Probiotics/Synbiotics to Reduce Infectious Complications after Colorectal Surgery: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Review Design
2.2. Search Strategy
2.3. Inclusion Criteria
2.4. Data Extraction
2.5. Assessment of Methodological Quality
2.6. Subgroups Analyses
2.7. Certainty of Evidence Analyses
2.8. Data Synthesis and Statistical Analysis
3. Results
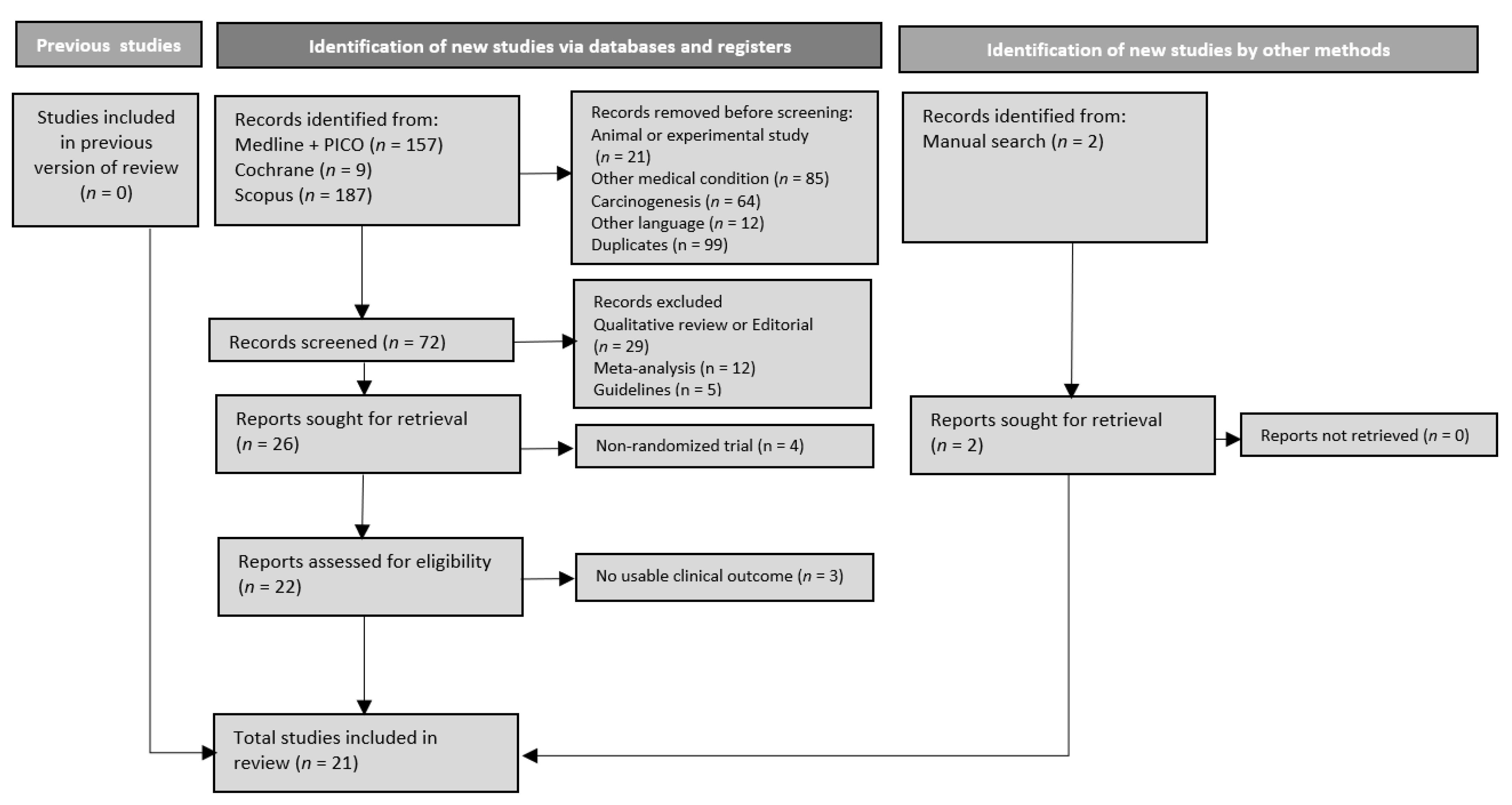
3.1. Protocol Deviations
3.2. Search Results and Trials’ Characteristics
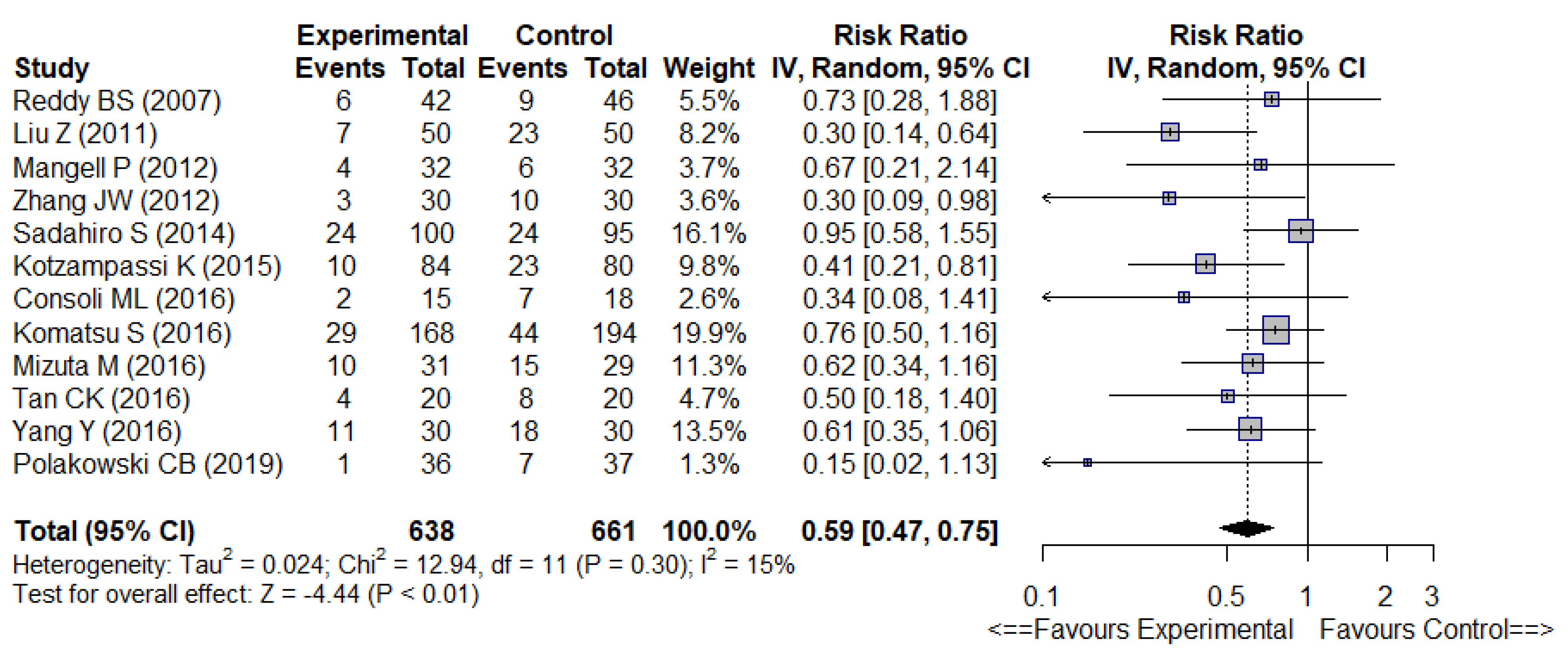
3.3. Overall Results
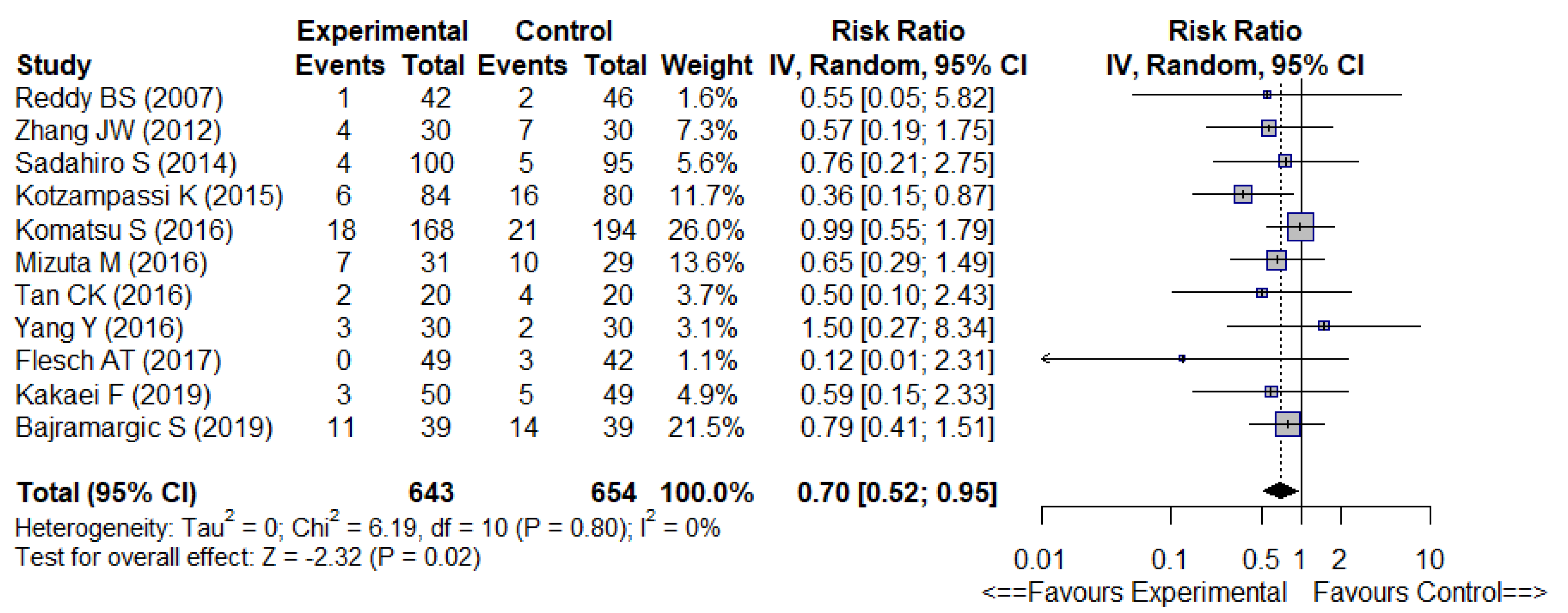
3.3.1. Primary Outcomes
3.3.2. Secondary Outcomes
3.4. Sensitivity (Subgroup) Analyses
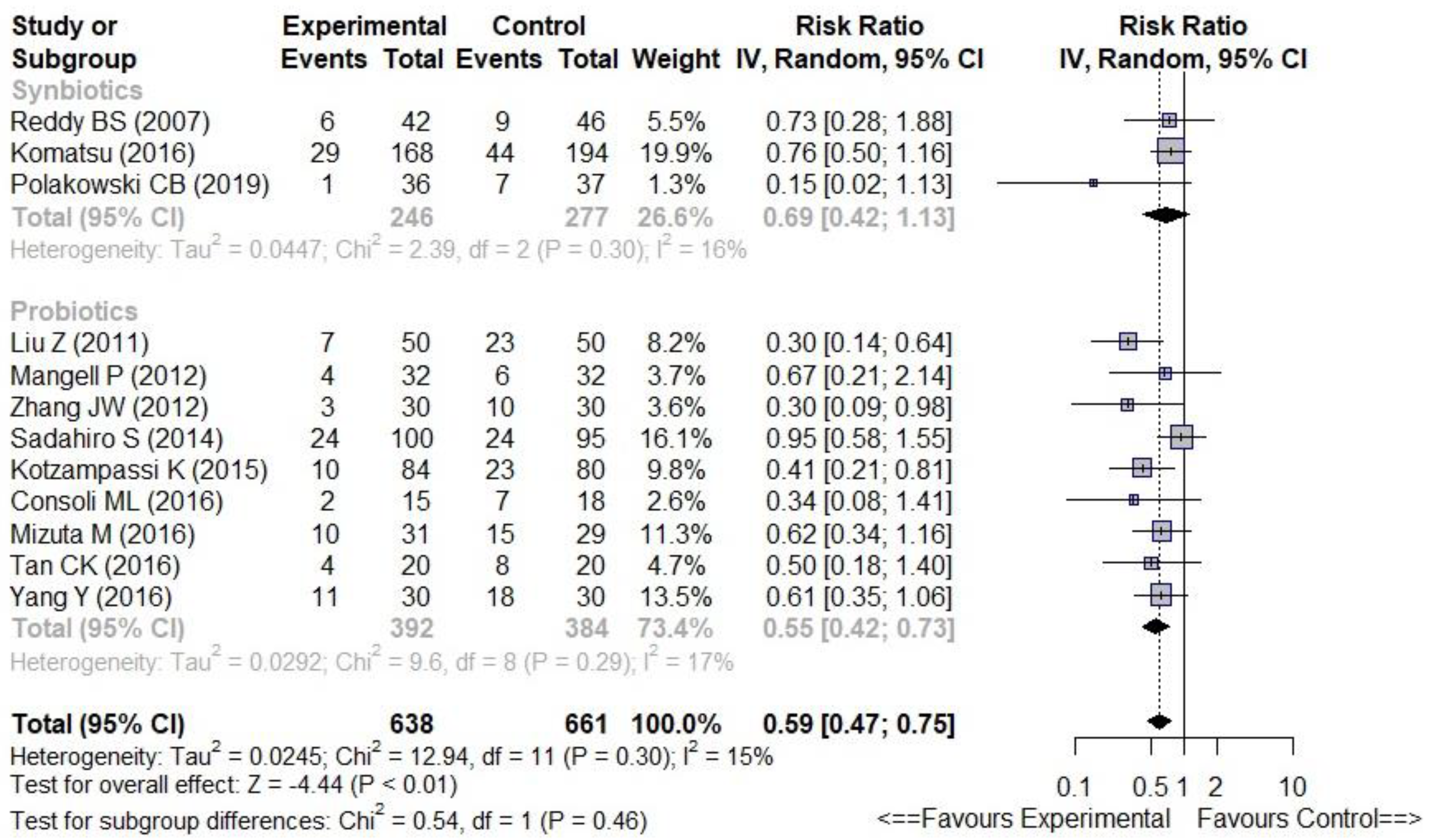
3.4.1. Probiotics versus Synbiotics
3.4.2. Multistrain vs. Non-Multistrain Formulations
3.4.3. Preoperative vs. Perioperative vs. Postoperative
3.4.4. Placebo vs. Standard Care Controls
3.4.5. Quality of RCTs
3.4.6. Ethnicity
3.4.7. Competing Interests
3.5. Bias of Publication
3.6. Certainty of the Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Emerging Infections Program Hospital Prevalence Survey Team. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Fuglestad, M.A.; Tracey, E.L.; Leinicke, J.A. Evidence-based Prevention of Surgical Site Infection. Surg. Clin. North Am. 2021, 101, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Kiran, R.P.; El-Gazzaz, G.H.; Vogel, J.D.; Remzi, F.H. Laparoscopic approach significantly reduces surgical site infections after colorectal surgery: Data from national surgical quality improvement program. J. Am. Coll. Surg. 2010, 211, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Blanc, M.C.; Slim, K.; Beyer-Berjot, L. Best practices in bowel preparation for colorectal surgery: A 2020 overview. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 681–688. [Google Scholar] [CrossRef]
- Espin Basany, E.; Solís-Peña, A.; Pellino, G.; Kreisler, E.; Fraccalvieri, D.; Muinelo-Lorenzo, M.; Maseda-Díaz, O.; García-González, J.M.; Santamaría-Olabarrieta, M.; Codina-Cazador, A. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): A multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2020, 5, 729–738. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.V.; Ockhuizen, T. New insights into the impact of the intestinal microbiota on health and disease: A symposium report. Br. J. Nutr. 2012, 107 (Suppl. S1), S1–S13. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, S.; Yokoyama, Y.; Nagino, M. Gut microbiota and bacterial translocation in digestive surgery: The impact of probiotics. Langenbecks Arch. Surg. 2017, 402, 401–416. [Google Scholar] [CrossRef]
