Surface Layer Protein Pattern of Levilactobacillus brevis Strains Investigated by Proteomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. DNA Extraction and Biotypization of Strains
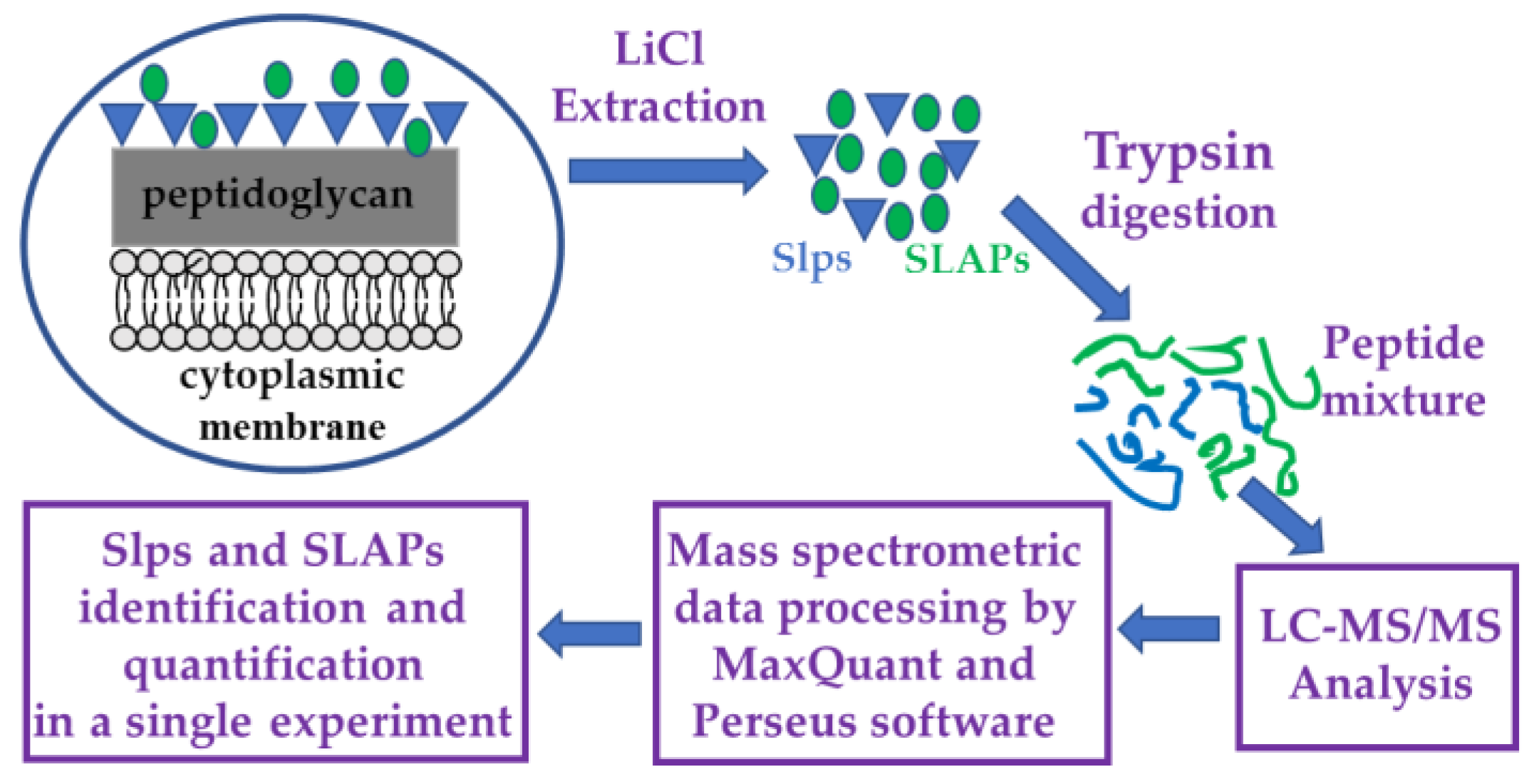
2.3. Extraction and Digestion of Surface Proteins for Proteomic Analyses
2.4. LC-MS/MS Analysis, Protein Identification, and Label-Free Quantification (LFQ) Analyses
2.5. Bioinformatics and Functional Analyses
3. Results and Discussion
3.1. Proteomic Analysis of Surface Layer Proteins (Slps) of L. brevis Strains
3.2. Proteomic Analysis of Surface Layer Associated Proteins (SLAPS) of L. brevis Strains
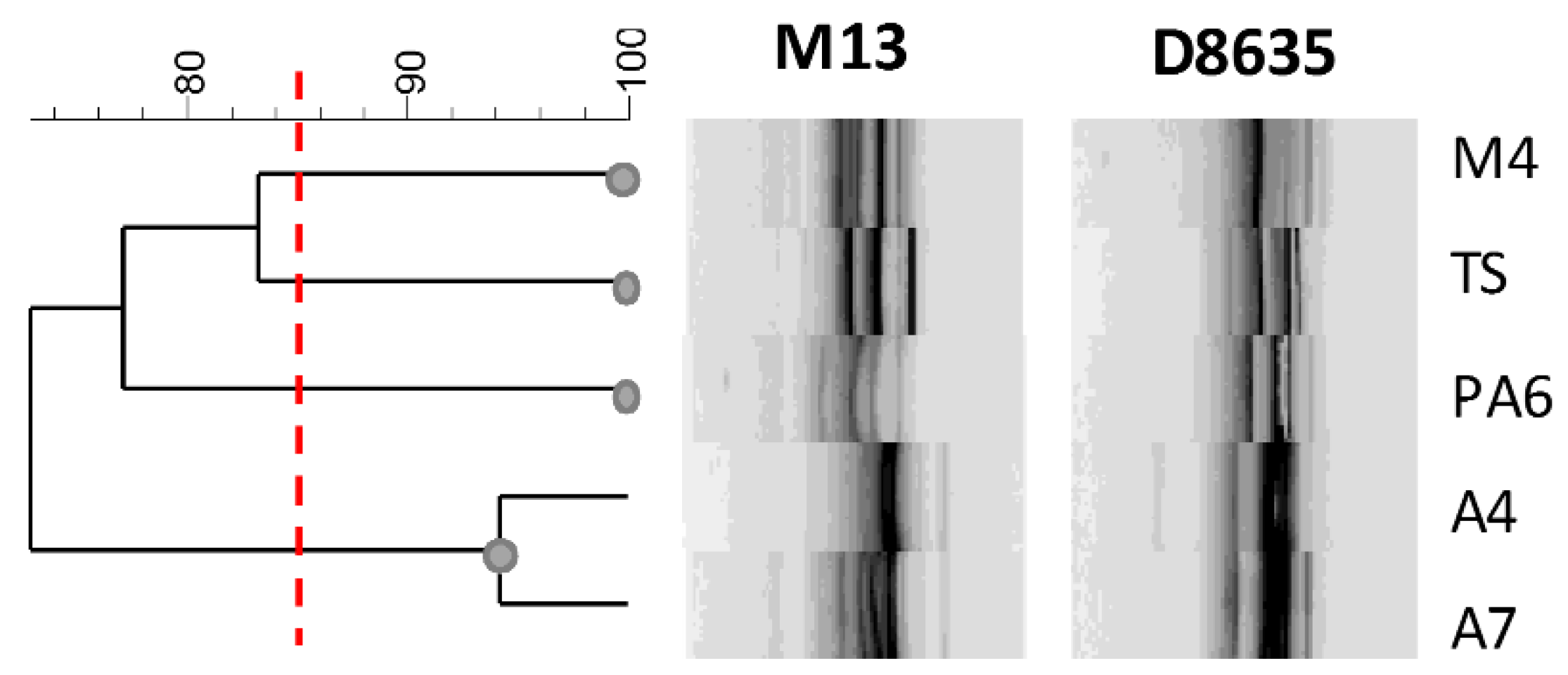
3.3. Biotypization of Levilactobacillus brevis Strains
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010, 8, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Fagan, R.P.; Fairweather, N.F. Biogenesis and functions of bacterial S-layers. Nat. Rev. Microbiol. 2014, 12, 211–222. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Schuster, B.; Egelseer, E.M.; Pum, D. S-layers: Principles and applications. FEMS Microbiol. Rev. 2014, 38, 823–864. [Google Scholar] [CrossRef] [PubMed]
- Hynönen, U.; Palva, A. Lactobacillus surface layer proteins: Structure.; function and applications. Appl. Microbiol. Biotechnol. 2013, 97, 5225–5243. [Google Scholar] [CrossRef]
- Konstantinov, S.R.; Smidt, H.; de Vos, W.M.; Bruijns, S.C.; Singh, S.K.; Valence, F.; Molle, D.; Lortal, S.; Altermann, E.; Klaenhammer, T.R.; et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. USA 2008, 105, 19474–19479. [Google Scholar] [CrossRef]
