Extended Inter-Meal Interval Negatively Impacted the Glycemic and Insulinemic Responses after Both Lunch and Dinner in Healthy Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Ethics
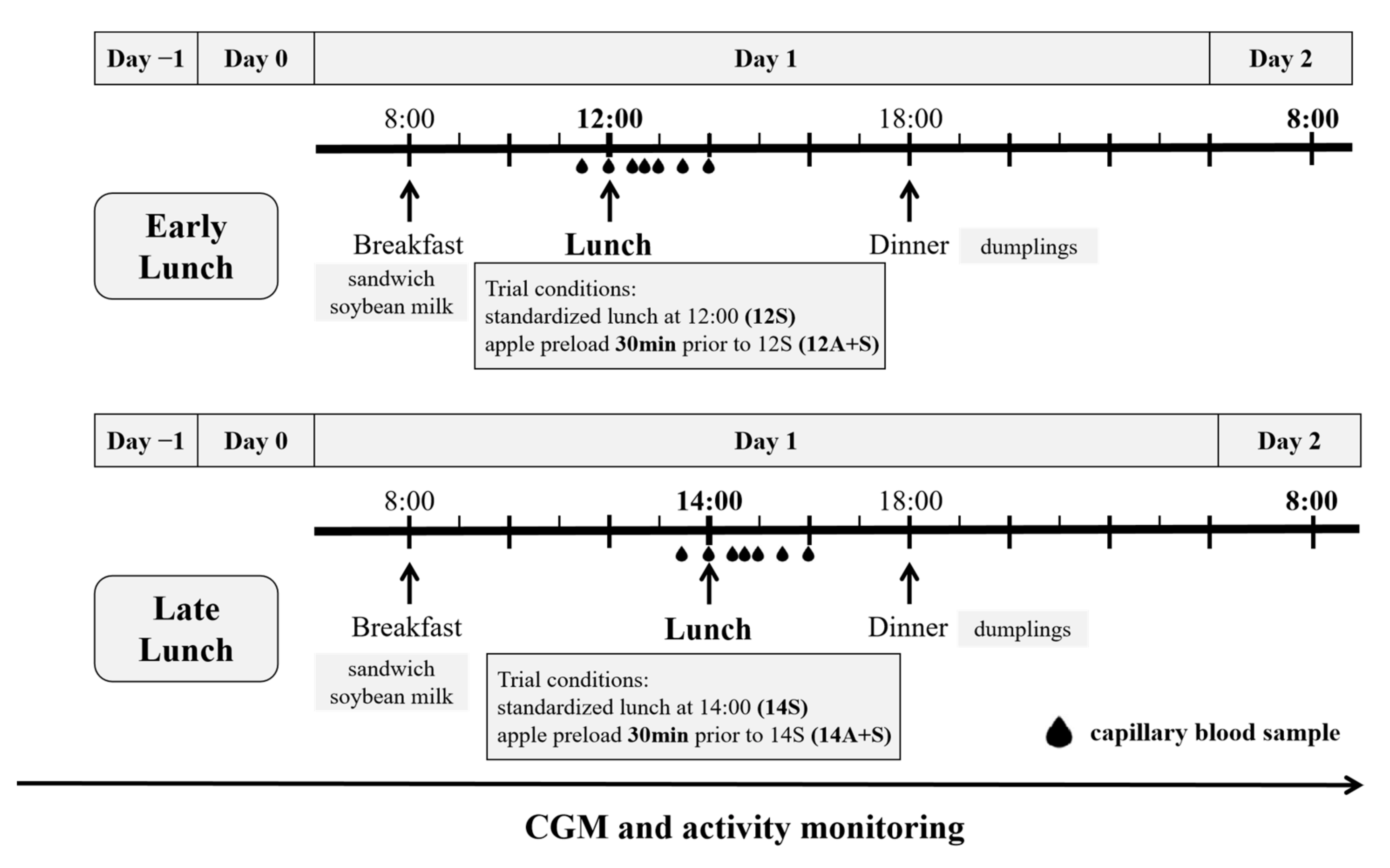
2.2. Study Design and Procedures
2.3. Test Meal Components
2.4. Continuous Glucose Monitoring
2.5. Blood Collection and Analysis
2.6. Data Processing and Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
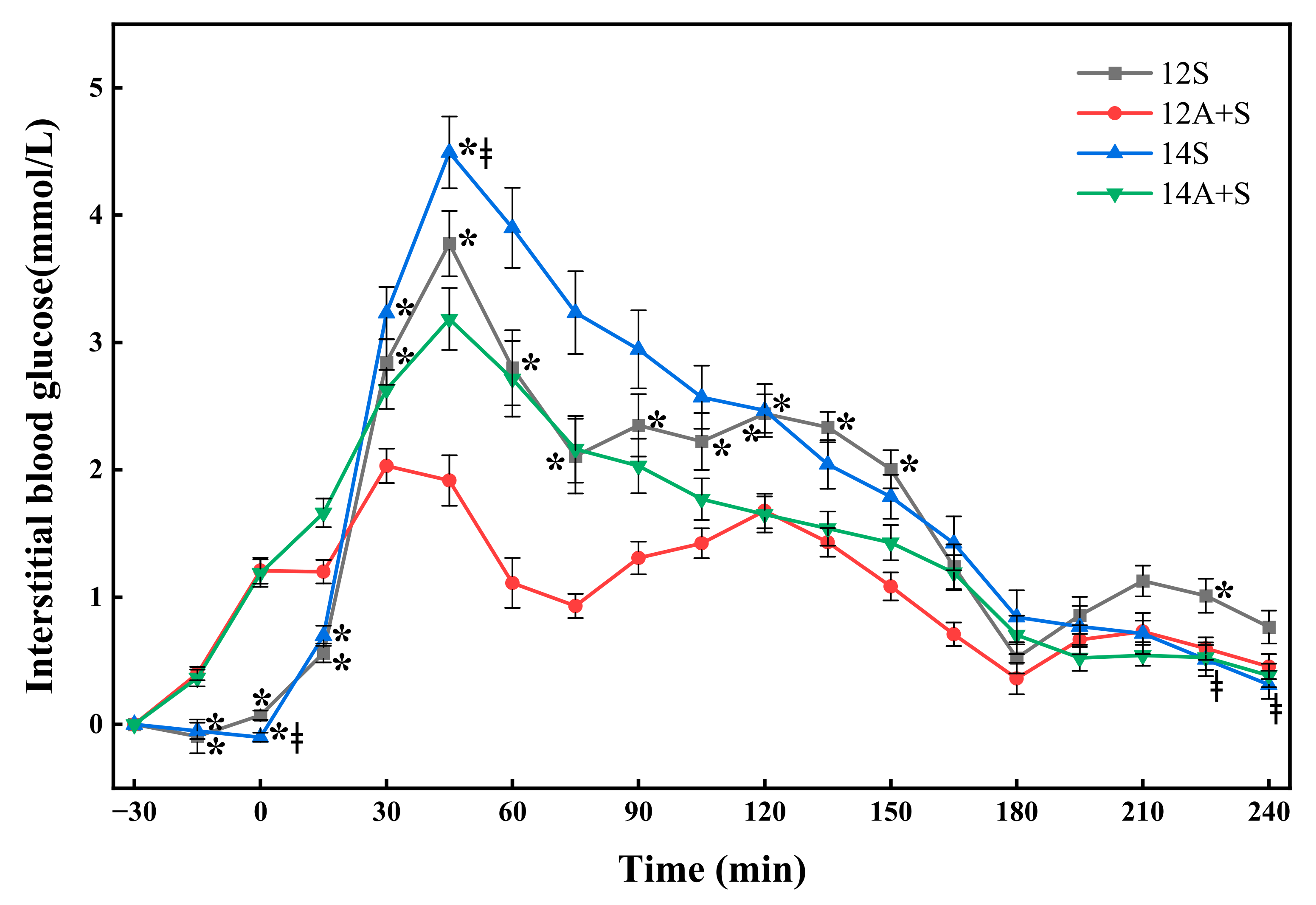
3.2. Postprandial Interstitial Glycemic Responses Following the Lunch Test Meals
3.3. Postprandial Capillary Glucose and Insulin Responses Following the Lunch Test Meals
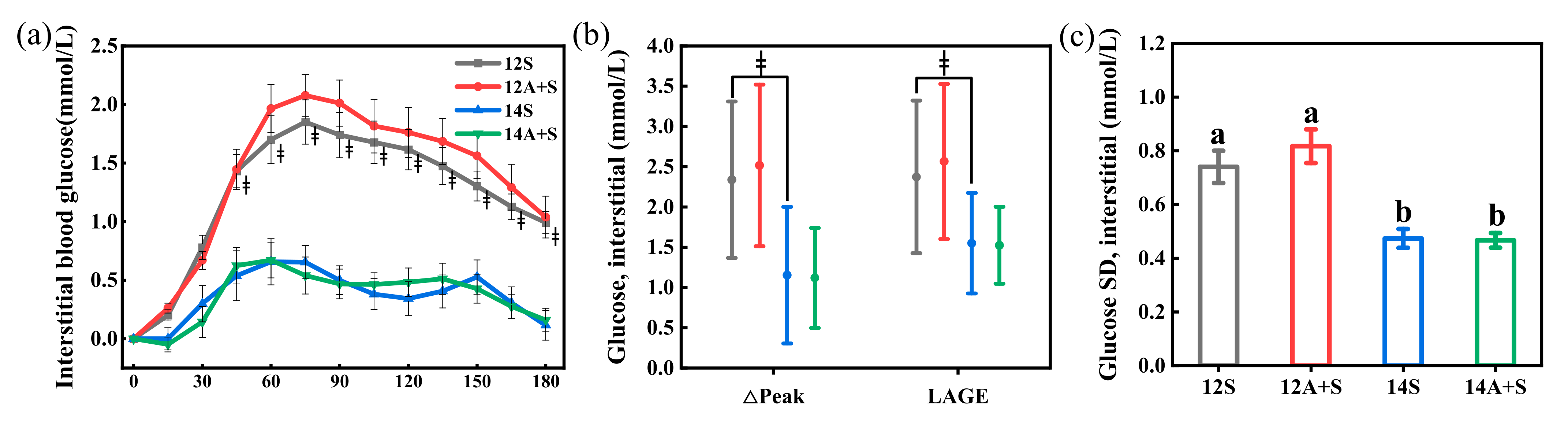
3.4. Postprandial Interstitial Glycemic Responses Following the Subsequent Meals
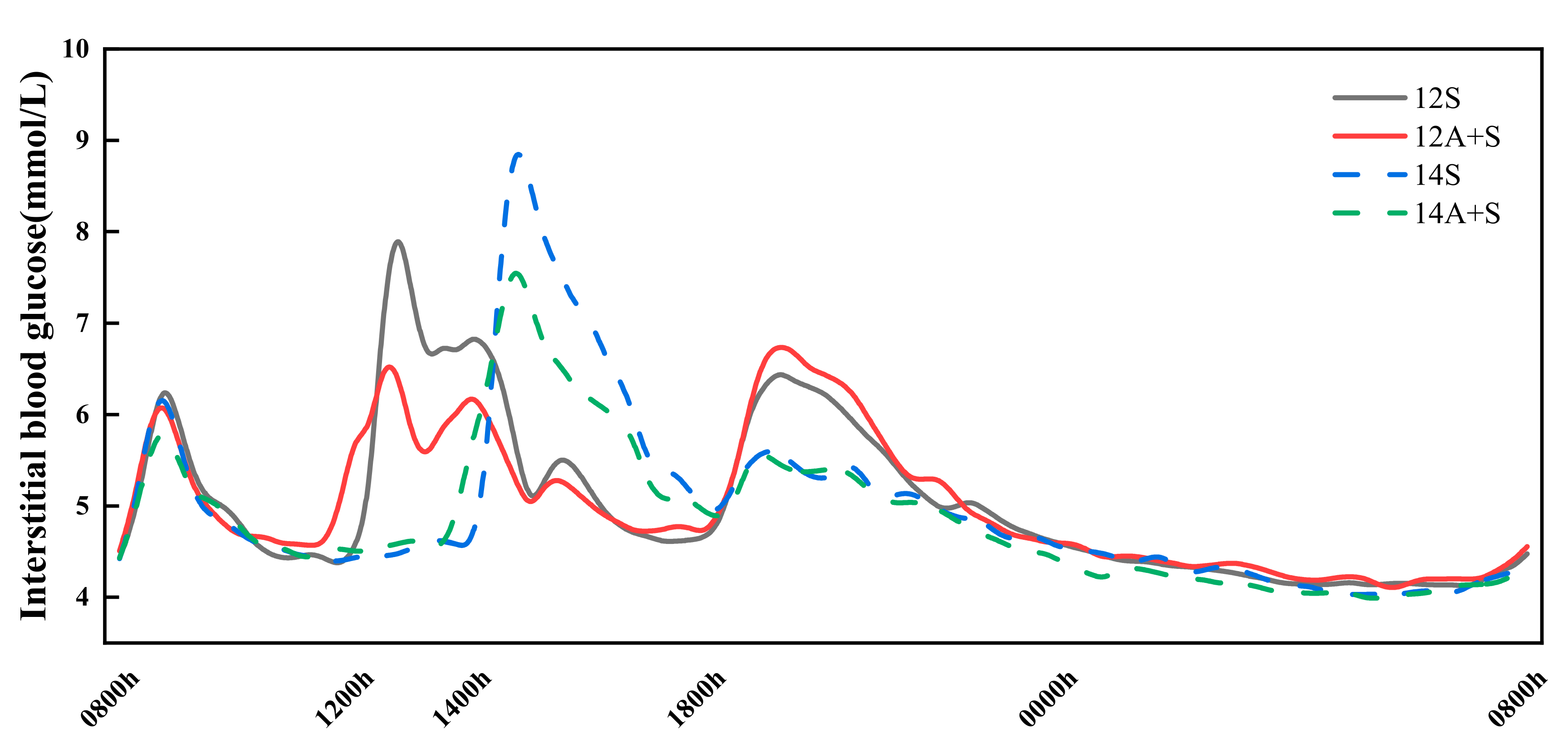
3.5. 24 h Interstitial Glucose Trace
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ford, N.D.; Patel, S.A.; Narayan, M.V. Obesity in Low- and Middle-Income Countries: Burden, Drivers, and Emerging Challenges. Annu. Rev. Public Health 2017, 38, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G.D. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on Postprandial Glycemia and Insulin Resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef]
- Davis, R.; Rogers, M.; Coates, A.M.; Leung, G.K.W.; Bonham, M.P. The Impact of Meal Timing on Risk of Weight Gain and Development of Obesity: A Review of the Current Evidence and Opportunities for Dietary Intervention. Curr. Diabetes Rep. 2022, 22, 147–155. [Google Scholar] [CrossRef]
- Dashti, H.S.; Gomez-Abellan, P.; Qian, J.; Esteban, A.; Morales, E.; Scheer, F.; Garaulet, M. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am. J. Clin. Nutr. 2020, 113, 154–161. [Google Scholar] [CrossRef]
- Martínez-Lozano, N.; Tvarijonaviciute, A.; Ríos, R.; Barón, I.; Scheer, F.A.J.L.; Garaulet, M. Late Eating Is Associated with Obesity, Inflammatory Markers and Circadian-Related Disturbances in School-Aged Children. Nutrients 2020, 12, 2881. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Phillips, A.J.