- He, D.; Wang, H.Y.; Feng, J.Y.; Zhang, M.M.; Zhou, Y.; Wu, X.T. Use of pro-/synbiotics as prophylaxis in patients undergoing colorectal resection for cancer: A meta-analysis of randomized controlled trials. Clin. Res. Hepatol. Gastroenterol. 2013, 37, 406–415. [Google Scholar] [CrossRef]
- de Andrade Calaça, P.R.; Pedrosa Bezerra, R.; Campos Albuquerque, W.W.; Figueiredo Porto, A.L.; Holanda Cavalcanti, M.T. Probiotics as a preventive strategy for surgical infection in colorectal cancer patients: A systematic review and meta-analysis of randomized trials. Transl. Gastroenterol. Hepatol. 2017, 2, 67. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.C.; Yan, Y.K.; Ma, Y.J.; Wang, X.W.; Geng, J.; Wang, M.C.; Wei, F.X.; Zhang, Y.W.; Xu, X.D.; Zhang, Y.C. Probiotics Reduce Postoperative Infections in Patients Undergoing Colorectal Surgery: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2017, 2017, 6029075. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.D.; Xu, W.; Liu, M.M.; Hu, K.J.; Sun, Y.Y.; Yang, X.F.; Zhu, G.Q.; Wang, Z.W.; Huang, W. Efficacy of prophylactic probiotics in combination with antibiotics versus antibiotics alone for colorectal surgery: A meta-analysis of randomized controlled trials. J. Surg. Oncol. 2018, 117, 1394–1404. [Google Scholar] [CrossRef]
- Chen, C.; Wen, T.; Zhao, Q. Probiotics Used for Postoperative Infections in Patients Undergoing Colorectal Cancer Surgery. Biomed. Res. Int. 2020, 2020, 5734718. [Google Scholar] [CrossRef]
- Ouyang, X.; Li, Q.; Shi, M.; Niu, D.; Song, W.; Nian, Q.; Li, X.; Ding, Z.; Ai, X.; Wang, J. Probiotics for preventing postoperative infection in colorectal cancer patients: A systematic review and meta-analysis. Int. J. Colorectal. Dis. 2019, 34, 459–469. [Google Scholar] [CrossRef]
- Amitay, E.L.; Carr, P.R.; Gies, A.; Laetsch, D.C.; Brenner, H. Probiotic/Synbiotic Treatment and Postoperative Complications in Colorectal Cancer Patients: Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin. Transl. Gastroenterol. 2020, 11, e00268. [Google Scholar] [CrossRef]
- Zeng, J.; Ji, Y.; Liang, B.; Zhang, G.; Chen, D.; Zhu, M.; Wu, S.; Kuang, W. The effect of pro/synbiotics on postoperative infections in colorectal cancer patients: A systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2021, 43, 101370. [Google Scholar] [CrossRef]
