Impact of Sarcopenia on Clinical Outcomes in a Cohort of Caucasian Active Crohn’s Disease Patients Undergoing Multidetector CT-Enterography
Abstract
1. Introduction
2. Materials and Methods
2.1. Sarcopenia, Nutritional Status, and Body Composition Assessment
2.2. CT Protocol
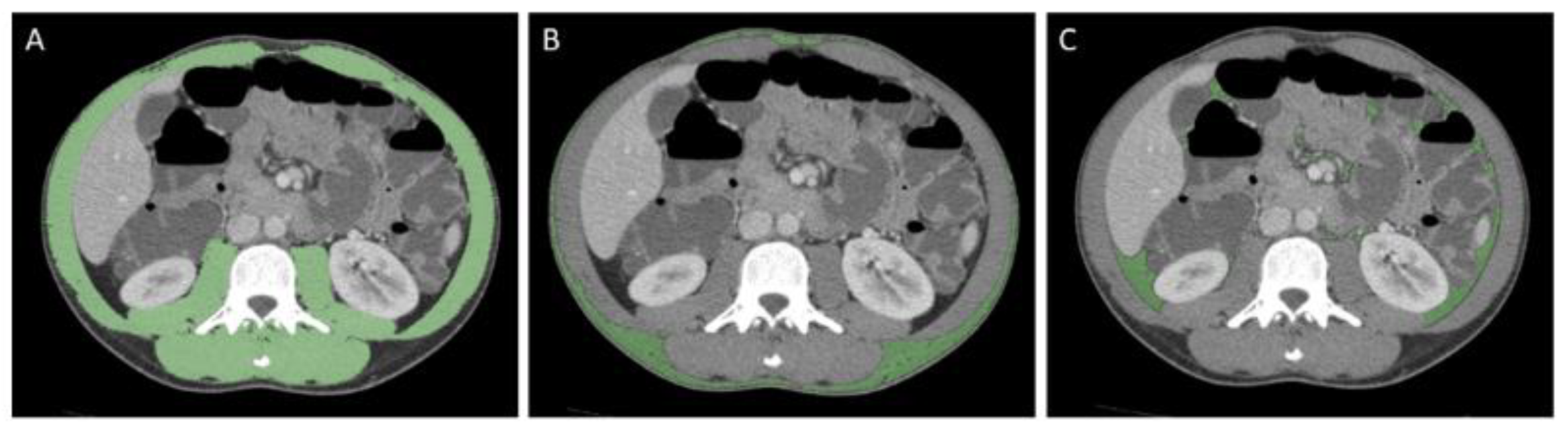
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Clinical Outcomes
3.3. Assessment of Body Composition Measures and Association with Clinical Parameters
3.4. Predictors of Sarcopenia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, M.; Gönczi, L.; Lakatos, P.L.; Burisch, J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohn’s Colitis 2021, 15, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and Risk Factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Windsor, J.W. The Four Epidemiological Stages in the Global Evolution of Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Gao, X.; Dai, C.; Huang, Y.; Wu, Y.; Zhou, W.; Cao, Q.; Jing, X.; Jiang, H.; et al. Validation of the GLIM Criteria for Diagnosis of Malnutrition and Quality of Life in Patients with Inflammatory Bowel Disease: A Multicenter, Prospective, Observational Study. Clin. Nutr. 2022, 41, 1297–1306. [Google Scholar] [CrossRef]
- Bamba, S.; Sasaki, M.; Takaoka, A.; Takahashi, K.; Imaeda, H.; Nishida, A.; Inatomi, O.; Sugimoto, M.; Andoh, A. Sarcopenia Is a Predictive Factor for Intestinal Resection in Admitted Patients with Crohn’s Disease. PLoS ONE 2017, 12, e0180036. [Google Scholar] [CrossRef]
- Fiorindi, C.; Luceri, C.; Dragoni, G.; Piemonte, G.; Scaringi, S.; Staderini, F.; Nannoni, A.; Ficari, F.; Giudici, F. GLIM Criteria for Malnutrition in Surgical IBD Patients: A Pilot Study. Nutrients 2020, 12, 2222. [Google Scholar] [CrossRef]
- Fiorindi, C.; Dragoni, G.; Scaringi, S.; Staderini, F.; Nannoni, A.; Ficari, F.; Giudici, F. Relationship between Nutritional Screening Tools and GLIM in Complicated IBD Requiring Surgery. Nutrients 2021, 13, 3899. [Google Scholar] [CrossRef]
- Cioffi, I.; Imperatore, N.; Di Vincenzo, O.; Pagano, M.C.; Santarpia, L.; Pellegrini, L.; Testa, A.; Marra, M.; Contaldo, F.; Castiglione, F.; et al. Evaluation of Nutritional Adequacy in Adult Patients with Crohn’s Disease: A Cross-Sectional Study. Eur. J. Nutr. 2020, 59, 3647–3658. [Google Scholar] [CrossRef]
- Cioffi, I.; Imperatore, N.; Di Vincenzo, O.; Santarpia, L.; Rispo, A.; Marra, M.; Testa, A.; Contaldo, F.; Castiglione, F.; Pasanisi, F. Association between Health-Related Quality of Life and Nutritional Status in Adult Patients with Crohn’s Disease. Nutrients 2020, 12, 746. [Google Scholar] [CrossRef]
