Short Chain Fatty Acids (SCFAs) Are the Potential Immunomodulatory Metabolites in Controlling Staphylococcus aureus-Mediated Mastitis
Abstract
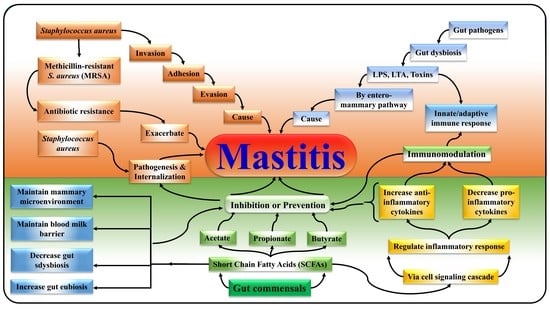
:1. Introduction
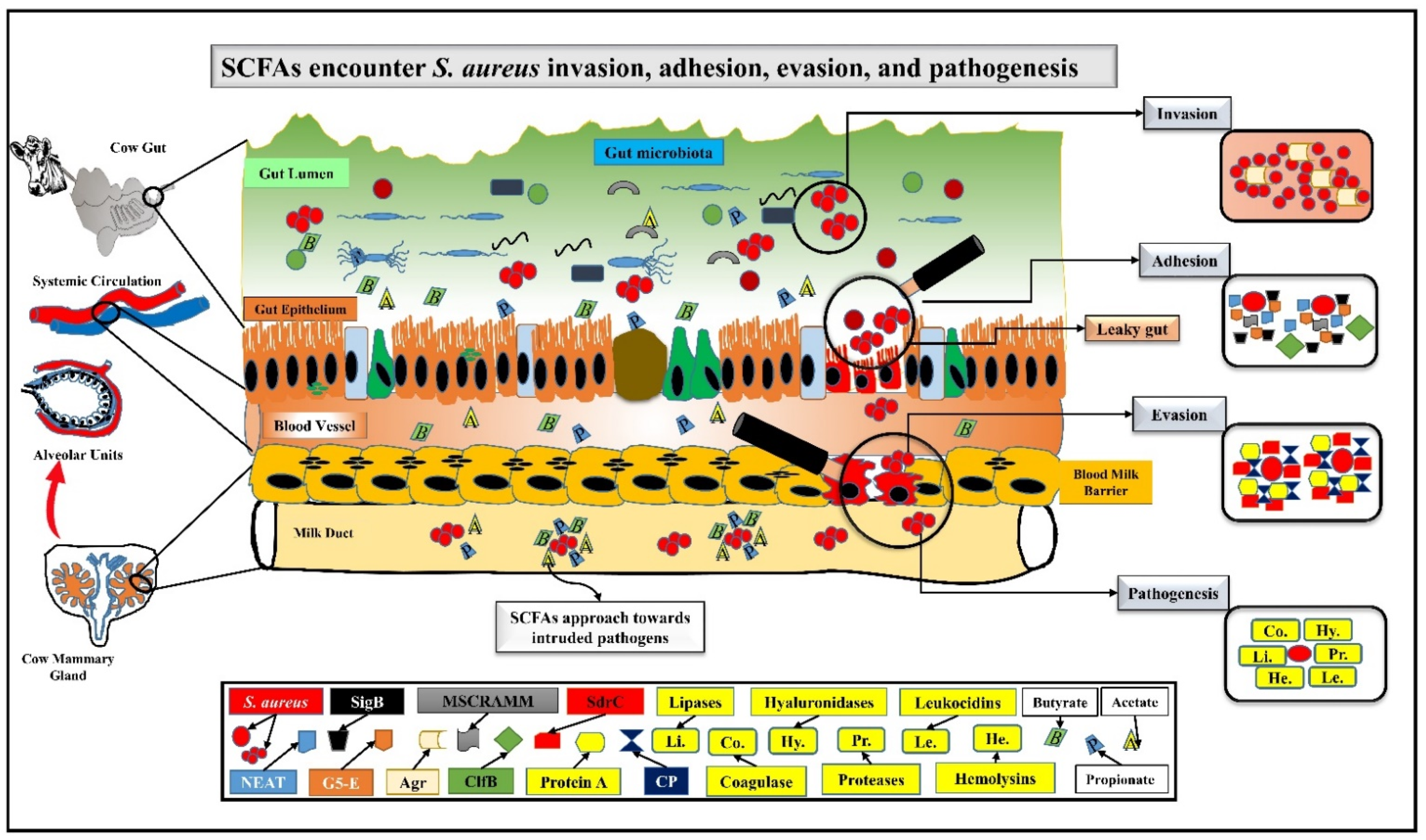
2. SCFAs Prevent Mastitis by Encountering S. aureus Pathogenesis via Challenging Its Adhesion, Invasion, and Evasion
3. SCFAs Ameliorate Mastitis by Modulating Gut-Mammary Dysbiosis
4. SCFAs Distinctly Participate in the Maintenance of Blood Milk Barrier and Mammary Gland Structure
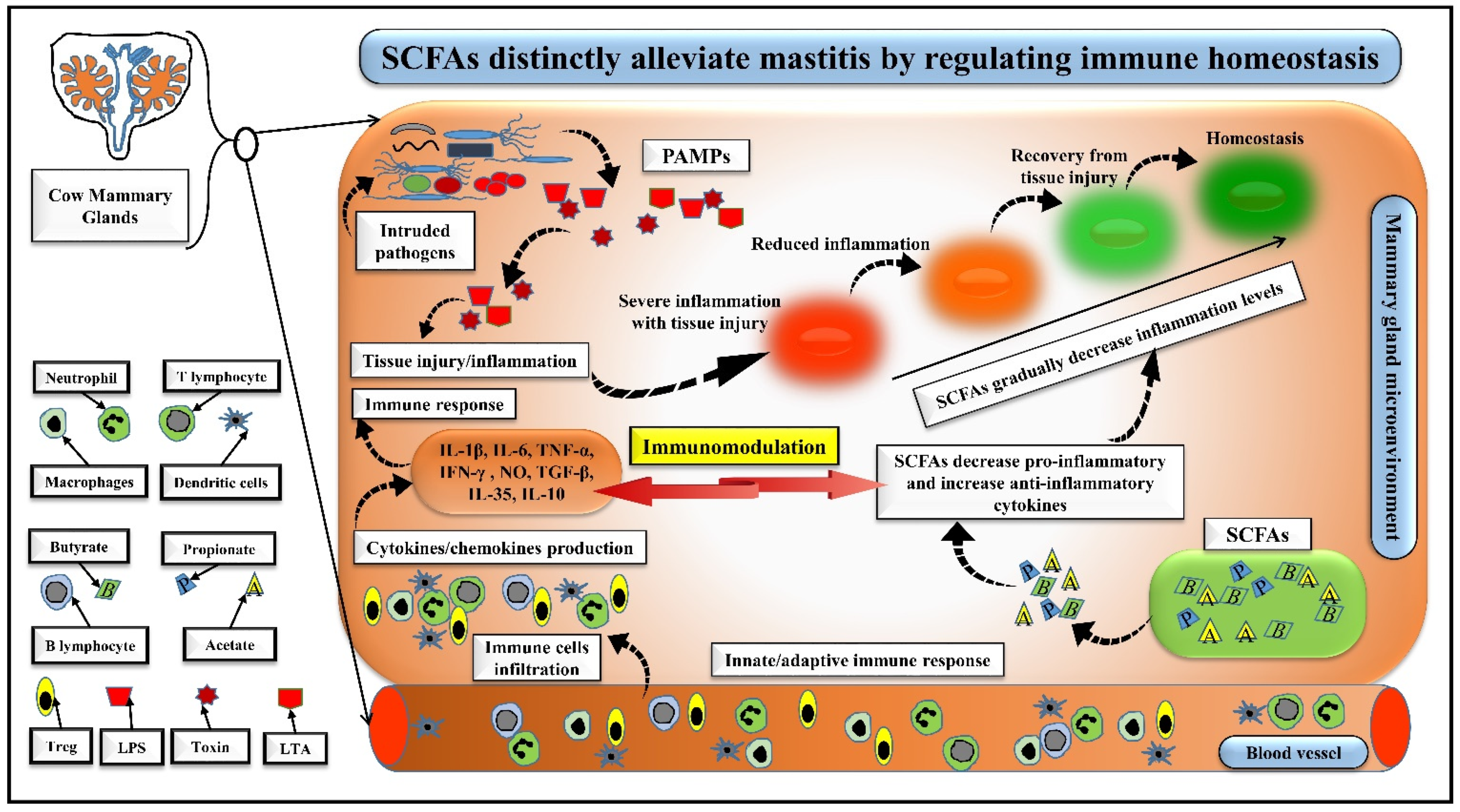
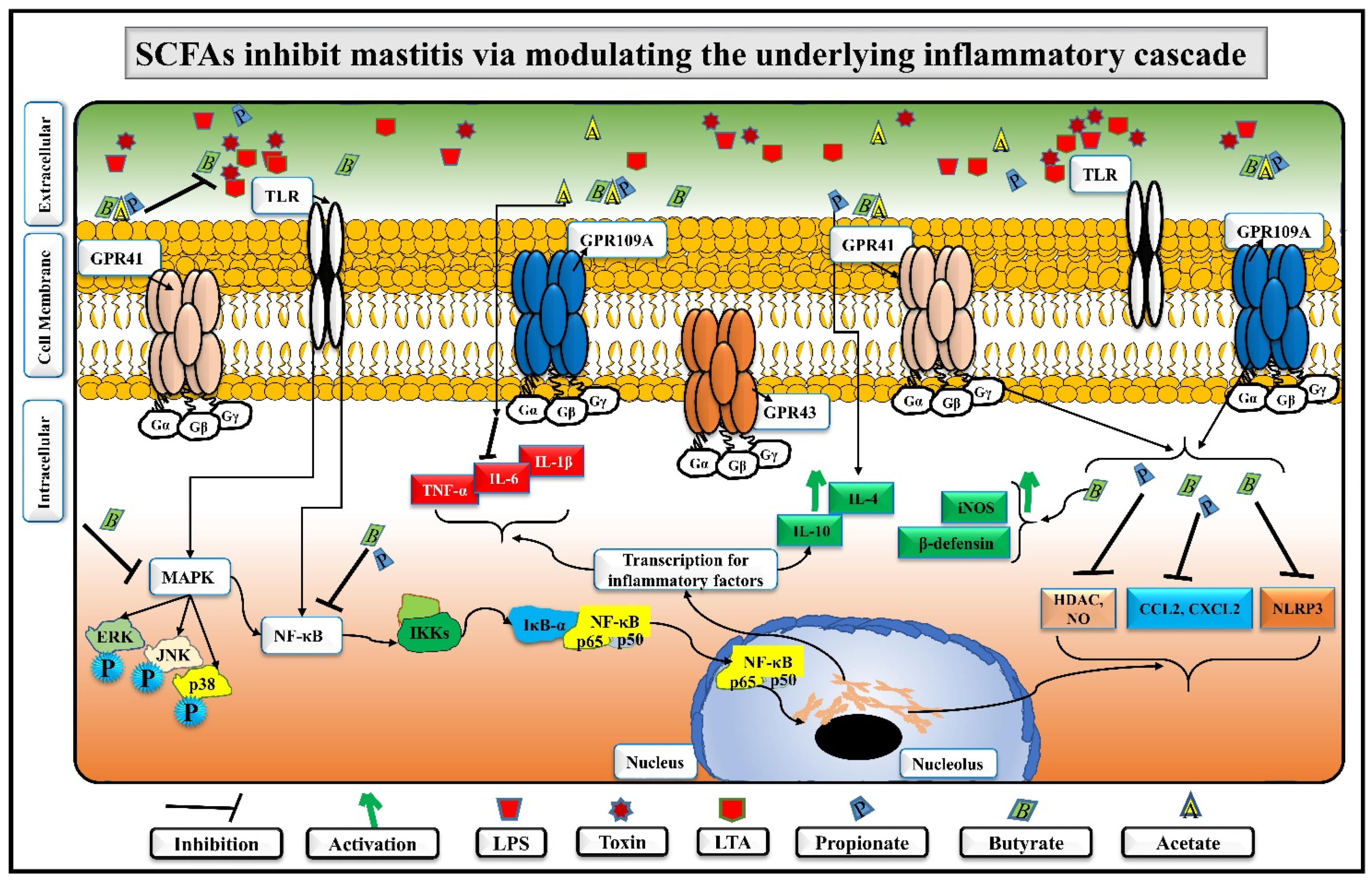
5. SCFAs Distinctly Alleviate Mastitis by Regulating Immune Homeostasis
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, T.; Kamran; Raziq, A.; Wazir, I.; Ullah, R.; Shah, P.; Ali, M.I.; Han, B.; Liu, G. Prevalence of Mastitis Pathogens and Antimicrobial Susceptibility of Isolates From Cattle and Buffaloes in Northwest of Pakistan. Front. Vet. Sci. 2021, 8, 746755. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.; Watson, C.J. The Mammary Microenvironment in Mastitis in Humans, Dairy Ruminants, Rabbits and Rodents: A One Health Focus. J. Mammary Gland Biol. Neoplasia 2018, 23, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Benić, M.; Mačešić, N.; Cvetnić, L.; Habrun, B.; Cvetnić, Ž.; Turk, R.; Đuričić, D.; Lojkic, M.; Dobranić, V.; Valpotic, H.; et al. Bovine mastitis: A persistent and evolving problem requiring novel approaches for its control—A review. Vet. Arh. 2018, 88, 535–557. [Google Scholar] [CrossRef]
- Lamari, I.; Mimoune, N.; Khelef, D. Effect of feed additive supplementation on bovine subclinical mastitisUčinak dodatka prehrani na supklinički mastitis krava. Vet. Stanica 2021, 52, 445–460. [Google Scholar] [CrossRef]
- Akhtar, M.; Guo, S.; Guo, Y.-f.; Zahoor, A.; Shaukat, A.; Chen, Y.; Umar, T.; Deng, P.G.; Guo, M. Upregulated-gene expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via TLRs following NF-κB and MAPKs in bovine mastitis. Acta Trop. 2020, 207, 105458. [Google Scholar] [CrossRef]
- Sordillo, L.M. Factors affecting mammary gland immunity and mastitis susceptibility. Livest. Prod. Sci. 2005, 98, 89–99. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef]
- Algammal, A.M.; Enany, M.E.; El-Tarabili, R.M.; Ghobashy, M.O.I.; Helmy, Y.A. Prevalence, Antimicrobial Resistance Profiles, Virulence and Enterotoxins-Determinant Genes of MRSA Isolated from Subclinical Bovine Mastitis in Egypt. Pathogens 2020, 9, 362. [Google Scholar] [CrossRef]
- Mimoune, N.; Saidi, R.; Benadjel, O.; Khelef, D.; Kaidi, R. Alternative treatment of bovine mastitis. Vet. Stn. 2021, 52, 639–649. [Google Scholar] [CrossRef]
