LCHF | HCLF | ||
|---|---|---|---|
| Baseline | (p Value) | ||
| Age M/F ratio | 43.8 ± 10.4 4/4 | 44.6 ± 15.27 5/3 | 0.895 - |
| Height (cm) | 176 ± 11.6 | 168 ± 11.02 | - |
| Mass (kg) | 89.53 ± 14.85 | 72.48 ± 14.72 | 0.037 |
| BMI (kg/m2) | 28.75 ± 2.15 | 24.56 ± 3.83 | 0.018 |
| FM (kg) | 32.08 ± 4.28 | 19.89 ± 7.46 | 0.001 |
| FFM (kg) | 57.39 ± 11.62 | 52.59 ± 11.63 | 0.423 |
| FM (%) $ | 35.65 (7.43) | 25.5 (2.5) | 0.010 |
| FFM (%) $ | 64.2 (7.40) | 74.5 (2.50) | 0.010 |
| SkM (%) | 32.05 ± 4.92 | 35.8 ± 4.20 | 0.071 |
| SBP (mmHg) | 130 ± 10 | 127 ± 12 | 0.673 |
| DBP (mmHg) | 83 ± 9 | 78 ± 8 | 0.373 |
| VAT (I) $ | 3.21 (3.43) | 2.65 (1.35) | 0.328 |
| WC (cm) $ | 101.2 (15.87) | 93.75 (22.34) | 0.054 |
| Glucose (mmol/L) $ | 5.47 (0.75) | 5.72 (0.59) | 0.520 |
| Adiponectin (ug/mL) $ | 2.11 (1.98) | 2.69 (3.27) | 0.442 |
| CRP (ng/mL) | 1.57 ± 0.83 | 1.25 ± 0.50 | 0.359 |
| Ferritin (ng/mL) | 172.61 ± 168.17 | 155.38 ± 83.3 | 0.799 |
| IL-6 (pg/mL) $ | 1.16 (1.14) | 1.11 (1.61) | 0.574 |
| Insulin (pmol/L) | 67.21 ± 27.64 | 60.26 ± 15.48 | 0.545 |
| HOMA IR | 2.9 ± 1.30 | 2.5 ± 0.60 | 0.482 |
| rQUICKI | 0.35 ± 0.03 | 0.35 ± 0.03 | 0.924 |
| PAI-1 (ng/mL) $ | 7.19 (10.04) | 5.14 (8.02) | 0.721 |
| Resistin (ng/mL) $ | 2.76 (2.41) | 3.82 (2.57) | >0.999 |
| TNFa (pg/mL) | 6.41 ± 0.69 | 6.52 ± 1.81 | 0.868 |
| D3-Hydroxybutarate (mmol/L) | 0.07 ± 0.02 | 0.08 ± 0.05 | 0.478 |
| Cystatin C (pg/mL) | 0.35 ± 0.11 | 0.41 ± 0.13 | 0.470 |
| Leptin (ng/mL) $ | 3.69 (2.01) | 1.31 (0.71) | 0.007 |
| LAR (ng/ug) $ | 1.58 (2.63) | 0.37 (0.41) | 0.007 |
| FGF21 (pg/mL) | 147.9 ± 114.6 | 180.7 ± 138.5 | 0.614 |
| LCHF | HCLF | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | Week 0 | Week 4 | Week 8 | Week 0 | Week 4 | Week 8 | TIME | T X G | Group |
| SBP (mmHg) | 130 ± 10 | 120 ± 10 b | 123 ± 9 b | 127 ± 12 | 128 ± 12 | 129 ± 14 | 0.040 | 0.010 | 0.431 |
| DBP (mmHg) | 83 ± 9 | 77 ± 9 | 75 ± 9 bb | 78 ± 8 | 79 ± 8 | 78 ± 9 | 0.009 | 0.009 | 0.870 |
| Glucose (mmol/L) | 5.69 ± 0.41 | 5.78 ± 0.47 | 5.68 ± 0.47 | 5.79 ± 0.35 | 5.60 ± 0.54 | 5.80 ± 0.53 | 0.844 | 0.159 | 0.925 |
| Cystatin C (ug/mL) | 0.36 ± 0.11 | 0.31 ± 0.06 | 0.36 ± 0.09 | 0.41 ± 0.13 | 0.43 ± 0.17 | 0.43 ± 0.15 | 0.620 | 0.201 | 0.183 |
| NEFA (mmol/) | 0.79 ± 0.34 | 0.96 ± 0.30 | 0.80 ± 0.24 | 0.76 ± 0.25 | 0.79 ± 0.22 | 0.62 ± 0.17 | 0.078 | 0.587 | 0.309 |
| Ferritin (ng/mL) | 172.61 ± 168.17 | 162.49 ± 159.66 | 173.14 ± 162.87 | 155.38 ± 83.30 | 128.14 ± 72.66 | 141.38 ± 89.82 | 0.039 | 0.290 | 0.718 |
| Non-parametric testing | LCHF (Time) | HCLF (Time) | |||||||
| WC (cm) | 101 (15.8) | 99.1 (17.2) | 98.4 (16.9) b | 93.8 (22.4) | 93.1 (21.9) | 92.0 (22.1) | 0.052 | 0.346 | |
| VAT (l) | 4.23 (3.09) | 3.83 (3.09) | 3.19 (3.06) b | 2.65 (1.98) | 2.46 (1.90) | 2.46 (1.84) | 0.052 | 0.544 | |
| Adiponectin (ug/mL) | 2.69 (3.27) | 3.33 (1.91) | 3.19 (3.33) | 2.01 (2.09) | 2.12 (1.51) | 2.12 (1.85) | 0.964 | 0.079 | |
| CRP (ug/mL) | 1.10 (1.62) | 1.69 (1.66) ** | 1.24 (1.29) | 1.06 (0.79) | 0.88 (0.51) | 0.98 (0.86) | 0.305 | 0.236 | |
| IL-6 (pg/mL) | 1.30 (1.37) | 1.23 (1.27) | 1.54 (0.38) | 1.11 (1.61) | 0.83 (0.92) | 1.04 (1.99) | 0.964 | 0.794 | |