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.A.; Zaman, A.; Cornier, M.; Catenacci, V.A.; Tussey, E.J.; Grau, L.; Arbet, J.; Broussard, J.L.; Rynders, C.A. Later Meal and Sleep Timing Predicts Higher Percent Body Fat. Nutrients 2021, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Dalla Man, C.; Nandy, D.K.; Levine, J.A.; Bharucha, A.E.; Rizza, R.A.; Basu, R.; Carter, R.E.; Cobelli, C.; Kudva, Y.C.; et al. Diurnal Pattern to Insulin Secretion and Insulin Action in Healthy Individuals. Diabetes 2012, 61, 2691–2700. [Google Scholar] [CrossRef]
- Leung, G.K.W.; Huggins, C.E.; Bonham, M.P. Effect of meal timing on postprandial glucose responses to a low glycemic index meal: A crossover trial in healthy volunteers. Clin. Nutr. 2019, 38, 465–471. [Google Scholar] [CrossRef]
- Van Cauter, E.; Shapiro, E.T.; Tillil, H.; Polonsky, K.S. Circadian modulation of glucose and insulin responses to meals: Relationship to cortisol rhythm. Am. J. Physiol. 1992, 262, E467–E475. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Brereton, N.; Schweitzer, A.; Cotter, M.; Duan, D.; Børsheim, E.; Wolfe, R.R.; Pham, L.V.; Polotsky, V.Y.; Jun, J.C. Metabolic Effects of Late Dinner in Healthy Volunteers—A Randomized Crossover Clinical Trial. J. Clin. Endocrinol. Metab. 2020, 105, 2789–2802. [Google Scholar] [CrossRef]
- Kajiyama, S.; Imai, S.; Hashimoto, Y.; Yamane, C.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Tanaka, M.; Kajiyama, S.; Fukui, M. Divided consumption of late-night-dinner improves glucose excursions in young healthy women: A randomized cross-over clinical trial. Diabetes Res. Clin. Pract. 2018, 136, 78–84. [Google Scholar] [CrossRef]
- Nakamura, K.; Tajiri, E.; Hatamoto, Y.; Ando, T.; Shimoda, S.; Yoshimura, E. Eating Dinner Early Improves 24-h Blood Glucose Levels and Boosts Lipid Metabolism after Breakfast the Next Day: A Randomized Cross-Over Trial. Nutrients 2021, 13, 2424. [Google Scholar] [CrossRef] [PubMed]
- Che, T.; Yan, C.; Tian, D.; Zhang, X.; Liu, X.; Wu, Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: A randomised controlled trial. Nutr. Metab. 2021, 18, 1–10. [Google Scholar] [CrossRef]
- Lopez-Minguez, J.; Gómez-Abellán, P.; Garaulet, M. Timing of Breakfast, Lunch, and Dinner. Effects on Obesity and Metabolic Risk. Nutrients 2019, 11, 2624. [Google Scholar] [CrossRef]