- Uroić, K.; Novak, J.; Hynönen, U.; Pietilä, T.; Pavunc, A.; Kant, R.; Kos, B.; Palva, A.; Šušković, J. The role of S-layer in adhesive and immunomodulating properties of probiotic starter culture Lactobacillus brevis D6 isolated from artisanal smoked fresh cheese. LWT—Food Sci. Technol. 2016, 69, 623–632. [Google Scholar] [CrossRef]
- Johnson, B.; Selle, K.; O’Flaherty, S.; Goh, Y.J.; Klaenhammer, T. Identification of extracellular surface-layer associated proteins in Lactobacillus acidophilus NCFM. Microbiology 2013, 159, 2269–2282. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, F.L.R.; Rabah, H.; De Oliveira Carvalho, R.D.; Gaucher, F.; Cordeiro, B.F.; da Silva, S.H.; Le Loir, Y.; Azevedo, V.; Jan, G. Extractable bacterial surface proteins in probiotic-host interaction. Front. Microbiol. 2018, 9, 645. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Lippolis, R.; Mazzeo, M.F. Proteomics for the investigation of surface-exposed proteins in probiotics. Front. Nutr. 2019, 6, 52. [Google Scholar] [CrossRef]
- Klotz, C.; O’Flaherty, S.; Goh, Y.J.; Barrangou, R. Investigating the effect of growth phase on the surface-layer associated proteome of Lactobacillus acidophilus using quantitative proteomics. Front. Microbiol. 2017, 8, 2174. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, C.J. Intracellular/surface moonlighting proteins that aid in the attachment of gut microbiota to the host. AIMS Microbiol. 2019, 5, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, M.; Mahony, J.; Kelleher, P.; Roberts, R.J.; O’Sullivan, T.; Geertman, J.A.; van Sinderen, D. Comparative genome analysis of the Lactobacillus brevis species. BMC Genomics 2019, 20, 416. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Reale, A. A holistic review on Euro-Asian lactic acid bacteria fermented cereals and vegetables. Microorganisms 2020, 8, 1176. [Google Scholar] [CrossRef]