- Nardone, O.M.; de Sire, R.; Petito, V.; Testa, A.; Villani, G.; Scaldaferri, F.; Castiglione, F. Inflammatory Bowel Diseases and Sarcopenia: The Role of Inflammation and Gut Microbiota in the Development of Muscle Failure. Front. Immunol. 2021, 12, 694217. [Google Scholar] [CrossRef]
- Pizzoferrato, M.; de Sire, R.; Ingravalle, F.; Mentella, M.C.; Petito, V.; Martone, A.M.; Landi, F.; Miggiano, G.A.D.; Mele, M.C.; Lopetuso, L.R.; et al. Characterization of Sarcopenia in an IBD Population Attending an Italian Gastroenterology Tertiary Center. Nutrients 2019, 11, 2281. [Google Scholar] [CrossRef]
- de Sire, R.; Rizzatti, G.; Ingravalle, F. Skeletal Muscle-Gut Axis: Emerging Mechanisms of Sarcopenia for Intestinal and Extra Intestinal Diseases. Skelet. Muscle Gut Axis Emerg. Mech. Sarcopenia Intest. Extra Intest. Dis. 2018, 64, 351–362. [Google Scholar] [CrossRef]
- Nishikawa, H.; Nakamura, S.; Miyazaki, T.; Kakimoto, K.; Fukunishi, S.; Asai, A.; Nishiguchi, S.; Higuchi, K. Inflammatory Bowel Disease and Sarcopenia: Its Mechanism and Clinical Importance. J. Clin. Med. 2021, 10, 4214. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Ryan, E.; McNicholas, D.; Creavin, B.; Kelly, M.E.; Walsh, T.; Beddy, D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2019, 25, 67–73. [Google Scholar] [CrossRef]
- Teigen, L.M.; Kuchnia, A.J.; Mourtzakis, M.; Earthman, C.P. The Use of Technology for Estimating Body Composition: Strengths and Weaknesses of Common Modalities in a Clinical Setting. Nutr. Clin. Pract. 2017, 32, 20–29. [Google Scholar] [CrossRef]
- Bamba, S.; Inatomi, O.; Takahashi, K.; Morita, Y.; Imai, T.; Ohno, M.; Kurihara, M.; Takebayashi, K.; Kojima, M.; Iida, H.; et al. Assessment of Body Composition from CT Images at the Level of the Third Lumbar Vertebra in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2021, 27, 1435–1442. [Google Scholar] [CrossRef]
- Muresan, B.T.; Sánchez Juan, C.; Artero, A.; Hernández Machancoses, A.; Almendros-Blanco, P.; Montoro, A.; Roselló Ferrando, J.; Íñigo Valdenebro, R.; Ríos Ríos, J.; Soriano del Castillo, J.M.; et al. Measurement of Body Composition in Cancer Patients Using CT Planning Scan at the Third Lumbar Vertebra. Nutr. Hosp. 2019, 36, 1307–1314. [Google Scholar] [CrossRef]
- Lee, C.S.; Won, D.D.; Oh, S.N.; Lee, Y.S.; Lee, I.K.; Kim, I.-H.; Choi, M.H.; Oh, S.T. Prognostic Role of Pre-Sarcopenia and Body Composition with Long-Term Outcomes in Obstructive Colorectal Cancer: A Retrospective Cohort Study. World J. Surg Oncol. 2020, 18, 230. [Google Scholar] [CrossRef]