- Slim, K.; Marquillier, T. Umbrella reviews: A new tool to synthesize scientific evidence in surgery. J. Visc. Surg. 2021, 28, 144–149. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials. 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [Green Version]
- van Aert, R.C.M.; Jackson, D. Multistep estimators of the between-study variance: The relationship with the Paule-Mandel estimator. Stat. Med. 2018, 37, 2616–2629. [Google Scholar] [CrossRef] [Green Version]
- Michael, B.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H. Introduction to Meta-Analysis; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Reddy, B.S.; Macfie, J.; Gatt, M.; Larsen, C.N.; Jensen, S.S.; Leser, T.D. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br. J. Surg. 2007, 94, 546–554. [Google Scholar] [CrossRef]
- Horvat, M.; Krebs, B.; Potrc, S.; Ivanecz, A.; Kompan, L. Preoperative synbiotic bowel conditioning for elective colorectal surgery. Wien. Klin. Wochenschr. 2010, 122 (Suppl. S2), 26–30. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, H.; Yang, Z.; Xia, Y.; Liu, W.; Yang, J.; Jiang, Y.; Zhang, H.; Yang, Z.; Wang, Y.; et al. Randomised clinical trial: The effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery-A double-blind study. Aliment. Pharmacol. Ther. 2011, 33, 50–63. [Google Scholar] [CrossRef]
- Mangell, P.; Thorlacius, H.; Syk, I.; Ahrné, S.; Molin, G.; Olsson, C.; Jeppsson, B. Lactobacillus plantarum 299v does not reduce enteric bacteria or bacterial translocation in patients undergoing colon resection. Dig. Dis. Sci. 2012, 57, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Du, P.; Gao, J.; Yang, B.R.; Fang, W.J.; Ying, C.M. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am. J. Med. Sci. 2012, 343, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Huang, M.J.; Zhang, X.W.; Wang, L.; Huang, N.Q.; Peng, H.; Lan, P.; Peng, J.S.; Yang, Z.; Xia, Y.; et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: A double-center and double-blind randomized clinical trial. Am. J. Clin. Nutr. 2013, 97, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, B.; Horvat, M.; Golle, A.; Krznaric, Z.; Papeš, D.; Augustin, G.; Arslani, N.; Potrč, S. A randomized clinical trial of synbiotic treatment before colorectal cancer surgery. Am. Surg. 2013, 79, E340–E342. [Google Scholar] [CrossRef]
- Sadahiro, S.; Suzuki, T.; Tanaka, A.; Okada, K.; Kamata, H.; Ozaki, T.; Koga, Y. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: Prospective randomized trial. Surgery 2014, 155, 493–503. [Google Scholar] [CrossRef]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J. Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef]