- Olekhnovich, E.I.; Batotsyrenova, E.G.; Yunes, R.A.; Kashuro, V.A.; Poluektova, E.U.; Veselovsky, V.A.; Ilina, E.N.; Danilenko, V.N.; Klimina, K.M. The effects of Levilactobacillus brevis on the physiological parameters and gut microbiota composition of rats subjected to desynchronosis. Microb. Cell Fact. 2021, 20, 226. [Google Scholar] [CrossRef]
- Reale, A.; Tremonte, P.; Succi, M.; Sorrentino, E.; Coppola, R. Exploration of lactic acid bacteria ecosystem of sourdoughs from the Molise region. Ann. Microbiol. 2005, 55, 17–22. [Google Scholar]
- Wu, Q.; Shah, N.P. High γ-aminobutyric acid production from lactic acid bacteria: Emphasis on Lactobacillus brevis as a functional dairy starter. Crit. Rev. Food. Sci. Nutr. 2017, 57, 3661–3672. [Google Scholar] [CrossRef]
- Abdelazez, A.; Abdelmotaal, H.; Evivie, S.E.; Melak, S.; Jia, F.F.; Khoso, M.H.; Zhu, Z.T.; Zhang, L.J.; Sami, R.; Meng, X.C. Screening potential probiotic characteristics of Lactobacillus brevis strains in vitro and intervention effect on type I diabetes in vivo. Biomed. Res. Int. 2018, 2018, 7356173. [Google Scholar] [CrossRef]
- Fang, F.; Xu, J.; Li, Q.; Xia, X.; Du, G. Characterization of a Lactobacillus brevis strain with potential oral probiotic properties. BMC Microbiol. 2018, 18, 221. [Google Scholar] [CrossRef]
- Rönkä, E.; Malinen, E.; Saarela, M.; Rinta-Koski, M.; Aarnikunnas, J.; Palva, A. Probiotic and milk technological properties of Lactobacillus brevis. Int. J. Food Microbiol. 2003, 83, 63–74. [Google Scholar] [CrossRef]