- Shoaib, M.; Rahman, S.; Aqib, A.; Ashfaq, K.; Naveed, A.; Kulyar, M.; Bhutta, Z.; Younas, M.; Sarwar, I.; Naseer, M.; et al. Diversified Epidemiological Pattern and Antibiogram of mecA Gene in Staphylococcus aureus Isolates of Pets, Pet Owners and Environment. Pak. Vet. J. 2020, 40, 331–336. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Fu, Y.; Zhang, N. Targeting gut microbiota as a possible therapy for mastitis. Eur. J. Clin. Microbiol. 2019, 38, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Alva-Murillo, N.; Ochoa-Zarzosa, A.; López-Meza, J.E. Short chain fatty acids (propionic and hexanoic) decrease Staphylococcus aureus internalization into bovine mammary epithelial cells and modulate antimicrobial peptide expression. Vet. Microbiol. 2012, 155, 324–331. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Li, X.; Sun, B. Dynamic balancing of intestinal short-chain fatty acids: The crucial role of bacterial metabolism. Trends Food Sci. Technol. 2020, 100, 118–130. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. Chapter Three—The Role of Short-Chain Fatty Acids in Health and Disease. In Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 121, pp. 91–119. [Google Scholar]
- Colquhoun, C.; Duncan, M.; Grant, G. Inflammatory Bowel Diseases: Host-Microbial-Environmental Interactions in Dysbiosis. Diseases 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Guo, J.; Zhao, C.; Jiang, P.; Maimai, T.; Yanyi, L.; Cao, Y.; Fu, Y.; Zhang, N. The gut microbiota contributes to the development of Staphylococcus aureus-induced mastitis in mice. ISME J. 2020, 14, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Alva-Murillo, N.; López-Meza, J.E.; Ochoa-Zarzosa, A. Nonprofessional Phagocytic Cell Receptors Involved in Staphylococcus aureus Internalization. Biomed Res. Int. 2014, 2014, 538546. [Google Scholar] [CrossRef]
- Rainard, P.; Foucras, G.; Fitzgerald, J.R.; Watts, J.L.; Koop, G.; Middleton, J.R. Knowledge gaps and research priorities in Staphylococcus aureus mastitis control. Transbound. Emerg. Dis. 2018, 65 (Suppl. S1), 149–165. [Google Scholar] [CrossRef]
- Saidi, R.; Cantekin, Z.; Mimoune, N.; Ergun, Y.; Solmaz, H.; Khelef, D.; Kaidi, R. Investigation of the presence of slime production, VanA gene and antiseptic resistance genes in Staphylococci isolated from bovine mastitis in AlgeriaIstraživanje prisutnosti proizvodnje sluzi, VanA gena i gena zarezistenciju na antiseptike u stafilokoka izoliranih iz goveđegmastitisa u Alžiru. Vet. Stn. 2020, 52, 57–63. [Google Scholar] [CrossRef]
- Foster, T.J. Surface Proteins of Staphylococcus aureus. Microbiol. Spectr. 2019, 7, GPP3–GPP46. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Bischoff, M.; Lattar, S.M.; Noto Llana, M.; Pförtner, H.; Niemann, S.; Geraci, J.; Van de Vyver, H.; Fraunholz, M.J.; Cheung, A.L.; et al. Sigma Factor SigB Is Crucial to Mediate Staphylococcus aureus Adaptation during Chronic Infections. PLoS Pathog. 2015, 11, e1004870. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, E.A.L.; Picech, F.; Renna, M.S.; Baravalle, C.; Andreotti, C.S.; Russi, R.; Calvinho, L.F.; Diez, C.; Dallard, B.E. Detection of Staphylococcus aureus adhesion and biofilm-producing genes and their expression during internalization in bovine mammary epithelial cells. Vet. Microbiol. 2016, 183, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Josse, J.; Laurent, F.; Diot, A. Staphylococcal Adhesion and Host Cell Invasion: Fibronectin-Binding and Other Mechanisms. Front. Microbiol. 2017, 8, 2433. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.; Chen, W.; Ji, Q. Structural Basis of Staphylococcus aureus Surface Protein SdrC. Biochemistry 2020, 59, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Gogoi-Tiwari, J.; Williams, V.; Waryah, C.B.; Mathavan, S.; Tiwari, H.K.; Costantino, P.; Mukkur, T. Intramammary Immunization of Pregnant Mice with Staphylococcal Protein A Reduces the Post-Challenge Mammary Gland Bacterial Load but Not Pathology. PLoS ONE 2016, 11, e0148383. [Google Scholar] [CrossRef]
- Zaatout, N.; Ayachi, A.; Kecha, M. Staphylococcus aureus persistence properties associated with bovine mastitis and alternative therapeutic modalities. J. Appl. Microbiol. 2020, 129, 1102–1119. [Google Scholar] [CrossRef]
- Wei, Z.; Xiao, C.; Guo, C.; Zhang, X.; Wang, Y.; Wang, J.; Yang, Z.; Fu, Y. Sodium acetate inhibits Staphylococcus aureus internalization into bovine mammary epithelial cells by inhibiting NF-κB activation. Microb. Pathog. 2017, 107, 116–121. [Google Scholar] [CrossRef]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef]
- Nair, M.K.M.; Joy, J.; Vasudevan, P.; Hinckley, L.; Hoagland, T.A.; Venkitanarayanan, K.S. Antibacterial Effect of Caprylic Acid and Monocaprylin on Major Bacterial Mastitis Pathogens. J. Dairy Sci. 2005, 88, 3488–3495. [Google Scholar] [CrossRef]
- Assis, B.S.; Germon, P.; Silva, A.; Even, S.; Nicoli, J.; Loir, Y.L. Lactococcus lactis V7 inhibits the cell invasion of bovine mammary epithelial cells by Escherichia coli and Staphylococcus aureus. Benef. Microbes 2015, 6, 879–886. [Google Scholar] [CrossRef]
- Huang, W.; Guo, H.L.; Deng, X.; Zhu, T.T.; Xiong, J.F.; Xu, Y.H.; Xu, Y. Short-Chain Fatty Acids Inhibit Oxidative Stress and Inflammation in Mesangial Cells Induced by High Glucose and Lipopolysaccharide. Exp. Clin. Endocrinol. Diabetes 2017, 125, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; van Esch, B.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.; Jiang, N.; Zhang, A. Sodium Phenylbutyrate Ameliorates Inflammatory Response Induced by Staphylococcus aureus Lipoteichoic Acid via Suppressing TLR2/NF-κB/NLRP3 Pathways in MAC-T Cells. Molecules 2018, 23, 3056. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Zarzosa, A.; Villarreal-Fernández, E.; Cano-Camacho, H.; López-Meza, J.E. Sodium butyrate inhibits Staphylococcus aureus internalization in bovine mammary epithelial cells and induces the expression of antimicrobial peptide genes. Microb. Pathog. 