- Consoli, M.L.; da Silva, R.S.; Nicoli, J.R.; Bruña-Romero, O.; da Silva, R.G.; de Vasconcelos Generoso, S.; Correia, M.I. Randomized Clinical Trial: Impact of Oral Administration of Saccharomyces boulardii on Gene Expression of Intestinal Cytokines in Patients Undergoing Colon Resection. JPEN J. Parenter. Enteral Nutr. 2016, 40, 1114–1121. [Google Scholar] [CrossRef]
- Komatsu, S.; Sakamoto, E.; Norimizu, S.; Shingu, Y.; Asahara, T.; Nomoto, K.; Nagino, M. Efficacy of perioperative synbiotics treatment for the prevention of surgical site infection after laparoscopic colorectal surgery: A randomized controlled trial. Surg. Today 2016, 46, 479–490. [Google Scholar] [CrossRef]
- Mizuta, M.; Endo, I.; Yamamoto, S.; Inokawa, H.; Kubo, M.; Udaka, T.; Sogabe, O.; Maeda, H.; Shirakawa, K.; Okazaki, E.; et al. Perioperative supplementation with bifidobacteria improves postoperative nutritional recovery, inflammatory response, and fecal microbiota in patients undergoing colorectal surgery: A prospective, randomized clinical trial. Biosci. Microbiota Food Health 2016, 35, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.K.; Said, S.; Rajandram, R.; Wang, Z.; Roslani, A.C.; Chin, K.F. Pre-surgical Administration of Microbial Cell Preparation in Colorectal Cancer Patients: A Randomized Controlled Trial. World J. Surg. 2016, 40, 1985–1992. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Chen, H.; Hong, L.; Feng, J.; Yang, J.; Yang, Z.; Shi, C.; Wu, W.; Gao, R.; et al. The effect pf perioperative probiotics treatment for colorectal cancer: Short-term outcomes of a randomized controlled trial. Oncotarget 2016, 7, 8432–8440. [Google Scholar] [CrossRef] [Green Version]
- Flesch, A.T.; Tonial, S.T.; DE Carvalho Contu, P.; Damin, D.C. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: A randomized, double-blind clinical trial. Rev. Col. Bras. Cir. 2017, 44, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Kakaei, F.; Shahrasbi, M.; Asvadi Kermani, T.; Taheri, S.; Tarvirdizade, K. Assessment of probiotic effects on colorectal surgery complications: A double blind, randomized clinical trial. Biomed. Res. Ther. 2016, 6, 3067–3072. [Google Scholar] [CrossRef] [Green Version]
- Polakowski, C.B.; Kato, M.; Preti, V.B.; Schieferdecker, M.E.M.; Ligocki Campos, A.C. Impact of the preoperative use of synbiotics in colorectal cancer patients: A prospective, randomized, double-blind, placebo-controlled study. Nutrition 2019, 58, 40–46. [Google Scholar] [CrossRef]