- Singh, V.; Ganger, S.; Patil, S. Characterization of Lactobacillus brevis with potential probiotic properties and biofilm inhibition against Pseudomonas aeruginosa. Proceedings 2020, 66, 14. [Google Scholar] [CrossRef]
- Banic, M.; Uroic, K.; Leboš Pavunc, A.; Novak, J.; Zoric, K.; Durgo, K.; Petkovic, H.; Jamnik, P.; Kazazic, S.; Kazazic, S.; et al. Characterization of S-layer proteins of potential probiotic starter culture Lactobacillus brevis SF9B isolated from sauerkraut. LWT-Food Sci. Technol. 2018, 93, 257–267. [Google Scholar] [CrossRef]
- Suzuki, S.; Yokota, K.; Igimi, S.; Kajikawa, A. Comparative analysis of immunological properties of S-layer proteins isolated from Lactobacillus strains. Microbiology 2019, 165, 188–196. [Google Scholar] [CrossRef]
- Fraunhofer, M.E.; Geissler, A.J.; Jakob, F.; Vogel, R.F. Multiple genome sequences of exopolysaccharide-producing, brewery associated Lactobacillus brevis strains. Genome Announc. 2017, 5, e00585-17. [Google Scholar] [CrossRef]
- Jakava-Viljanen, M.; Avall-Jääskeläinen, S.; Messner, P.; Sleytr, U.B.; Palva, A. Isolation of three new surface layer protein genes (slp) from Lactobacillus brevis ATCC 14869 and characterization of the change in their expression under aerated and anaerobic conditions. J. Bacteriol. 2002, 184, 6786–6795. [Google Scholar] [CrossRef]
- Avall-Jääskeläinen, S.; Hynönen, U.; Ilk, N.; Pum, D.; Sleytr, U.B.; Palva, A. Identification and characterization of domains responsible for self-assembly and cell wall binding of the surface layer protein of Lactobacillus brevis ATCC 8287. BMC Microbiol. 