- Service, F.J.; Hall, L.D.; Westland, R.E.; O’Brien, P.C.; Go, V.L.; Haymond, M.W.; Rizza, R.A. Effects of size, time of day and sequence of meal ingestion on carbohydrate tolerance in normal subjects. Diabetologia 1983, 25, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, Z.; Fan, Z.; Wu, Y.; Lou, X.; Liu, A.; Lu, X. Diurnal differences in glycemic responses, insulin responses and cognition after rice-based meals. Asia Pac. J. Clin. Nutr. 2022, 31, 57–65. [Google Scholar] [PubMed]
- Garaulet, M.; Gomez-Abellan, P.; Alburquerque-Bejar, J.J.; Lee, Y.C.; Ordovas, J.M.; Scheer, F.A. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. Lond 2013, 37, 604–611. [Google Scholar] [CrossRef]
- Ruiz-Lozano, T.; Vidal, J.; de Hollanda, A.; Scheer, F.A.J.L.; Garaulet, M.; Izquierdo-Pulido, M. Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clin. Nutr. 2016, 35, 1308–1314. [Google Scholar] [CrossRef] [Green Version]
- Garaulet, M.; Vera, B.; Bonnet-Rubio, G.; Gómez-Abellán, P.; Lee, Y.; Ordovás, J.M. Lunch eating predicts weight-loss effectiveness in carriers of the common allele at PERILIPIN1: The ONTIME (Obesity, Nutrigenetics, Timing, Mediterranean) study. Am. J. Clin. Nutr. 2016, 104, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, B.; Sharma, N.; Pant, S.; Sharma, L.; Pahuja, B.; Singh, P. PCOS patients differ in meal timings rather than total caloric or macronutrient intake in comparison to weight matched controls. Eur. J. Obstet. Gyn. R. B. 2022, 270, 11–16. [Google Scholar] [CrossRef]
- Bandin, C.; Scheer, F.A.; Luque, A.J.; Avila-Gandia, V.; Zamora, S.; Madrid, J.A.; Gomez-Abellan, P.; Garaulet, M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes. Lond 2015, 39, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Engen, P.A.; Bandín, C.; Cabrera Rubio, R.; Voigt, R.M.; Green, S.J.; Naqib, A.; Keshavarzian, A.; Scheer, F.A.J.L.; Garaulet, M. Timing of food intake impacts daily rhythms of human salivary microbiota: A randomized, crossover study. FASEB J. 2018, 32, 2060–2072. [Google Scholar] [CrossRef] [PubMed]
- Nesti, L.; Mengozzi, A.; Tricò, D. Impact of Nutrient Type and Sequence on Glucose Tolerance: Physiological Insights and Therapeutic Implications. Front. Endocrinol. 2019, 10, 144. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Henry, C.J. Reducing the glycemic impact of carbohydrates on foods and meals: Strategies for the food industry and consumers with special focus on Asia. Compr. Rev. Food Sci. F. 2020, 19, 670–702. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhao, W.; Wang, L.; Fan, Z.; Zhu, R.; Wu, Y.; Zhou, Y. Apple Preload Halved the Postprandial Glycaemic Response of Rice Meal in Healthy Subjects. Nutrients 2019, 11, 2912. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, L.; Fan, Z.; Lu, J.; Zhu, R.; Wu, Y.; Lu, X. Co-ingested vinegar-soaked or preloaded dried apple mitigated acute postprandial glycemia of rice meal in healthy subjects under equicarbohydrate conditions. Nutr. Res. 2020, 83, 108–118. [Google Scholar] [CrossRef]
- Lu, X.; Lu, J.; Fan, Z.; Liu, A.; Zhao, W.; Wu, Y.; Zhu, R. Both Isocarbohydrate and Hypercarbohydrate Fruit Preloads Curbed Postprandial Glycemic Excursion in Healthy Subjects. Nutrients 2021, 13, 2470. [Google Scholar] [CrossRef]
- Bei-Fan, Z.; Cooperative, M.A.G.W. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: Study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Asia Pac. J. Clin. Nutr. 2002, 11, S685–S693. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing—Comparison with the euglycemic insulin clamp. Diabetes Care. 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Brynes, A.E.; Edwards, C.M.; Ghatei, M.A.; Dornhorst, A.; Morgan, L.M.; Bloom, S.R.; Frost, G.S. A randomised four-intervention crossover study investigating the effect of carbohydrates on daytime profiles of insulin, glucose, non-esterified fatty acids and triacylglycerols in middle-aged men. Brit. J. Nutr. 2003, 89, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Caumo, A.; Bergman, R.N.; Cobelli, C. Insulin sensitivity from meal tolerance tests in normal subjects: A minimal model index. J. Clin. Endocrinol. Metab. 2000, 85, 4396–4402. [Google Scholar] [CrossRef] [PubMed]
- Aloulou, I.; Brun, J.; Mercier, J. Evaluation of insulin sensitivity and glucose effectiveness during a standardized breakfast test: Comparison with the minimal model analysis of an intravenous glucose tolerance test. Metab. Clin. Exp. 2006, 55, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Nakamura, K.; Ogata, H.; Miyashita, A.; Nagasaka, S.; Omi, N.; Yamaguchi, S.; Hibi, M.; Umeda, T.; Nakaji, S.; et al. Acute effect of late evening meal on diurnal variation of blood glucose and energy metabolism. Obes. Res. Clin. Pract. 2011, 5, e220–e228. [Google Scholar] [CrossRef]