2009, 47, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Frutis-Murillo, M.; Sandoval-Carrillo, M.A.; Alva-Murillo, N.; Ochoa-Zarzosa, A.; López-Meza, J.E. Immunomodulatory molecules regulate adhesin gene expression in Staphylococcus aureus: Effect on bacterial internalization into bovine mammary epithelial cells. Microb. Pathog. 2019, 131, 15–21. [Google Scholar] [CrossRef]
- Alva-Murillo, N.; Medina-Estrada, I.; Báez-Magaña, M.; Ochoa-Zarzosa, A.; López-Meza, J.E. The activation of the TLR2/p38 pathway by sodium butyrate in bovine mammary epithelial cells is involved in the reduction of Staphylococcus aureus internalization. Mol. Immunol. 2015, 68, 445–455. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Rakoff-Nahoum, S. Understanding Competition and Cooperation within the Mammalian Gut Microbiome. Curr. Biol. 2019, 29, R538–R544. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, J.; Xi, X.; Ding, J.; Wang, H.; Zhang, H.; Kwok, L.Y. Bovine mastitis may be associated with the deprivation of gut Lactobacillus. Benef. Microbes 2016, 7, 95–102. [Google Scholar] [CrossRef]
- Chopyk, D.M.; Grakoui, A. Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders. Gastroenterology 2020, 159, 849–863. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, M.; Wang, H.; Zhang, F.; Xue, F.; Hua, D.; Liu, J.; et al. Rumen microbiome structure and metabolites activity in dairy cows with clinical and subclinical mastitis. J. Anim. Sci. Biotechnol. 2021, 12, 36. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Mu, R.; Guo, J.; Zhao, C.; Cao, Y.; Zhang, N.; Fu, Y. The Rumen Microbiota Contributes to the Development of Mastitis in Dairy Cows. Microbiol. Spectr. 2022, 10, e0251221. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Álvarez-Mercado, A.I.; Ruiz-Marín, C.M.; Reina-Pérez, I.; Pérez-Alonso, A.J.; Sánchez-Andujar, M.B.; Torné, P.; Gallart-Aragón, T.; Sánchez-Barrón, M.T.; Reyes Lartategui, S.; et al. Association of breast and gut microbiota dysbiosis and the risk of breast cancer: A case-control clinical study. BMC Cancer 2019, 19, 495. [Google Scholar] [CrossRef]
- Pang, M.; Xie, X.; Bao, H.; Sun, L.; He, T.; Zhao, H.; Zhou, Y.; Zhang, L.; Zhang, H.; Wei, R.; et al. Insights Into the Bovine Milk Microbiota in Dairy Farms With Different Incidence Rates of Subclinical Mastitis. Front. Microbiol. 2018, 9, 2379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Y.; Peng, Z.; Sun, X.; Sun, G.; Yuan, X.; Li, X.; Liu, G. Subacute ruminal acidosis suppressed the expression of MCT1 in rumen of cows. J. Cell. Physiol. 2019, 234, 11734–11745. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Xu, Z.; Shen, Z.; Lu, Z. The Regulation of Ruminal Short-Chain Fatty Acids on the Functions of Rumen Barriers. Front. Physiol. 2019, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.N.; Istiaq, A.; Clement, R.A.; Sultana, M.; Crandall, K.A.; Siddiki, A.Z.; Hossain, M.A. Metagenomic deep sequencing reveals association of microbiome signature with functional biases in bovine mastitis. Sci. Rep. 2019, 9, 13536. [Google Scholar] [CrossRef] [PubMed]