2008, 8, 165. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Zotta, T.; Ianniello, R.G.; Mamone, G.; Di Renzo, T. Selection criteria of lactic acid bacteria to be used as starter for sweet and salty leavened baked products. LWT 2020, 133, 110092. [Google Scholar] [CrossRef]
- Reale, A.; Di Renzo, T.; Boscaino, F.; Nazzaro, F.; Fratianni, F.; Aponte, M. Lactic acid bacteria biota and aroma profile of italian traditional sourdoughs from the irpinian area in Italy. Front. Microbiol. 2019, 10, 1621. [Google Scholar] [CrossRef]
- Reale, A.; Di Renzo, T.; Succi, M.; Tremonte, P.; Coppola, R.; Sorrentino, E. Identification of lactobacilli isolated in traditional ripe wheat sourdoughs by using molecular methods. World J. Microbiol. Biotechnol. 2011, 27, 237–244. [Google Scholar] [CrossRef]
- Querol, A.; Barrio, E.; Ramon, D. A comparative study of different methods of yeast strains characterization. Syst. Appl. Microbiol. 1992, 15, 439–446. [Google Scholar] [CrossRef]
- Huey, B.; Hall, J. Hypervariable DNA fingerprinting in Escherichia coli. Minisatellite probe from bacteriophage M13. J. Bacteriol. 1989, 171, 2528–2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akopyanz, N.; Bukanov, N.O.; Westblom, T.U.; Kresovich, S.; Berg, D.E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR based RAPD fingerprinting. Nucleic Acids Res. 1992, 20, 5137–5142. [Google Scholar] [CrossRef] [PubMed]