- Bajramagic, S.; Hodzic, E.; Mulabdic, A.; Holjan, S.; Smajlovic, S.V.; Rovcanin, A. Usage of Probiotics and its Clinical Significance at Surgically Treated Patients Sufferig from Colorectal Carcinoma. Med. Arch. 2019, 73, 316–320. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, P.; Cen, Y.; Li, W. Effects of preoperative oral administration of glucose solution combined with postoperative probiotics on inflammation and intestinal barrier function in patients after colorectal cancer surgery. Oncol. Lett. 2019, 18, 694–698. [Google Scholar] [CrossRef] [Green Version]
- Park, I.J.; Lee, J.H.; Kye, B.H.; Oh, H.K.; Cho, Y.B.; Kim, Y.T.; Kim, J.Y.; Sung, N.Y.; Kang, S.B.; Seo, J.M.; et al. Effects of PrObiotics on the Symptoms and Surgical ouTComes after Anterior REsection of Colon Cancer (POSTCARE): A Randomized, double-blind, placebo-controlled trial. J. Clin. Med. 2020, 9, 2181. [Google Scholar] [CrossRef]
- Wang, P.; Yin, X.; Chen, G.; Li, L.; Le, Y.; Xie, Z.; Ouyang, W.; Tong, J. Perioperative probiotic treatment decreased the incidence of postoperative cognitive impairment in elderly patients following non-cardiac surgery: A randomised double-blind and placebo-controlled trial. Clin. Nutr. 2021, 40, 64–71. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, A.; Teng, D.; Li, S.; Yan, Y.; Hu, S.; Du, X. Probiotics and synbiotics for preventing postoperative infectious complications in colorectal cancer patients: A systematic review and meta-analysis. Tech. Coloproctol. 2022, 26, 425–436. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Adiamah, A.; Kushairi, A.; Varadhan, K.K.; Krznaric, Z.; Kulkarni, A.D.; Neal, K.R.; Lobo, D.N. Perioperative probiotics or synbiotics in adults undergoing elective abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. Ann. Surg. 2020, 271, 1036–1047. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Neugent, M.L.; Hulyalkar, N.V.; Nguyen, V.H.; Zimmern, P.E.; De Nisco, N.J. Advances in Understanding the Human Urinary Microbiome and Its Potential Role in Urinary Tract Infection. mBio 2020, 11, e00218-20. [Google Scholar] [CrossRef]
- Slim, K.; Theissen, A. Enhanced recovery after elective surgery. A revolution that reduces post-operative morbidity and mortality. J. Visc. Surg. 2020, 157, 487–491. [Google Scholar] [CrossRef]
- Ljungqvist, O.; de Boer, H.D.; Balfour, A.; Fawcett, W.J.; Lobo, D.N.; Nelson, G.; Scott, M.J.; Wainwright, T.W.; Demartines, N. Opportunities and Challenges for the Next Phase of Enhanced Recovery After Surgery: A Review. JAMA Surg. 2021, 156, 775–784. [Google Scholar] [CrossRef]