- Campbell, J.P.; Teigen, L.; Manski, S.; Blumhof, B.; Guglielmo, F.F.; Shivashankar, R.; Shmidt, E. Sarcopenia Is More Prevalent Among Inflammatory Bowel Disease Patients Undergoing Surgery and Predicts Progression to Surgery Among Medically Treated Patients. Inflamm. Bowel Dis. 2022, izac013. [Google Scholar] [CrossRef] [PubMed]
- Boparai, G.; Kedia, S.; Kandasamy, D.; Sharma, R.; Madhusudhan, K.S.; Dash, N.R.; Sahu, P.; Pal, S.; Sahni, P.; Panwar, R.; et al. Combination of Sarcopenia and High Visceral Fat Predict Poor Outcomes in Patients with Crohn’s Disease. Eur. J. Clin. Nutr. 2021, 75, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Galata, C.; Hodapp, J.; Weiß, C.; Karampinis, I.; Vassilev, G.; Reißfelder, C.; Otto, M. Skeletal Muscle Mass Index Predicts Postoperative Complications in Intestinal Surgery for Crohn’s Disease. J. Parenter. Enter. Nutr. 2020, 44, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.Q.; Moore, G.T.; Strauss, B.J.G. Visceral Adiposity Predicts Post-Operative Crohn’s Disease Recurrence. Aliment. Pharmacol. Ther. 2017, 45, 1255–1264. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and Clinical Implications of Sarcopenic Obesity in Patients with Solid Tumours of the Respiratory and Gastrointestinal Tracts: A Population-Based Study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Grillot, J.; D’Engremont, C.; Parmentier, A.-L.; Lakkis, Z.; Piton, G.; Cazaux, D.; Gay, C.; De Billy, M.; Koch, S.; Borot, S.; et al. Sarcopenia and Visceral Obesity Assessed by Computed Tomography Are Associated with Adverse Outcomes in Patients with Crohn’s Disease. Clin. Nutr. 2020, 39, 3024–3030. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Camera, L.; Pezzullo, F.; Acampora, A.; Liuzzi, R.; Rispo, A.; Nardone, O.M.; Luglio, G.; Bucci, L.; Castiglione, F.; Brunetti, A. Multi-detector CT enterography in active inflammatory bowel disease: Image quality and diagnostic efficacy of a low radiation high contrast protocol. Clin. Imaging 2019, 58, 27–33. [Google Scholar] [CrossRef]
- Maddalena, C.; Ponsiglione, A.; Camera, L.; Santarpia, L.; Pasanisi, F.; Bruzzese, D.; Panico, C.; Fiore, G.; Camardella, S.; Caramia, T.; et al. Prognostic Role of Sarcopenia in Metastatic Colorectal Cancer Patients during First-Line Chemotherapy: A Retrospective Study. World J. Clin. Oncol. 2021, 12, 355–366. [Google Scholar] [CrossRef]