- Cvetnić, L.; Samardžija, M.; Duvnjak, S.; Habrun, B.; Cvetnić, M.; Jaki Tkalec, V.; Đuričić, D.; Benić, M. Multi Locus Sequence Typing and spa Typing of Staphylococcus Aureus Isolated from the Milk of Cows with Subclinical Mastitis in Croatia. Microorganisms 2021, 9, 725. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Microbiol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Zhuge, A.; Li, B.; Yuan, Y.; Lv, L.; Li, Y.; Wu, J.; Yang, L.; Bian, X.; Wang, K.; Wang, Q.; et al. Lactobacillus salivarius LI01 encapsulated in alginate-pectin microgels ameliorates D-galactosamine-induced acute liver injury in rats. Appl. Microbiol. Biotechnol. 2020, 104, 7437–7455. [Google Scholar] [CrossRef]
- Rainard, P.; Foucras, G. A Critical Appraisal of Probiotics for Mastitis Control. Front. Vet. Sci. 2018, 5, 251. [Google Scholar] [CrossRef]
- Qiao, J.; Kwok, L.; Zhang, J.; Gao, P.; Zheng, Y.; Guo, Z.; Hou, Q.; Huo, D.; Huang, W.; Zhang, H. Reduction of Lactobacillus in the milks of cows with subclinical mastitis. Benef. Microbes 2015, 6, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-T.; Li, K.-Y.; Tu, P.-W.; Ho, S.-T.; Hsu, C.-C.; Hsieh, J.-C.; Chen, M.-J. Investigating the Reciprocal Interrelationships among the Ruminal Microbiota, Metabolome, and Mastitis in Early Lactating Holstein Dairy Cows. Animals 2021, 11, 3108. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xu, H.; Xu, J.; Zhao, H.; Lin, Q.; Zhou, Y.; Nie, Y. Sodium Butyrate Ameliorates Gut Microbiota Dysbiosis in Lupus-Like Mice. Front. Nutr. 2020, 7, 604283. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, A.S.; Pezo, R.C. Untapped “-omics”: The microbial metagenome, estrobolome, and their influence on the development of breast cancer and response to treatment. Breast Cancer Res. Treat. 2020, 179, 287–300. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Guo, W.; Liu, B.; Yin, Y.; Kan, X.; Gong, Q.; Li, Y.; Cao, Y.; Wang, J.; Xu, D.; Ma, H.; et al. Licochalcone A Protects the Blood-Milk Barrier Integrity and Relieves the Inflammatory Response in LPS-Induced Mastitis. Front. Immunol. 2019, 10, 287. [Google Scholar] [CrossRef]
- Nguyen, D.A.; Neville, M.C. Tight junction regulation in the mammary gland. J. Mammary Gland Biol. Neoplasia 1998, 3, 233–246. [Google Scholar] [CrossRef]
- Bogni, C.; Odierno, L.; Raspanti, C.; Giraudo, J.; Larriestra, A.; Reinoso, E.; Lasagno, M.; Ferrari, M.; Ducrós, E.; Frigerio, C. War against mastitis: Current concepts on controlling bovine mastitis pathogens. In Science against Microbial Pathogens: Communicafing Current Research Technological Advances; Formatex Research Center: Badajoz, Spain, 2011; pp. 483–494. [Google Scholar]