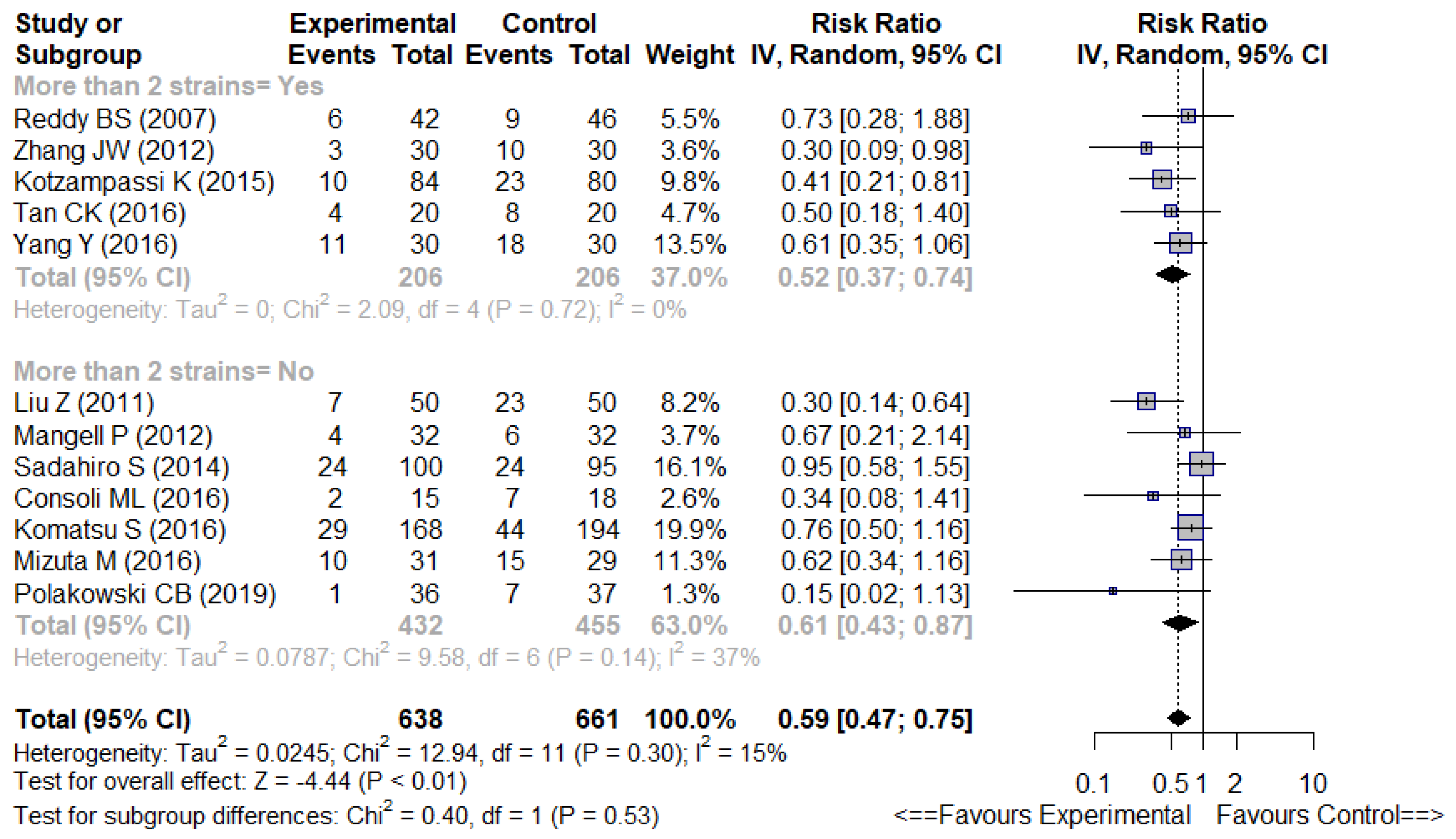
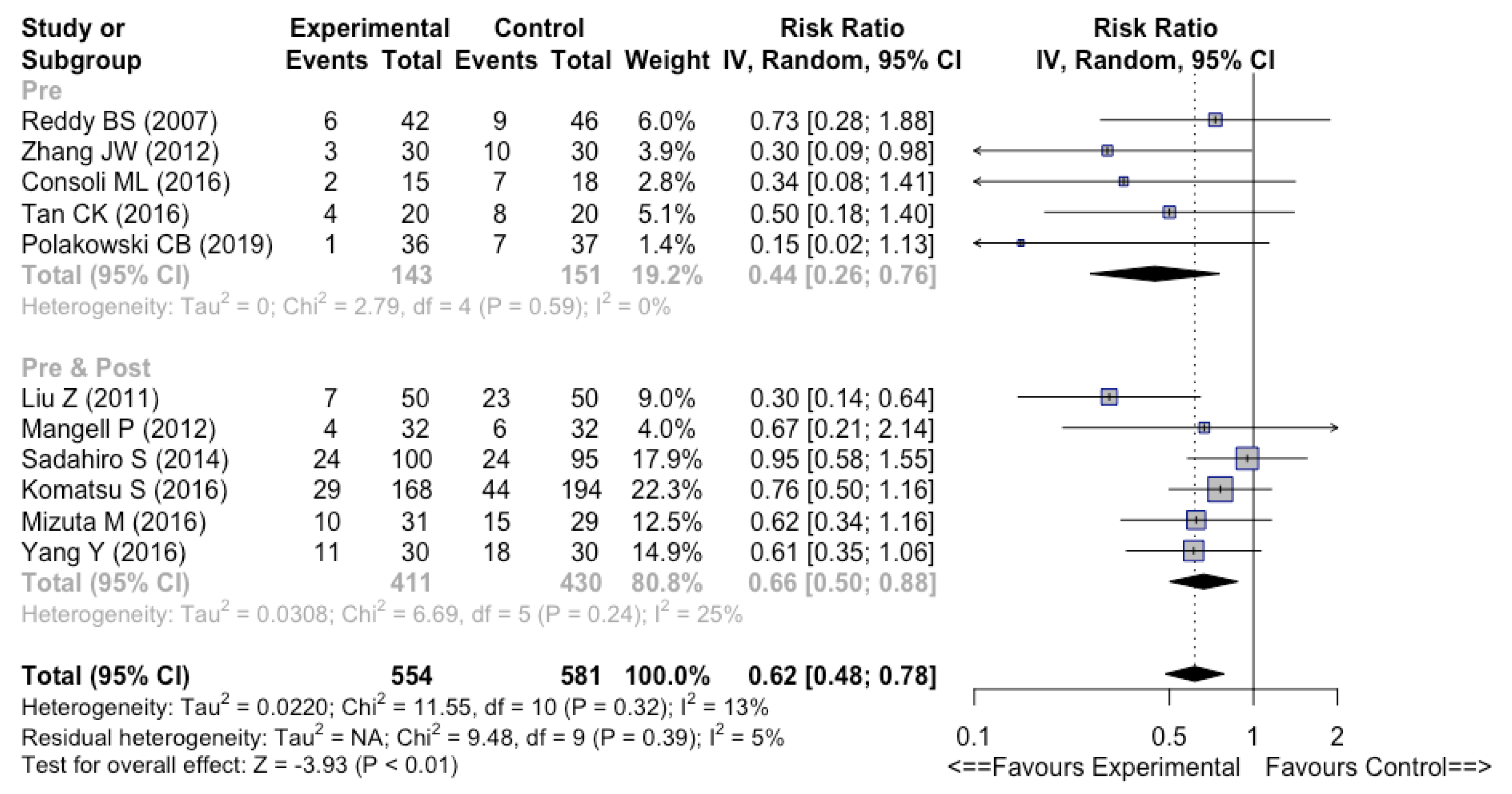
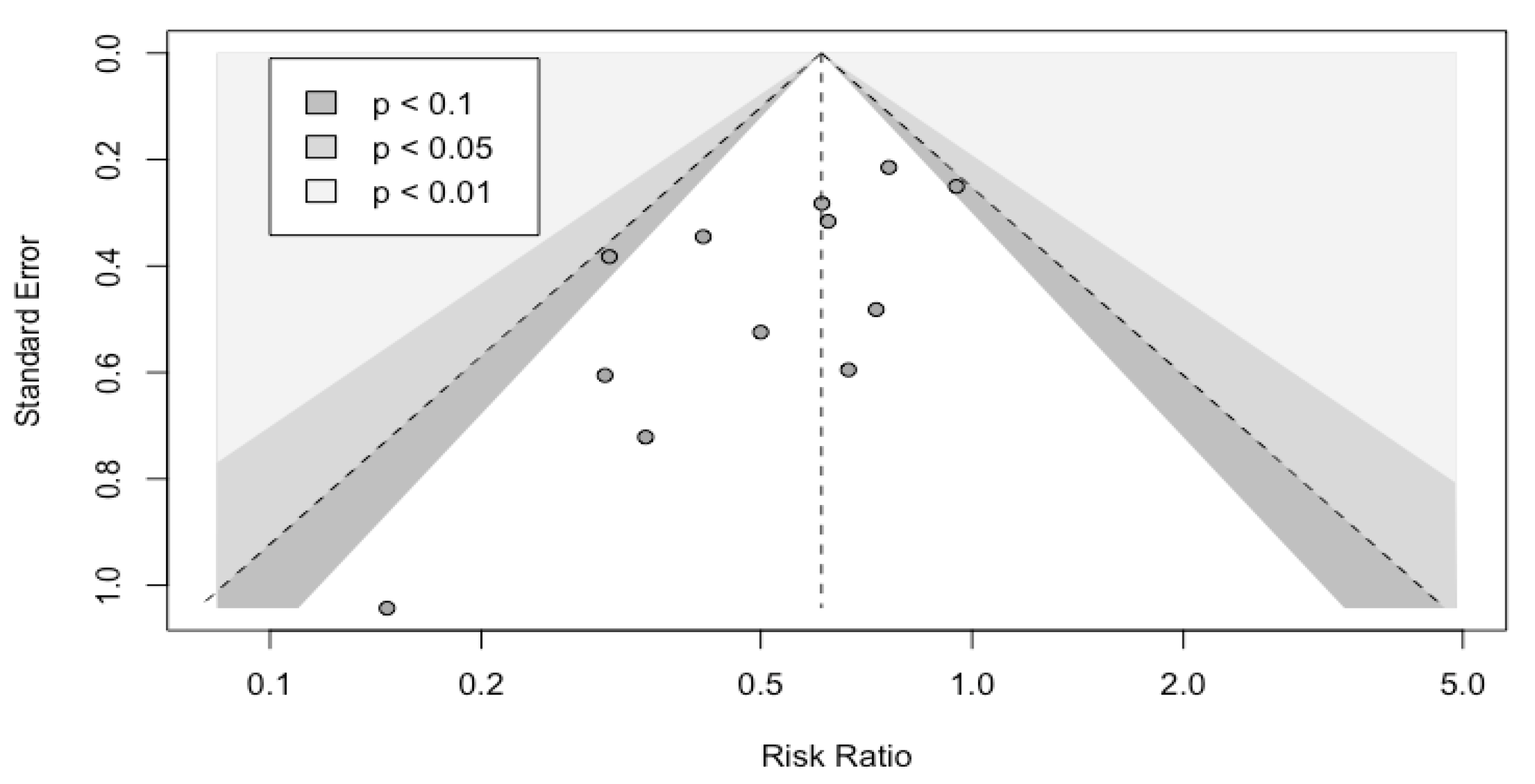
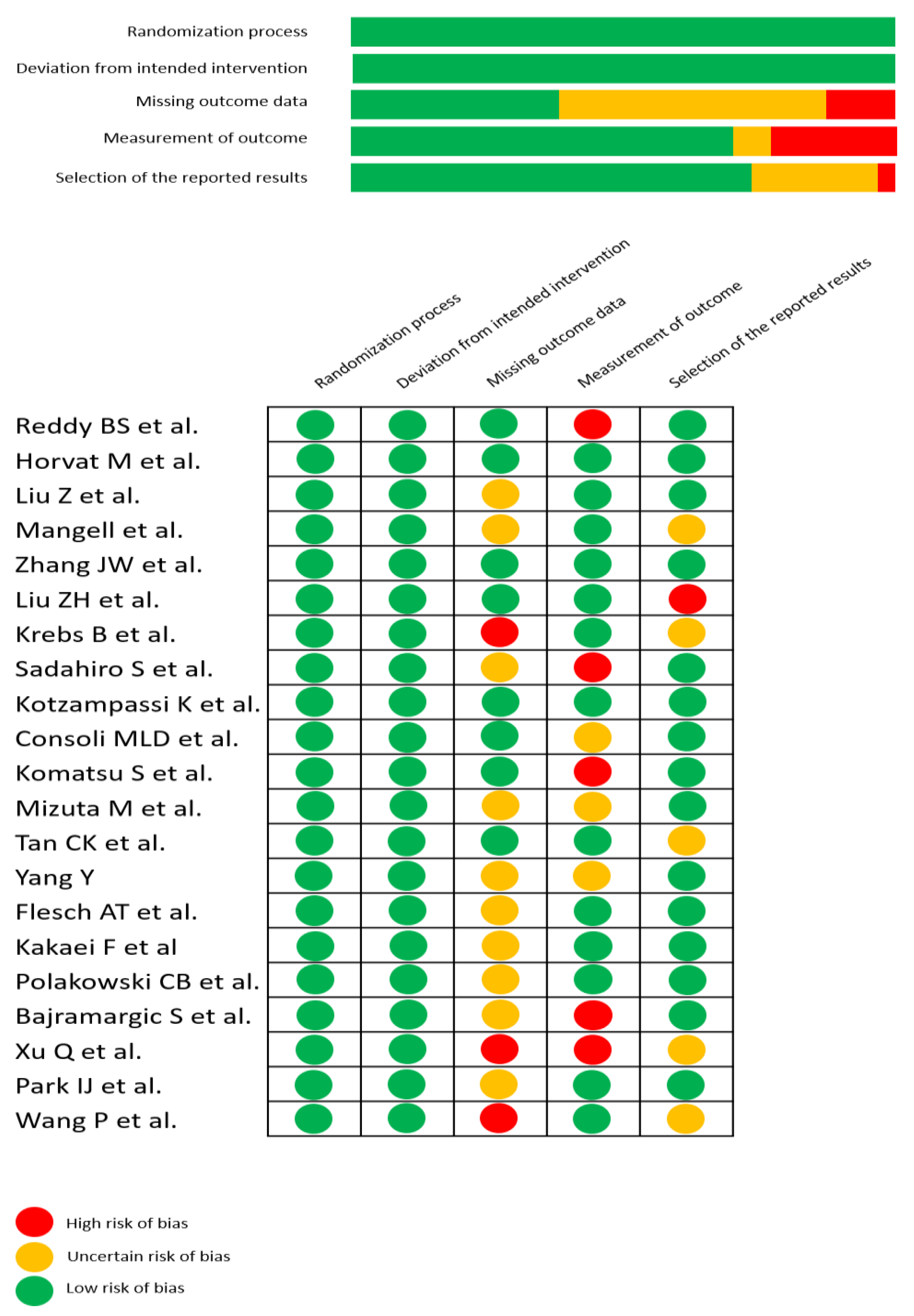
| First Author (Year) | Country | Number of Patients | Formulation | Control | Timing | Duration | Strains | Effect on Infectious Complications | Competing Interests | Jadad Scale |
|---|---|---|---|---|---|---|---|---|---|---|
| Reddy BS (2007) [24] | Denmark | 88 | Synbiot | SC | Pre | NA | L + B + S | No effect | No | 3 |
| Horvat M (2010) [25] | Slovenia | 40 | Synbiot | Placebo | Pre | NA | L | No effect | Yes | 5 |
| Liu Z (2011) [26] | China | 100 | Probiot | Placebo | Pre and Post | 6d & 10d | L + B | Decreased infectious complications | Yes | 5 |
| Mangell P (2012) [27] | Sweden | 64 | Probiot | Placebo | Pre and Post | 8d & 5d | L | No effect | No | 4 |
| Zhang JW (2012) [28] | China | 60 | Probiot | Placebo | Pre | 3d | L + B + E | Decreased infectious complications | Not declared | 4 |
| Liu ZH (2013) [29] | China | 150 | Probiot | Placebo | Pre and Post | 6d & 10d | L + B | Decreased infectious complications | No | 5 |
| Krebs B (2013) [30] | Slovenia | 34 | Synbiot | SC | Pre | 3d | L | No effect | Not declared | 4 |
| Sadahiro S (2014) [31] | Japan | 195 | Probiot | SC | Pre and Post | 7d & 5d | B | No effect | Not declared | 2 |
| Kotzampassi K (2015) [32] | Greece | 164 | Probiot | Placebo | Post | 14d | L + B + Y | Decreased infectious complications | No | 5 |
| Consoli ML (2016) [33] | Brazil | 33 | Probiot | SC | Pre | 7d | Y | No effect | Not declared | 3 |
| Komatsu S (2016) [34] | Japan | 362 | Synbiot | SC | Pre and Post | 7–11d & 2–7d | L + B | No effect | Yes | 3 |
| Mizuta M (2016) [35] | Japan | 60 | Probiot | SC | Pre and Post | 7–14d & 14d | B | No effect | Not declared | 4 |
| Tan CK (2016) [36] | Malaysia | 40 | Probiot | Placebo | Pre | 7d | L + B | No effect | Yes | 4 |
| Yang Y (2016) [37] | China | 60 | Probiot | Placebo | Pre and Post | 5d & 7d | L + B + E | No effect | No | 5 |
| Flesch AT (2017) [38] | Brazil | 91 | Synbiot | Placebo | Pre and Post | 5d & 14d | L + B | Decreased infectious complications | Not declared | 4 |
| Kakaei F (2019) [39] | Iran | 99 | Probiot | Placebo | Pre | 7d | L + B + S | No effect | No | 5 |
| Polakowski CB (2019) [40] | Brazil | 73 | Synbiot | Placebo | Pre | 7d. | L + B | Decreased infectious complications | Yes | 5 |