- Argeny, S.; Tamandl, D.; Scharitzer, M.; Stift, A.; Bergmann, M.; Riss, S. Visceral Fat Area Measured with Computed Tomography Does Not Predict Postoperative Course in Crohn’s Disease Patients. PLoS ONE 2018, 13, e0202220. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A simple index of crohn’s-disease activity. Lancet 1980, 315, 514. [Google Scholar] [CrossRef]
- Le Berre, C.; Ricciuto, A.; Peyrin-Biroulet, L.; Turner, D. Evolving Short- and Long-Term Goals of Management of Inflammatory Bowel Diseases: Getting It Right, Making It Last. Gastroenterology 2022, 162, 1424–1438. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Rispo, A.; Imbriaco, M.; Celentano, L.; Cozzolino, A.; Camera, L.; Mainenti, P.P.; Manguso, F.; Sabbatini, F.; DʼAmico, P.; Castiglione, F. Noninvasive Diagnosis of Small Bowel Crohn’s Disease: Combined Use of Bowel Sonography and Tc-99M-Hmpao Leukocyte Scintigraphy. Inflamm. Bowel Dis. 2005, 11, 376–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ananthakrishnan, A.N. Frailty in Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2021, 17, 263–268. [Google Scholar]
- Ünal, N.G.; Oruç, N.; Tomey, O.; Ömer Özütemiz, A. Malnutrition and Sarcopenia Are Prevalent among Inflammatory Bowel Disease Patients with Clinical Remission. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Rajabali, N.; Tandon, P.; Abraldes, J.G.; Peerani, F. Association Between Frailty or Sarcopenia and Adverse Outcomes in Inflammatory Bowel Disease: A Systematic Review. Gastroenterol. Hepatol. Adv. 2022, 1, 241–250. [Google Scholar] [CrossRef]
- Zhang, Y.; Weng, S.; Huang, L.; Shen, X.; Zhao, F.; Yan, S. Association of Sarcopenia with a Higher Risk of Infection in Patients with Type 2 Diabetes. Diabetes Metab. Res. 2022, 38, e3478. [Google Scholar] [CrossRef]
- Krell, R.W.; Kaul, D.R.; Martin, A.R.; Englesbe, M.J.; Sonnenday, C.J.; Cai, S.; Malani, P.N. Association between Sarcopenia and the Risk of Serious Infection among Adults Undergoing Liver Transplantation: Sarcopenia and Posttransplant Infection. Liver Transpl. 2013, 19, 1396–1402. [Google Scholar] [CrossRef]
- Takagi, K.; Yagi, T.; Yoshida, R.; Umeda, Y.; Nobuoka, D.; Kuise, T.; Fujiwara, T. Sarcopenia Predicts Postoperative Infection in Patients Undergoing Hepato-Biliary-Pancreatic Surgery. Int. J. Surg. Open 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Erhayiem, B.; Dhingsa, R.; Hawkey, C.J.; Subramanian, V. Ratio of Visceral to Subcutaneous Fat Area Is a Biomarker of Complicated Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2011, 9, 684–687.e1. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Feng, S.-T.; Cao, Q.-H.; Coffey, J.C.; Baker, M.E.; Huang, L.; Fang, Z.-N.; Qiu, Y.; Lu, B.-L.; Chen, Z.-H.; et al. Degree of Creeping Fat Assessed by Computed Tomography Enterography Is Associated with Intestinal Fibrotic Stricture in Patients with Crohn’s Disease: A Potentially Novel Mesenteric Creeping Fat Index. J. Crohn’s Colitis 2021, 15, 1161–1173. [Google Scholar] [CrossRef]