- Tsuchida, Y.; Hata, S.; Sone, Y. Effects of a late supper on digestion and the absorption of dietary carbohydrates in the following morning. J. Physiol. Anthropol. 2013, 32, 9. [Google Scholar] [CrossRef]
- Del Prato, S. Loss of early insulin secretion leads to postprandial hyperglycaemia. Diabetologia 2003, 46, M2–M8. [Google Scholar] [CrossRef]
- Tsujino, D.; Nishimura, R.; Taki, K.; Miyashita, Y.; Morimoto, A.; Tajima, N. Daily glucose profiles in Japanese people with normal glucose tolerance as assessed by continuous glucose monitoring. Diabetes Technol. Ther. 2009, 11, 457–460. [Google Scholar] [CrossRef]
- Nas, A.; Mirza, N.; Hägele, F.; Kahlhöfer, J.; Keller, J.; Rising, R.; Kufer, T.A.; Bosy-Westphal, A. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am. J. Clin. Nutr. 2017, n151332. [Google Scholar] [CrossRef]
- Kobayashi, F.; Ogata, H.; Omi, N.; Nagasaka, S.; Yamaguchi, S.; Hibi, M.; Tokuyama, K. Effect of breakfast skipping on diurnal variation of energy metabolism and blood glucose. Obes. Res. Clin. Pract. 2014, 8, e249–e257. [Google Scholar] [CrossRef]
- Ando, T.; Nakae, S.; Usui, C.; Yoshimura, E.; Nishi, N.; Takimoto, H.; Tanaka, S. Effect of diurnal variations in the carbohydrate and fat composition of meals on postprandial glycemic response in healthy adults: A novel insight for the second-meal phenomenon. Am. J. Clin. Nutr. 2018, 108, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Haymond, M.W.; Gerich, J.E.; Cryer, P.E.; Miles, J.M. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J. Clin. Invest. 1987, 79, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Kajiyama, S.; Hashimoto, Y.; Yamane, C.; Miyawaki, T.; Ozasa, N.; Tanaka, M.; Fukui, M. Divided consumption of late-night-dinner improves glycemic excursions in patients with type 2 diabetes: A randomized cross-over clinical trial. Diabetes Res. Clin. Pract. 2017, 129, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Ma, J.; Feng, B.; Zhang, H.; Alan-Diehl, J.; Eugene-Chin, Y.; Yan, W.; Xu, H. FFA-Induced Adipocyte Inflammation and Insulin Resistance: Involvement of ER Stress and IKKβ Pathways. Obesity 2011, 19, 483–491. [Google Scholar] [CrossRef]