- Abril, A.G.; Gonzalez-Villa, T.; Barros-Velázquez, J.; Cañas, B.; Sánchez-Pérez, A.; Calo-Mata, P.; Carrera, M. Staphylococcus aureus Exotoxins and Their Detection in the Dairy Industry and Mastitis. Toxins 2020, 12, 537. [Google Scholar] [CrossRef]
- Al-Asmakh, M.; Stukenborg, J.B.; Reda, A.; Anuar, F.; Strand, M.L.; Hedin, L.; Pettersson, S.; Söder, O. The gut microbiota and developmental programming of the testis in mice. PLoS ONE 2014, 9, e103809. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Wellnitz, O.; Bruckmaier, R.M. Invited review: The role of the blood–milk barrier and its manipulation for the efficacy of the mammary immune response and milk production. J. Dairy Sci. 2021, 104, 6376–6388. [Google Scholar] [CrossRef] [PubMed]
- Wellnitz, O.; Wall, S.K.; Saudenova, M.; Bruckmaier, R.M. Effect of intramammary administration of prednisolone on the blood-milk barrier during the immune response of the mammary gland to lipopolysaccharide. Am. J. Vet. Res. 2014, 75, 595–601. [Google Scholar] [CrossRef]
- Baumgartner, H.K.; Rudolph, M.C.; Ramanathan, P.; Burns, V.; Webb, P.; Bitler, B.G.; Stein, T.; Kobayashi, K.; Neville, M.C. Developmental Expression of Claudins in the Mammary Gland. J. Mammary Gland Biol. Neoplasia 2017, 22, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Spiljar, M.; Merkler, D.; Trajkovski, M. The Immune System Bridges the Gut Microbiota with Systemic Energy Homeostasis: Focus on TLRs, Mucosal Barrier, and SCFAs. Front. Immunol. 2017, 8, 1353. [Google Scholar] [CrossRef]
- Ali, I.; Yang, M.; Wang, Y.; Yang, C.; Shafiq, M.; Wang, G.; Li, L. Sodium propionate protect the blood-milk barrier integrity, relieve lipopolysaccharide-induced inflammatory injury and cells apoptosis. Life Sci. 2021, 270, 119138. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Oyama, S.; Numata, A.; Rahman, M.M.; Kumura, H. Lipopolysaccharide disrupts the milk-blood barrier by modulating claudins in mammary alveolar tight junctions. PLoS ONE 2013, 8, e62187. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Z.; Zhang, X.; Wang, Y.; Yang, Z.; Fu, Y. Propionate Protects against Lipopolysaccharide-Induced Mastitis in Mice by Restoring Blood-Milk Barrier Disruption and Suppressing Inflammatory Response. Front. Immunol. 2017, 8, 1108. [Google Scholar] [CrossRef]
- Wang, J.J.; Wei, Z.K.; Zhang, X.; Wang, Y.N.; Fu, Y.H.; Yang, Z.T. Butyrate protects against disruption of the blood-milk barrier and moderates inflammatory responses in a model of mastitis induced by lipopolysaccharide. Br. J. Pharmacol. 2017, 174, 3811–3822. [Google Scholar] [CrossRef]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef]