- Mao, R.; Kurada, S.; Gordon, I.O.; Baker, M.E.; Gandhi, N.; McDonald, C.; Coffey, J.C.; Rieder, F. The Mesenteric Fat and Intestinal Muscle Interface: Creeping Fat Influencing Stricture Formation in Crohn’s Disease. Inflamm. Bowel Dis. 2019, 25, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Xu, M.; Zhou, Y.; Geng, N.; Lin, N.; Song, W.; Li, S.; Piao, Y.; Han, Z.; Guo, R.; et al. Assessing Visceral Obesity and Abdominal Adipose Tissue Distribution in Healthy Populations Based on Computed Tomography: A Large Multicenter Cross-Sectional Study. Front. Nutr. 2022, 9, 871697. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Rieder, F.; Kucharzik, T.; Peyrin-Biroulet, L.; Schoepfer, A.M.; Rubin, D.T.; Vavricka, S.R. Emerging Treatment Options for Extraintestinal Manifestations in IBD. Gut 2021, 70, 796–802. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.; Rogler, G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1982–1992. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Guillo, L.; Abreu, M.; Panaccione, R.; Sandborn, W.J.; Azevedo, V.F.; Gensler, L.; Moghaddam, B.; Ahuja, V.; Ali, S.A.; Allez, M.; et al. Endpoints for Extraintestinal Manifestations in Inflammatory Bowel Disease Trials: The EXTRA Consensus from the International Organization for the Study of Inflammatory Bowel Diseases. Lancet Gastroenterol. Hepatol. 2022, 7, 254–261. [Google Scholar] [CrossRef]
- Zundler, S.; Günther, C.; Kremer, A.E.; Zaiss, M.M.; Rothhammer, V.; Neurath, M.F. Gut Immune Cell Trafficking: Inter-Organ Communication and Immune-Mediated Inflammation. Nat. Rev. Gastroenterol. Hepatol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Schett, G.; McInnes, I.B.; Neurath, M.F. Reframing Immune-Mediated Inflammatory Diseases through Signature Cytokine Hubs. N. Engl. J. Med. 2021, 385, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, K.; Fallon, K.; Ruut, T.; Lane, D.; McKay, R.; Shadbolt, B.; Ang, S.; Cook, M.; Platten, J.; Pavli, P.; et al. Infliximab Reverses Inflammatory Muscle Wasting (Sarcopenia) in Crohn’s Disease. Aliment. Pharmacol. Ther. 2015, 41, 419–428. [Google Scholar] [CrossRef]