- Lortal, S.; Van Heijenoort, J.; Gruber, K.; Sleytr, U.B. S-layer of Lactobacillus helveticus ATCC 12046: Isolation, chemical characterization and re-formation after extraction with lithium chloride. J. Gen. Microbiol. 1992, 138, 611–618. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protocols 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Zybailov, B.; Mosley, A.L.; Sardiu, M.E.; Coleman, M.K.; Florens, L.; Washburn, M.P. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J. Proteome Res. 2006, 5, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Chen, C.; Liu, H.; Zabad, S.; Rivera, N.; Rowin, E.; Hassan, M.; Gomez De Jesus, S.M.; Llinás Santos, P.S.; Kravchenko, K.; Mikhova, M.; et al. MoonProt 3.0: An update of the moonlighting proteins database. Nucleic Acids Res. 2021, 49, D368–D372. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Vidgrén, G.; Palva, I.; Pakkanen, R.; Lounatmaa, K.; Palva, A. S-layer protein gene of Lactobacillus brevis: Cloning by polymerase chain reaction and determination of the nucleotide sequence. J. Bacteriol. 1992, 174, 7419–7427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Leeuw, E.; Li, X.; Lu, W. Binding characteristics of the Lactobacillus brevis ATCC 8287 surface layer to extracellular matrix proteins. FEMS Microbiol. Lett. 2006, 260, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Prado Acosta, M.; Goyette-Desjardins, G.; Scheffel, J.; Dudeck, A.; Ruland, J.; Lepenies, B. S-layer from Lactobacillus brevis modulates antigen-presenting cell functions via the Mincle-Syk-Card9 axis. Front. Immunol. 2021, 12, 602067. [Google Scholar] [CrossRef] [PubMed]
- Prado Acosta, M.; Ruzal, S.M.; Cordo, S.M. S-layer proteins from Lactobacillus sp. inhibit bacterial infection by blockage of DC-SIGN cell receptor. Int. J. Biol. Macromol. 2016, 92, 998–1005. [Google Scholar] [CrossRef]