| Bajramargic S (2019) [41] | Bosnia and Herzegovina | 78 | Probiot | SC | Post | 1 year | L + B + S | No effect | No | 1 |
| Xu Q (2019) [42] | China | 60 | Probiot | Placebo | Post | 7d | B | No effect | No | 1 |
| Park IJ (2020) [43] | Korea | 59 | Probiot | Placebo | Pre and Post | 7d & 21d | L + B | No effect | Yes | 5 |
| Wang P (2020) [44] | China | 51 | Probiot | Placebo | Pre and Post | NA | L + B + E | NA | No | 5 |
| Probiotic Strains | Prebiotic | |||||||
|---|---|---|---|---|---|---|---|---|
| Authors | Lactobacillus | Bifidobacterium | Streptococcus | Enterococcus | Yeast | Oligosaccharides | Oligofructose | Inulin |
| Reddy BS et al. [24] | X | X | X | X | ||||
| Horvat M et al. [25] | X | X | X | |||||
| Liu Z et al. [26] | X | X | ||||||
| Mangell B et al. [27] | X | |||||||
| Zhang JW et al. [28] | X | X | X | |||||
| Liu ZH et al. [29] | X | X | ||||||
| Krebs B et al. [30] | X | X | ||||||
| Sadahiro S et al. [31] | X | |||||||
| Kotzampassi K et al. [32] | X | X | X | |||||
| Consoli ML et al. [33] | X | |||||||
| Komatsu S et al. [34] | X | X | X | |||||
| Mizuta M et al. [35] | X | |||||||
| Tan CK et al. [36] | X | X | ||||||
| Yang Y et al. [37] | X | X | X | |||||
| Flesch AT et al. [38] | X | X | X | X | ||||
| KaKaei F et al. [39] | X | X | X | |||||
| Polakowski CB et al. [40] | X | X | X | X | ||||
| Bajramargic S et al. [41] | X | X | X | |||||
| Xu Q et al. [42] | X | |||||||
| Park IJ et al. [43] | X | X | ||||||
| Wang P et al. [44] | X | X | X | |||||
| Outcome | Effect Size (RR [95%CI]) | Heterogeneity (I2) | ARR | NNT | Factors of Confidence | Quality of Evidence | Comments |
|---|---|---|---|---|---|---|---|
| Overall infectious complications | 0.59 [0.47–0.75] | 15% | −9% | 11 | ⊕ ⊕ ⊕ − ⊕ | Moderate | |
| Surgical site infections | 0.70 [0.50–0.95] | 0% | −4.40% | 23 | ⊕ − ⊕ − ⊕ | Low | No or low heterogeneity. |
| Pulmonary infections | 0.35 [0.20–0.63] | 0% | −7% | 14 | ⊕ ⊕ ⊕ − ⊕ | Moderate | Significant ARR mainly for overall and non-surgical infectious complications. |
| Urinary infections | 0.41 [0.19–0.87] | 0% | −5.70% | 17 | ⊕ ⊕ ⊕ − ⊕ | Moderate | The data do not allow any particular timing or duration of probiotic or synbiotic use to be recommended |
| Anastomotic leak | 0.83 [0.47–1.48] | 29% | −0.40% | 2 | ⊕ − ⊕ − ⊕ | Low | |
| Wound infection | 0.74 [0.53–1.03] | 0% | −3.80% | 26 | ⊕ − ⊕ − ⊕ | Low |
| First Author | Year of Publication | RCT (n) | Regimen | Overall Infectious Complications (RR [95%CI]) | Surgical Site Infections (RR [95%CI]) | Pulmonary Infections (RR [95%CI]) | Urinary Infections (RR [95%CI]) | Subgroups Analyses |