- Murdaca, G.; Spanò, F.; Contatore, M.; Guastalla, A.; Penza, E.; Magnani, O.; Puppo, F. Infection Risk Associated with Anti-TNF-α Agents: A Review. Expert Opin. Drug Saf. 2015, 14, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.M.; Al-Jaouni, R.; Filippi, J.; Wiroth, J.-B.; Zeanandin, G.; Arab, K.; Hébuterne, X. Sarcopenia Is Prevalent in Patients with Crohn’s Disease in Clinical Remission. Inflamm. Bowel Dis. 2008, 14, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Nappi, C.; Megna, R.; Volpe, F.; Ponsiglione, A.; Caiazzo, E.; Piscopo, L.; Mainolfi, C.G.; Vergara, E.; Imbriaco, M.; Klain, M.; et al. Quantification of Coronary Artery Atherosclerotic Burden and Muscle Mass: Exploratory Comparison of Two Freely Available Software Programs. Appl. Sci. 2022, 12, 5468. [Google Scholar] [CrossRef]
- Nappi, C.; Ponsiglione, A.; Acampa, W.; Gaudieri, V.; Zampella, E.; Assante, R.; Cuocolo, R.; Mannarino, T.; Dell’Aversana, S.; Petretta, M.; et al. Relationship between Epicardial Adipose Tissue and Coronary Vascular Function in Patients with Suspected Coronary Artery Disease and Normal Myocardial Perfusion Imaging. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1379–1387. [Google Scholar] [CrossRef]
| Total (n = 63) | |
|---|---|
| Age | 44.2 ± 17.0 |
| Sex | |
| Male | 34 (54.0) |
| Female | 29 (46.0) |
| BMI (kg/m2) | 21.2 ± 3.6 |
| SMI (cm2/m2) | 43.6 ± 9.1 |
| SFA (cm2) | 111.8 (91.6) |
| Sarcopenic patients | 43 (68.3) |
| VFA (cm2) | 57.0 ± 35.0 |
| VFA/SFA | 0.62 ± 0.58 |
| Disease duration (months) | 140.0 ± 106.5 |
| CD Montreal disease localization | |
| Ileal | 13 (20.6) |
| Colonic | 3 (4.8) |
| Ileo-colonic | 46 (73.0) |
| Isolated upper-GI | 1 (1.6) |
| CD Montreal disease behavior | |
| Inflammatory | 2 (3.2) |
| Stricturing | 36 (57.1) |
| Penetrating | 25 (39.7) |
| Perianal disease | 15 (23.8) |
| HBI | 7.8 ± 1.7 |
| SES-CD | 7.8 ± 5.9 |
| BWT (mm) | 7.0 ± 2.2 |
| CDE (cm) | 17.8 ± 14.6 |
| Extraintestinal manifestations | 13 (20.6) |
| Musculoskeletal manifestations | 11 (17.5) |
| Cutaneous manifestations | 1 (1.6) |
| Ocular Manifestations | 1 (1.6) |
| Baseline anti-TNF-α therapy | 18 (28.6) |
| Baseline CCS therapy | 15 (23.8) |
| Baseline ISS therapy | 14 (22.2) |
| Previous anti-TNF-α therapy | 26 (41.3) |
| CRP (mg/L) | 2.9 ± 4.0 |
| FC (μg/g) | 302.7 ± 163.4 |
| Serum albumin (g/dL) | 3.6 ± 0.6 |
| Patients with Sarcopenia (n = 43) | Patients without Sarcopenia (n = 20) | p-Value | |
|---|---|---|---|
| BMI (kg/m2) | 20.3 ± 3.1 | 23.3 ± 3.8 | 0.002 |
| SFA (cm2) | 94.1 ± 90.4 | 149.9 ± 84.2 | 0.009 |
| VFA (cm2) | 54.0 ± 63.1 | 63.4 ± 62.7 | 0.54 |
| VFA/SFA ratio | 0.7 ± 0.6 | 0.4 ± 0.4 | 0.04 |
| Age | 45.6 ± 18.0 | 41.1 ± 14.8 | 0.49 |
| CRP (mg/L) | 2.91 ± 4.1 | 2.98 ± 3.8 | 0.54 |