- Herry, V.; Gitton, C.; Tabouret, G.; Répérant, M.; Forge, L.; Tasca, C.; Gilbert, F.B.; Guitton, E.; Barc, C.; Staub, C.; et al. Local immunization impacts the response of dairy cows to Escherichia coli mastitis. Sci. Rep. 2017, 7, 3441. [Google Scholar] [CrossRef]
- Pérez-Reytor, D.; Puebla, C.; Karahanian, E.; García, K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef] [PubMed]
- Soderholm, A.T.; Pedicord, V.A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Weber, G.J.; van Sambeek, D.M.; Soares, J.W.; Racicot, K.; Breault, D.T. Intestinal enteroids recapitulate the effects of short-chain fatty acids on the intestinal epithelium. PLoS ONE 2020, 15, e0230231. [Google Scholar] [CrossRef]
- Gonçalves, P.; Araújo, J.R.; Di Santo, J.P. A Cross-Talk Between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Crispi, K.; Atalla, H.; Miglior, F.; Mallard, B.A. Bovine mastitis: Frontiers in immunogenetics. Front. Immunol. 2014, 5, 493. [Google Scholar] [CrossRef] [PubMed]
- Rainard, P.; Cunha, P.; Gilbert, F.B. Innate and Adaptive Immunity Synergize to Trigger Inflammation in the Mammary Gland. PLoS ONE 2016, 11, e0154172. [Google Scholar] [CrossRef]
- Meijer, K.; de Vos, P.; Priebe, M.G. Butyrate and other short-chain fatty acids as modulators of immunity: What relevance for health? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 715–721. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Liu, Z.; Jiang, A.; Li, S.; Wu, D.; Zhang, Y.; Zhu, X.; Zhou, E.; Wei, Z.; et al. Sodium Butyrate Alleviates Lipopolysaccharide-Induced Inflammatory Responses by Down-Regulation of NF-κB, NLRP3 Signaling Pathway, and Activating Histone Acetylation in Bovine Macrophages. Front. Vet. Sci. 2020, 7, 579674. [Google Scholar] [CrossRef]
- Ranjbar, R.; Vahdati, S.N.; Tavakoli, S.; Khodaie, R.; Behboudi, H. Immunomodulatory roles of microbiota-derived short-chain fatty acids in bacterial infections. Biomed. Pharm. 2021, 141, 111817. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Laborda-Illanes, A.; Sanchez-Alcoholado, L.; Dominguez-Recio, M.E.; Jimenez-Rodriguez, B.; Lavado, R.; Comino-Méndez, I.; Alba, E.; Queipo-Ortuño, M.I. Breast and Gut Microbiota Action Mechanisms in Breast Cancer Pathogenesis and Treatment. Cancers 2020, 12, 2465. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell. Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, Y.; Zhang, R.; He, T.; Huang, G.; Tian, K.; Liu, J.; Chen, J.; Dong, G. Sodium Butyrate More Effectively Mitigates the Negative Effects of High-Concentrate Diet in Dairy Cows than Sodium β-Hydroxybutyrate via Reducing Free Bacterial Cell Wall Components in Rumen Fluid and Plasma. Toxins 2021, 13, 352. [Google Scholar] [CrossRef]