|---|---|---|---|---|---|---|---|---|
| He D. [9] | 2013 | 6 | probiotics/ synbiotics | 0.39 [0.22–0.68] | NA | 0.32 [0.11–0.93] | NA | Quality of RCT, publications bias |
| De Abdrade Calaça PR. [10] | 2017 | 7 | probiotics | 0.53 [0.40–0.71] | NA | NA | NA | None |
| Liu PC. [11] | 2017 | 9 | probiotics | 0.59 [0.43–0.83] | 0.67 [0.49–0.93] | 0.25 [0.11–0.60] | 0.39 [0.16–0.96] | Probiotic formulations |
| Wu XD. [12] | 2017 | 14 | probiotics | NA | 0.72 [0.56–0.92] | 0.50 [0.29–0.84] | 0.50 [0.25–0.98] | Publication bias |
| Chen C. [13] | 2019 | 6 | probiotics | 0.31 [0.15–0.64] | 0.62 [0.39–0.99] | 0.36 [0.18–0.71] | 0.26 [0.11–0.60] | None |
| Ouyang K. [14] | 2019 | 10 | probiotics | 0.51 [0.38–0.68] | NA | 0.56 [0.32–0.98] | 0.61 [0.32–1.19] | Publication bias |
| Amitay EL. [15] | 2020 | 11 | probiotics/ synbiotics | 0.34 [0.21–0.54] | NA | NA | NA | None |
| Zeng J. [16] | 2021 | 19 | probiotics/ synbiotics | 0.37 [0.27–0.53] | 0.43 [0.31–0.58] | 0.31 [0.18–0.55] | 0.41 [0.19–0.87] | Intervention type, strain type, intervention time |
| Our meta-analysis | 2022 | 21 | probiotics/ synbiotics | 0.59 [0.47–0.75] | 0.70 [0.50–0.95] | 0.35 [0.20–0.63] | 59 [0.47–0.75] | Intervention type, strain type, intervention time, controls, quality of RCT, competing interests, ethnicity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veziant, J.; Bonnet, M.; Occean, B.V.; Dziri, C.; Pereira, B.; Slim, K. Probiotics/Synbiotics to Reduce Infectious Complications after Colorectal Surgery: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2022, 14, 3066. https://doi.org/10.3390/nu14153066
Veziant J, Bonnet M, Occean BV, Dziri C, Pereira B, Slim K. Probiotics/Synbiotics to Reduce Infectious Complications after Colorectal Surgery: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients. 2022; 14(15):3066. https://doi.org/10.3390/nu14153066
Chicago/Turabian StyleVeziant, Julie, Mathilde Bonnet, Bob V. Occean, Chadly Dziri, Bruno Pereira, and Karem Slim. 2022. "Probiotics/Synbiotics to Reduce Infectious Complications after Colorectal Surgery: A Systematic Review and Meta-Analysis of Randomised Controlled Trials" Nutrients 14, no. 15: 3066. https://doi.org/10.3390/nu14153066
APA StyleVeziant, J., Bonnet, M., Occean, B. V., Dziri, C., Pereira, B., & Slim, K. (2022). Probiotics/Synbiotics to Reduce Infectious Complications after Colorectal Surgery: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients, 14(15), 3066. https://doi.org/10.3390/nu14153066