- Boden, G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997, 46, 3–10. [Google Scholar] [CrossRef]
- Morgan, L.; Arendt, J.; Owens, D.; Folkard, S.; Hampton, S.; Deacon, S.; English, J.; Ribeiro, D.; Taylor, K. Effects of the endogenous clock and sleep time on melatonin, insulin, glucose and lipid metabolism. J. Endocrinol. 1998, 157, 443–451. [Google Scholar] [CrossRef]
- Shea, S.A.; Hilton, M.F.; Orlova, C.; Ayers, R.T.; Mantzoros, C.S. Independent Circadian and Sleep/Wake Regulation of Adipokines and Glucose in Humans. J. Clin. Endocrinol. Metab. 2005, 90, 2537–2544. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef]
- Adafer, R.; Messaadi, W.; Meddahi, M.; Patey, A.; Haderbache, A.; Bayen, S.; Messaadi, N. Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating’s Effects on Human Health. Nutrients 2020, 12, 3770. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Cioffi, I.; Evangelista, A.; Ponzo, V.; Goitre, I.; Ciccone, G.; Ghigo, E.; Bo, S. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 17–33. [Google Scholar] [CrossRef]
- Almiron-Roig, E.; Palla, L.; Guest, K.; Ricchiuti, C.; Vint, N.; Jebb, S.A.; Drewnowski, A. Factors that determine energy compensation: A systematic review of preload studies. Nutr. Rev. 2013, 71, 458–473. [Google Scholar] [CrossRef]
- Heacock, P.M.; Hertzler, S.R.; Wolf, B.W. Fructose prefeeding reduces the glycemic response to a high-glycemic index, starchy food in humans. J. Nutr. 2002, 132, 2601–2604. [Google Scholar] [CrossRef]
- Pullicin, A.J.; Glendinning, J.I.; Lim, J. Cephalic phase insulin release: A review of its mechanistic basis and variability in humans. Physiol. Behav. 2021, 239, 113514. [Google Scholar] [CrossRef]
- Dhillon, J.; Lee, J.Y.; Mattes, R.D. The cephalic phase insulin response to nutritive and low-calorie sweeteners in solid and beverage form. Physiol. Behav. 2017, 181, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Laurent, D.; Yu, C.; Cline, G.W.; Shulman, G.I. Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes 2001, 50, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Geidl-Flueck, B.; Gerber, P. Insights into the Hexose Liver Metabolism—Glucose versus Fructose. Nutrients 2017, 9, 1026. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Fan, Z.; Dong, Y.; Liu, M.; Wang, L.; Pan, H. Postprandial Glycaemic Responses of Dried Fruit-Containing Meals in Healthy Adults: Results from a Randomised Trial. Nutrients 2018, 10, 694. [Google Scholar] [CrossRef]