- Akhtar, M.; Shaukat, A.; Zahoor, A.; Chen, Y.; Wang, Y.; Yang, M.; Umar, T.; Guo, M.; Deng, G. Hederacoside-C Inhibition of Staphylococcus aureus-Induced Mastitis via TLR2 & TLR4 and Their Downstream Signaling NF-κB and MAPKs Pathways In Vivo and In Vitro. Inflammation 2020, 43, 579–594. [Google Scholar] [CrossRef]
- Sun, X.; Luo, S.; Jiang, C.; Tang, Y.; Cao, Z.; Jia, H.; Xu, Q.; Zhao, C.; Loor, J.J.; Xu, C. Sodium butyrate reduces bovine mammary epithelial cell inflammatory responses induced by exogenous lipopolysaccharide, by inactivating NF-κB signaling. J. Dairy Sci. 2020, 103, 8388–8397. [Google Scholar] [CrossRef]
- Silva, L.G.; Ferguson, B.S.; Avila, A.S.; Faciola, A.P. Sodium propionate and sodium butyrate effects on histone deacetylase (HDAC) activity, histone acetylation, and inflammatory gene expression in bovine mammary epithelial cells. J. Anim. Sci. 2018, 96, 5244–5252. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Liu, Y.; Xiao, N.; Suo, H.; Xie, K.; Yang, C.; Wu, C. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation 2012, 35, 1676–1684. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, H.Y.; Kim, M.G.; Jeong, S.; Yun, C.H.; Han, S.H. Short-chain Fatty Acids Inhibit Staphylococcal Lipoprotein-induced Nitric Oxide Production in Murine Macrophages. Immune Netw. 2019, 19, e9. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Koch, L.; Frommhold, D.; Buschmann, K.; Kuss, N.; Poeschl, J.; Ruef, P. LPS- and LTA-induced expression of IL-6 and TNF-α in neonatal and adult blood: Role of MAPKs and NF-κB. Mediat. Inflamm. 2014, 2014, 283126. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, M.; Naqvi, S.U.-A.-S.; Liu, Q.; Pan, H.; Ma, Z.; Kong, N.; Chen, Y.; Shi, D.; Kulyar, M.F.-e.-A.; Khan, J.A.; et al. Short Chain Fatty Acids (SCFAs) Are the Potential Immunomodulatory Metabolites in Controlling Staphylococcus aureus-Mediated Mastitis. Nutrients 2022, 14, 3687. https://doi.org/10.3390/nu14183687
Akhtar M, Naqvi SU-A-S, Liu Q, Pan H, Ma Z, Kong N, Chen Y, Shi D, Kulyar MF-e-A, Khan JA, et al. Short Chain Fatty Acids (SCFAs) Are the Potential Immunomodulatory Metabolites in Controlling Staphylococcus aureus-Mediated Mastitis. Nutrients. 2022; 14(18):3687. https://doi.org/10.3390/nu14183687
Chicago/Turabian StyleAkhtar, Muhammad, Syed Umair-Ali-Shah Naqvi, Qiyao Liu, Hong Pan, Ziyu Ma, Na Kong, Yan Chen, Deshi Shi, Muhammad Fakhar-e-Alam Kulyar, Jawaria Ali Khan, and et al. 2022. "Short Chain Fatty Acids (SCFAs) Are the Potential Immunomodulatory Metabolites in Controlling Staphylococcus aureus-Mediated Mastitis" Nutrients 14, no. 18: 3687. https://doi.org/10.3390/nu14183687
APA StyleAkhtar, M., Naqvi, S. U.-A.-S., Liu, Q., Pan, H., Ma, Z., Kong, N., Chen, Y., Shi, D., Kulyar, M. F.-e.-A., Khan, J. A., & Liu, H. (2022). Short Chain Fatty Acids (SCFAs) Are the Potential Immunomodulatory Metabolites in Controlling Staphylococcus aureus-Mediated Mastitis. Nutrients, 14(18), 3687. https://doi.org/10.3390/nu14183687