- Vollmer, W.; Joris, B.; Charlier, P.; Foster, S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 2008, 32, 259–286. [Google Scholar] [CrossRef] [Green Version]
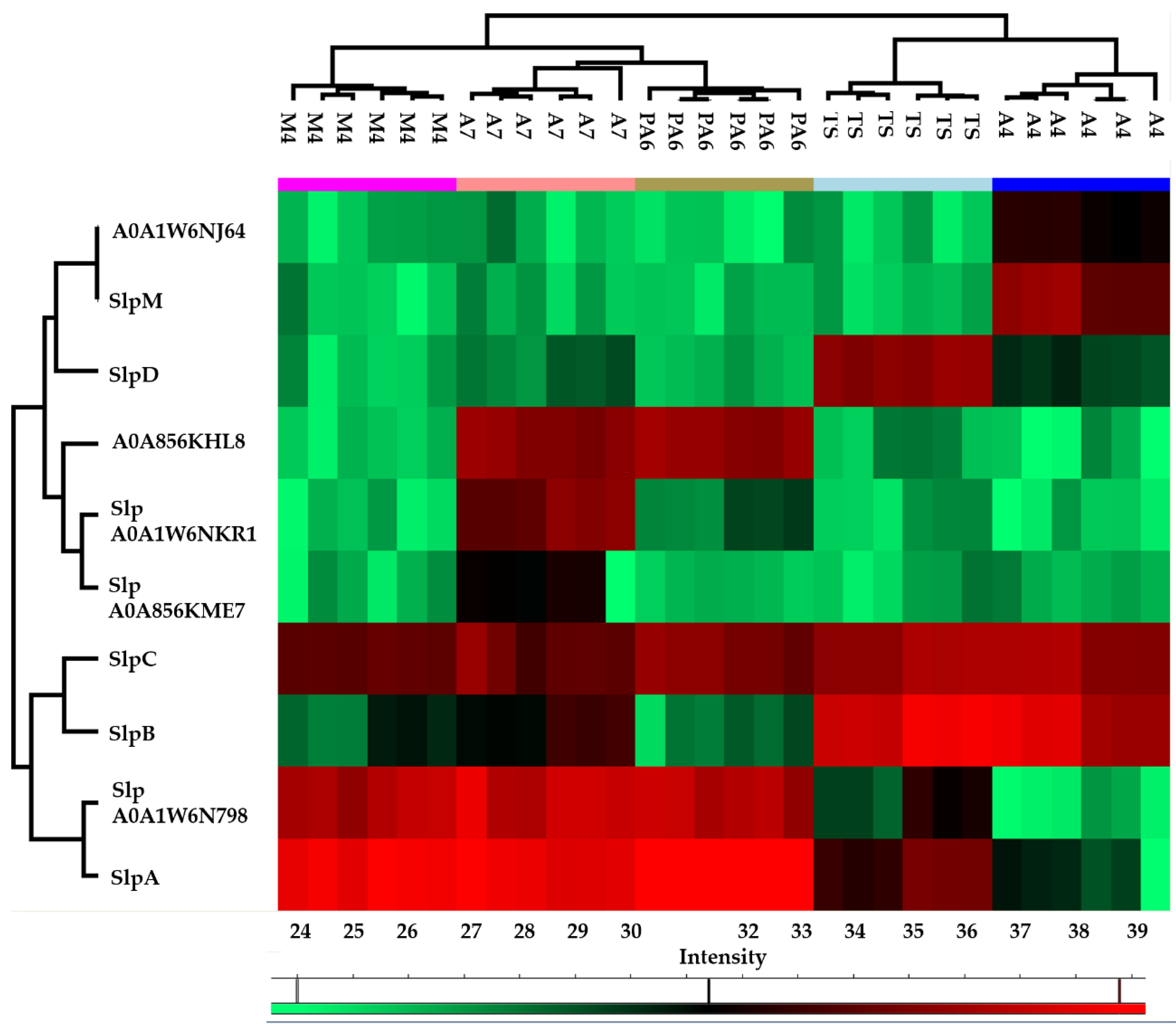
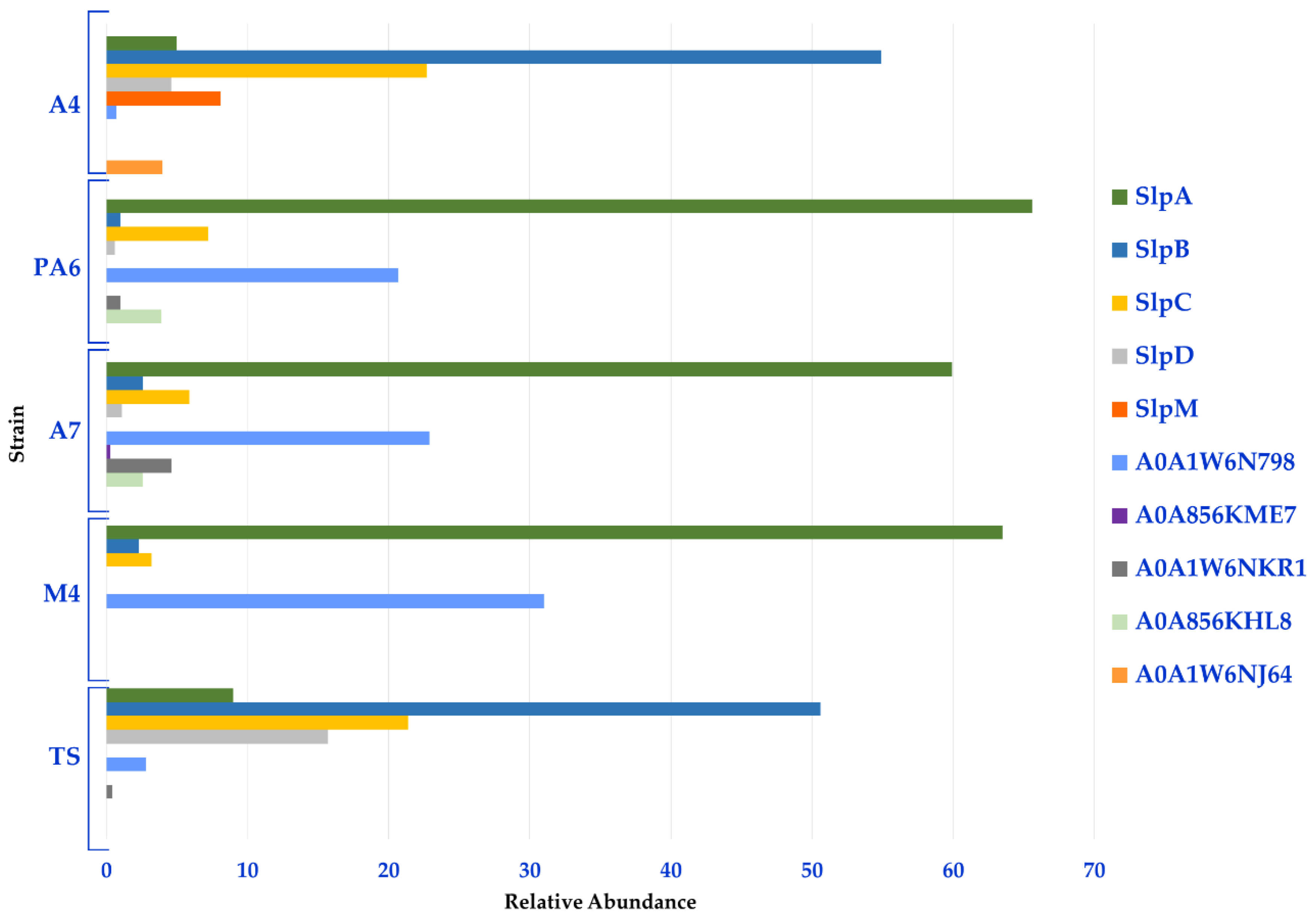

| Accession | Protein Name | Gene Name | Seq. Cov. [%] | Mol. Weight [kDa] | pI | A4 1 | PA6 1 | A7 1 | M4 1 | TS 1 | n. of Strains |
|---|---|---|---|---|---|---|---|---|---|---|---|
| G1UE81 | Surface layer protein A | SlpA | 66.1 | 48.17 | 9.54 | x | x | x | x | x | 5 |
| Q8GFE5 | Surface layer protein B | slpB | 92.1 | 50.92 | 9.54 | x | x | x | x | x | 5 |
| Q8GFE4 | Surface layer protein C | slpC | 84.6 | 48.86 | 9.66 | x | x | x | x | x | 5 |
| Q8GFE3 | Surface layer protein D | SlpD | 77.5 | 44.58 | 9.66 | x | x | x | x | 4 | |
| H9BQS1 | Surface layer protein M | slpM | 63.3 | 51.46 | 9.54 | x | 1 | ||||
| A0A1W6N798 | S-layer protein | AZI09_01470 | 84.3 | 47.76 | 9.60 | x | x | x | x | x | 5 |
| A0A856KME7 | S-layer protein | UCCLB556_pD0019 | 13.8 | 47.89 | 9.68 | x | 1 | ||||
| A0A1W6NKR1 | S-layer protein | AZI11_14340 | 27.8 | 49.17 | 9.48 | x | x | x | 3 | ||
| A0A856KHL8 | Uncharacterized protein | UCCLB556_2008 | 75.3 | 47.68 | 9.56 | x | x | 2 | |||
| A0A1W6NJ64 | Uncharacterized protein | AZI11_11480 | 55.2 | 48.42 | 9.65 | x | 1 |
| Query | Protein Name | Sequence Identity (%) | Similar Protein Accession | Protein Name |
|---|---|---|---|---|
| H9BQS1 | Surface layer protein SlpM | 93.6 | Q8GFE5 | Surface layer protein SlpB |
| A0A856KME7 | S-layer protein | 57.7 | G1UE81 | Surface layer protein A |
| A0A1W6NKR1 | S-layer protein | 58.0 | G1UE81 | Surface layer protein A |