| TNFα (pg/mL) | 6.74 (1.28) | 5.83 (1.93) | 5.97 (1.74) | 6.50 (3.19) | 6.23 (3.21) | 6.15 (3.45) | 0.620 | 0.531 | |
| Leptin (ng/mL) | 3.98 (1.86) ** | 1.98 (1.84) b ** | 1.20 (2.42) bb | 1.31 (0.71) | 0.95 (0.54) | 1.07 (0.86) | 0.001 | 0.285 | |
| LAR (ng/ug) | 1.70 (2.63) ** | 0.96 (1.4) b ** | 0.70 (1.37) bb | 0.37 (0.41) | 0.23 (0.28) | 0.31 (0.7) | 0.001 | 0.029 | |
| Resistin (ng/mL) | 2.75 (1.15) | 2.76 (1.47) | 2.60 (1.51) | 3.82 (2.57) | 2.93 (1.78) | 2.97 (1.70) | 0.964 | 0.150 | |
| PAI-1 (ng/mL) | 7.30 (9.61) | 6.53 (4.45) | 6.32 (5.20) | 5.14 (8.02) | 4.11 (5.37) | 4.47 (9.99) | 0.112 | 0.794 | |
| FGF21 (pg/mL) | 148.16 (203.49) | 99.4 (92.43) | 167.38 (152.08) | 138 (243.56) | 136.4 (233.41) | 201.3 (406.55) | 0.051 | 0.935 | |
| D-3-Hydroxybutyrate (mmol/L) | 0.07 (0.01) | 0.31 (0.06) b * | 0.19 (0.27) | 0.09 (0.05) | 0.13 (0.11) | 0.08 (0.03) | 0.035 | 0.580 | |
| Fold Change (%) | |||
|---|---|---|---|
| Metabolite | Description | LCHF | HCLF |
| Hydroxybutyrlcarnitine | Lipid metabolism | 121.83 | −5.03 |
| 1-Aminocyclohexanecarboxylic acid | L-α-amino acid metabolism | −86.27 | 15.44 |
| N-(1-Deoxy-1-fructosyl)histidine | Histidine metabolism | 73.44 | 3.28 |
| Palmitoleyl linolenate | Lipid Metabolism | 52.13 | −14.21 |
| Succinylcholine | Acyl choline metabolism | −15.12 | 43.1 |
| Acetyl-L-Carnitine | Lipid Metabolism | 47.95 | 1.03 |
| Ursodeoxycholic acid 3-sulfate | Bile acid metabolism | −31.94 | −74.87 |
| Linoleic acid | Lipid metabolism | −49.42 | −6.62 |
| 5’-Hydroxymethyl meloxicam | Purine ribonucleoside metabolism | 6.56 | 44.66 |
| N(Pai)-Methyl-L-Histidine | Histidine metabolism | 49.25 | 86.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCullough, D.; Harrison, T.; Boddy, L.M.; Enright, K.J.; Amirabdollahian, F.; Schmidt, M.A.; Doenges, K.; Quinn, K.; Reisdorph, N.; Mazidi, M.; et al. The Effect of Dietary Carbohydrate and Fat Manipulation on the Metabolome and Markers of Glucose and Insulin Metabolism: A Randomised Parallel Trial. Nutrients 2022, 14, 3691. https://doi.org/10.3390/nu14183691
McCullough D, Harrison T, Boddy LM, Enright KJ, Amirabdollahian F, Schmidt MA, Doenges K, Quinn K, Reisdorph N, Mazidi M, et al. The Effect of Dietary Carbohydrate and Fat Manipulation on the Metabolome and Markers of Glucose and Insulin Metabolism: A Randomised Parallel Trial. Nutrients. 2022; 14(18):3691. https://doi.org/10.3390/nu14183691
Chicago/Turabian StyleMcCullough, Deaglan, Tanja Harrison, Lynne M. Boddy, Kevin J. Enright, Farzad Amirabdollahian, Michael A. Schmidt, Katrina Doenges, Kevin Quinn, Nichole Reisdorph, Mohsen Mazidi, and et al. 2022. "The Effect of Dietary Carbohydrate and Fat Manipulation on the Metabolome and Markers of Glucose and Insulin Metabolism: A Randomised Parallel Trial" Nutrients 14, no. 18: 3691. https://doi.org/10.3390/nu14183691
APA StyleMcCullough, D., Harrison, T., Boddy, L. M., Enright, K. J., Amirabdollahian, F., Schmidt, M. A., Doenges, K., Quinn, K., Reisdorph, N., Mazidi, M., Lane, K. E., Stewart, C. E., & Davies, I. G. (2022). The Effect of Dietary Carbohydrate and Fat Manipulation on the Metabolome and Markers of Glucose and Insulin Metabolism: A Randomised Parallel Trial. Nutrients, 14(18), 3691. https://doi.org/10.3390/nu14183691