| Serum albumin (g/L) | 3.49 ± 0.6 | 3.88 ± 0.5 | 0.029 |
| FC (μg/g) | 334.66 ± 167.2 | 238.95 ± 138.7 | 0.03 |
| Female gender | 18 (41.7) | 11 (55.0) | 0.33 |
| EIM | 12 (27.9) | 1 (5.0) | 0.04 |
| Baseline anti-TNF-α therapy | 10 (23.2) | 8 (40.0) | 0.17 |
| Baseline CCS therapy | 8 (18.6) | 7 (35.0) | 0.15 |
| Baseline ISS therapy | 9 (20.9) | 5 (25.0) | 0.72 |
| Previous anti-TNF-α therapy | 20 (46.5) | 6 (30.0) | 0.21 |
| Need for surgery | 21 (48.8) | 12 (60.0) | 0.41 |
| Infectious events | 18 (41.9) | 3 (15.0) | 0.03 |
| Low respiratory tract | 6 (13.9) | 1 (5.0) | |
| PICC-related | 8 (18.6) | 0 | |
| Urinary tract | 1 (2.3) | 1 (5.0) | |
| Herpesvirus-related | 2 (4.6) | 1 (5.0) | |
| Gynaecologic | 1 (2.3) | 0 |
| 95% C.I. per EXP(B) | ||||||
|---|---|---|---|---|---|---|
| Estimated Coefficient | Standard Error | p-Value | Exp(B) | Lower Limit | Upper Limit | |
| Body Mass Index | −0.309 | 0.122 | 0.011 | 0.734 | 0.578 | 0.932 |
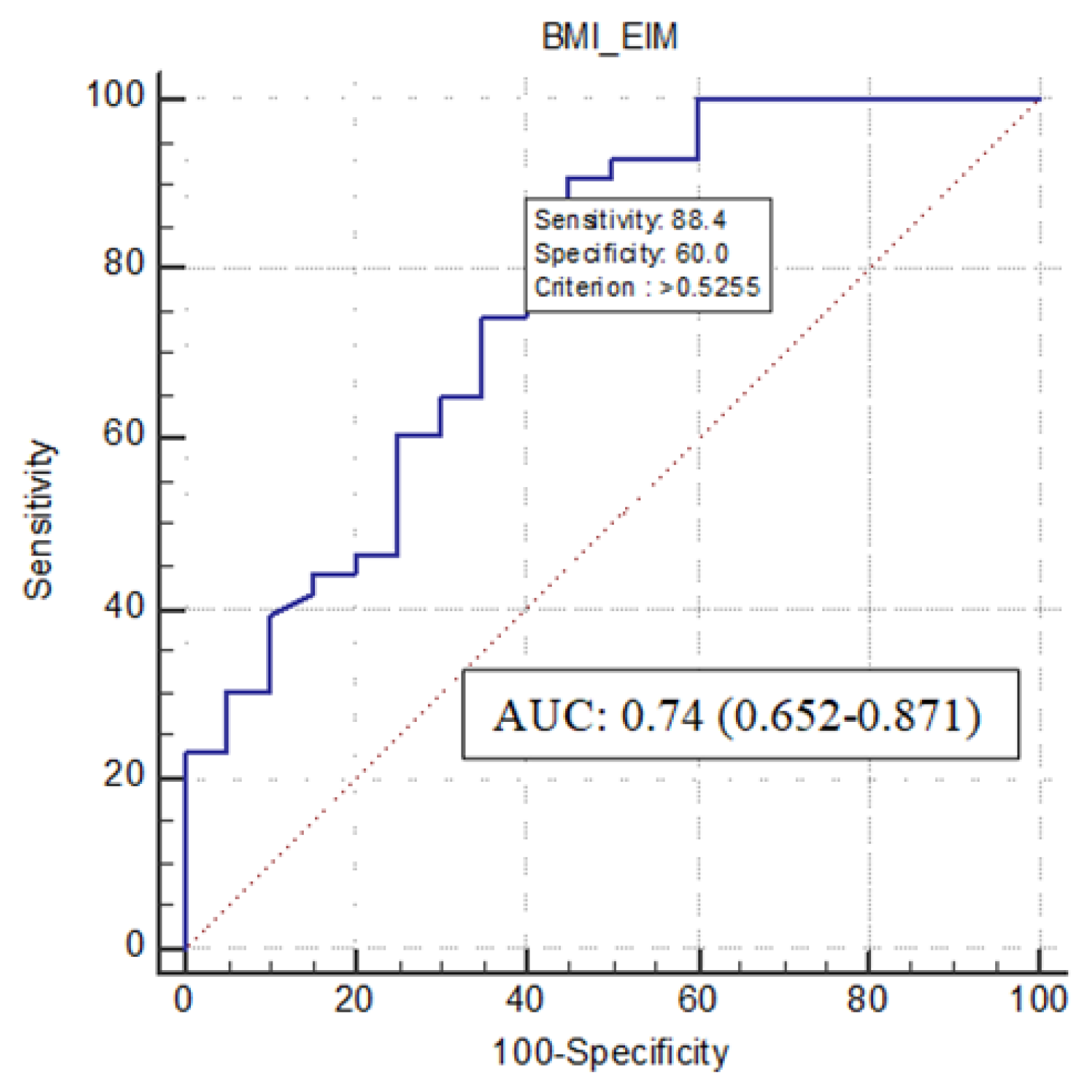
| EIM | 2.957 | 1.479 | 0.046 | 19.233 | 1.059 | 349.139 |
| Constant | 6.855 | 2.591 | 0.008 | 948.241 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardone, O.M.; Ponsiglione, A.; de Sire, R.; Calabrese, G.; Liuzzi, R.; Testa, A.; Guarino, A.D.; Olmo, O.; Rispo, A.; Camera, L.; et al. Impact of Sarcopenia on Clinical Outcomes in a Cohort of Caucasian Active Crohn’s Disease Patients Undergoing Multidetector CT-Enterography. Nutrients 2022, 14, 3460. https://doi.org/10.3390/nu14173460
Nardone OM, Ponsiglione A, de Sire R, Calabrese G, Liuzzi R, Testa A, Guarino AD, Olmo O, Rispo A, Camera L, et al. Impact of Sarcopenia on Clinical Outcomes in a Cohort of Caucasian Active Crohn’s Disease Patients Undergoing Multidetector CT-Enterography. Nutrients. 2022; 14(17):3460. https://doi.org/10.3390/nu14173460
Chicago/Turabian StyleNardone, Olga Maria, Andrea Ponsiglione, Roberto de Sire, Giulio Calabrese, Raffaele Liuzzi, Anna Testa, Alessia Dalila Guarino, Oriana Olmo, Antonio Rispo, Luigi Camera, and et al. 2022. "Impact of Sarcopenia on Clinical Outcomes in a Cohort of Caucasian Active Crohn’s Disease Patients Undergoing Multidetector CT-Enterography" Nutrients 14, no. 17: 3460. https://doi.org/10.3390/nu14173460
APA StyleNardone, O. M., Ponsiglione, A., de Sire, R., Calabrese, G., Liuzzi, R., Testa, A., Guarino, A. D., Olmo, O., Rispo, A., Camera, L., & Castiglione, F. (2022). Impact of Sarcopenia on Clinical Outcomes in a Cohort of Caucasian Active Crohn’s Disease Patients Undergoing Multidetector CT-Enterography. Nutrients, 14(17), 3460. https://doi.org/10.3390/nu14173460