- Angarita Dávila, L.; Bermúdez, V.; Aparicio, D.; Céspedes, V.; Escobar, M.; Durán-Agüero, S.; Cisternas, S.; de Assis Costa, J.; Rojas-Gómez, D.; Reyna, N.; et al. Effect of Oral Nutritional Supplements with Sucromalt and Isomaltulose versus Standard Formula on Glycaemic Index, Entero-Insular Axis Peptides and Subjective Appetite in Patients with Type 2 Diabetes: A Randomised Cross-Over Study. Nutrients 2019, 11, 1477. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care. 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Raphaelli, C.; Dos Santos Pereira, E.; Camargo, T.M.; Vinholes, J.; Rombaldi, C.V.; Vizzotto, M.; Nora, L. Apple Phenolic Extracts Strongly Inhibit α-Glucosidase Activity. Plant Food. Hum. Nutr. 2019, 74, 430–435. [Google Scholar] [CrossRef] [PubMed]


| Test Meals | Carbohydrate (g) | Protein (g) | Fat (g) | Energy (kcal) | Detail Content |
|---|---|---|---|---|---|
| S 2 | 97.2 | 24.2 | 18.0 | 648 | Lactose-free low-fat milk 200 g, roasted sesame dressing 25 mL, egg yolk 10 g, egg white 50 g, romaine Lettuce 25 g, cherry tomato 75 g, broccoli 50 g, sesame oil 2 g, uncooked rice 100 g, water119.2 g |
| A+S 3 | 97.2 | 24.1 | 18.1 | 648 | Lactose-free low-fat milk 200 g, roasted sesame dressing 25 mL, egg yolk 10 g, egg white 50 g, romaine Lettuce 25 g, cherry tomato 75 g, broccoli 50 g, sesame oil 1 g, uncooked rice 80.2 g, apple 140 g |
| Characteristics | Mean ± SD (Male/Female) |
|---|---|
| Number of participants (male/female) | 26(12/14) |
| Age, years | 20.8 ± 0.9 |
| Body composition | |
| BMI, kg/m2 | 21.4 ± 2.1/20.8 ± 1.7 |
| Waist: hip ratio | 0.7 ± 0.0/0.8 ± 0.0 |
| Waist: height ratio | 0.4 ± 0.0/0.4 ± 0.0 |
| Fat mass, % | 15.6 ± 4.1/24.1 ± 4.0 |
| Visceral fat index | 4.8 ± 2.0/2.3 ± 1.2 |
| Basal metabolic rate (BMR), kcal/day | 1391.6 ± 203.4 |
| Systolic blood pressure, mmHg | 114.0 ± 12.3 |
| Diastolic blood pressure, mmHg | 67.0 ± 9.0 |
| Habitual meal timing | |
| Breakfast | 8:02 ± 0:42 |
| Lunch | 11:42 ± 0:25 |
| Dinner | 17:41 ± 0:22 |
| Test Meals | iAUC0-270 (mmol·min/L) | ∆Peak270 (mmol/L) | LAGE270 (mmol/L) | SD | CV (%) | CONGA-1 | J-Index |