| A0A1W6N798 | S-layer protein | 60.6 | G1UE81 | Surface layer protein A |
| A0A1W6NJ64 | Uncharacterized protein | 88.5 | Q8GFE4 | Surface layer protein SlpC |
| A0A856KHL8 | Uncharacterized protein | 61.5 | G1UE81 | Surface layer protein A |
| Accession | Protein Name | Gene Names | A4 vs. TS Fold Change | A6 vs. TS Fold Change | A7 vs. TS Fold Change | M4 vs. TS Fold Change |
|---|---|---|---|---|---|---|
| G1UE81 | SlpA | slpA | −4.76 | 5.20 | 4.41 | 4.62 |
| Q8GFE5 | SlpB | slpB | NDA | −9.98 | −5.67 | −8.57 |
| Q8GFE4 | SlpC | slpC | NDA | NDA | −1.56 | −1.83 |
| Q8GFE3 | SlpD | SlpD | −5.77 | −9.30 | −7.24 | nd |
| A0A1W6N798 | Slp A0A1W6N798 | AZI09_01470 | −5.44 | 5.95 | 6.52 | 5.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzeo, M.F.; Reale, A.; Di Renzo, T.; Siciliano, R.A. Surface Layer Protein Pattern of Levilactobacillus brevis Strains Investigated by Proteomics. Nutrients 2022, 14, 3679. https://doi.org/10.3390/nu14183679
Mazzeo MF, Reale A, Di Renzo T, Siciliano RA. Surface Layer Protein Pattern of Levilactobacillus brevis Strains Investigated by Proteomics. Nutrients. 2022; 14(18):3679. https://doi.org/10.3390/nu14183679
Chicago/Turabian StyleMazzeo, Maria Fiorella, Anna Reale, Tiziana Di Renzo, and Rosa Anna Siciliano. 2022. "Surface Layer Protein Pattern of Levilactobacillus brevis Strains Investigated by Proteomics" Nutrients 14, no. 18: 3679. https://doi.org/10.3390/nu14183679
APA StyleMazzeo, M. F., Reale, A., Di Renzo, T., & Siciliano, R. A. (2022). Surface Layer Protein Pattern of Levilactobacillus brevis Strains Investigated by Proteomics. Nutrients, 14(18), 3679. https://doi.org/10.3390/nu14183679