|---|---|---|---|---|---|---|---|
| 12S | 432.6 ± 29.7 a | 3.9 ± 0.2 a | 4.2 ± 0.3 ab | 1.3 ± 0.1 ab | 21.1 ± 1.1 ab | 2.0 ± 0.1 ab | 17.1 ± 0.8 ab |
| 12A+S | 287.0 ± 18.3 b | 2.4 ± 0.1 b | 2.5 ± 0.1 c | 0.7 ± 0.0 c | 12.7 ± 0.6 c | 1.1 ± 0.1 c | 13.0 ± 0.5 c |
| 14S | 482.1 ± 34.6 a | 4.7 ± 0.3 c | 5.0 ± 0.2 a | 1.6 ± 0.1 a | 24.6 ± 1.1 a | 2.5 ± 0.1 a | 20.5 ± 1.2 a |
| 14A+S | 392.0 ± 26.9 a | 3.4 ± 0.2 a | 3.5 ± 0.2 b | 1.0 ± 0.1 b | 17.4 ± 1.1 b | 1.6 ± 0.1 b | 15.9 ± 0.8 b |
| Test Meals | 24 h Mean (mmol/L) | 24 h tAUC (mmol·h/L) | 24 h Peak (mmol/L) | 24 h LAGE (mmol/L) | 24 h SD | GC > 2.5 (%) | GC > 5.0 (%) | ∆PL-D |
|---|---|---|---|---|---|---|---|---|
| 12S | 5.0 ± 0.1 | 120.8 ± 1.5 | 8.4 ± 0.2 a | 4.7 ± 0.3 a | 1.0 ± 0.0 ac | 6.1 ± 0.8 a | 0.2 ± 0.1 a | 1.4 ± 0.2 a |
| 12A+S | 5.0 ± 0.1 | 120.2 ± 1.8 | 7.4 ± 0.2 b | 3.6 ± 0.2 b | 0.9 ± 0.0 b | 4.0 ± 1.0 ab | 0.0 ± 0.0 a | 0.3 ± 0.2 b |
| 14S | 5.0 ± 0.1 | 119.5 ± 1.7 | 9.4 ± 0.3 c | 5.7 ± 0.3 c | 1.1 ± 0.1 c | 5.9 ± 0.7 a | 1.3 ± 0.4 b | 3.3 ± 0.2 c |
| 14A+S | 4.9 ± 0.1 | 116.6 ± 2.0 | 8.0 ± 0.2 ab | 4.1 ± 0.2 ab | 0.9 ± 0.0 ab | 3.6 ± 0.6 b | 0.2 ± 0.1 a | 2.1 ± 0.3 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Fan, Z.; Liu, A.; Liu, R.; Lou, X.; Hu, J. Extended Inter-Meal Interval Negatively Impacted the Glycemic and Insulinemic Responses after Both Lunch and Dinner in Healthy Subjects. Nutrients 2022, 14, 3617. https://doi.org/10.3390/nu14173617
Lu X, Fan Z, Liu A, Liu R, Lou X, Hu J. Extended Inter-Meal Interval Negatively Impacted the Glycemic and Insulinemic Responses after Both Lunch and Dinner in Healthy Subjects. Nutrients. 2022; 14(17):3617. https://doi.org/10.3390/nu14173617
Chicago/Turabian StyleLu, Xuejiao, Zhihong Fan, Anshu Liu, Rui Liu, Xinling Lou, and Jiahui Hu. 2022. "Extended Inter-Meal Interval Negatively Impacted the Glycemic and Insulinemic Responses after Both Lunch and Dinner in Healthy Subjects" Nutrients 14, no. 17: 3617. https://doi.org/10.3390/nu14173617
APA StyleLu, X., Fan, Z., Liu, A., Liu, R., Lou, X., & Hu, J. (2022). Extended Inter-Meal Interval Negatively Impacted the Glycemic and Insulinemic Responses after Both Lunch and Dinner in Healthy Subjects. Nutrients, 14(17), 3617. https://doi.org/10.3390/nu14173617







