Effect of Evening Primrose Oil Supplementation on Selected Parameters of Skin Condition in a Group of Patients Treated with Isotretinoin—A Randomized Double-Blind Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design
2.3. Experimental Procedure
2.3.1. Assessment of Skin Condition Parameters
Assessment of Transepidermal Hydration of the Skin (CORN)
Assessment of Skin Lubrication (Sebum)
Evaluation of Transepidermal Water Loss (TEWL)
2.3.2. Weight and BMI Measurements
2.4. Statistical Methods
3. Results
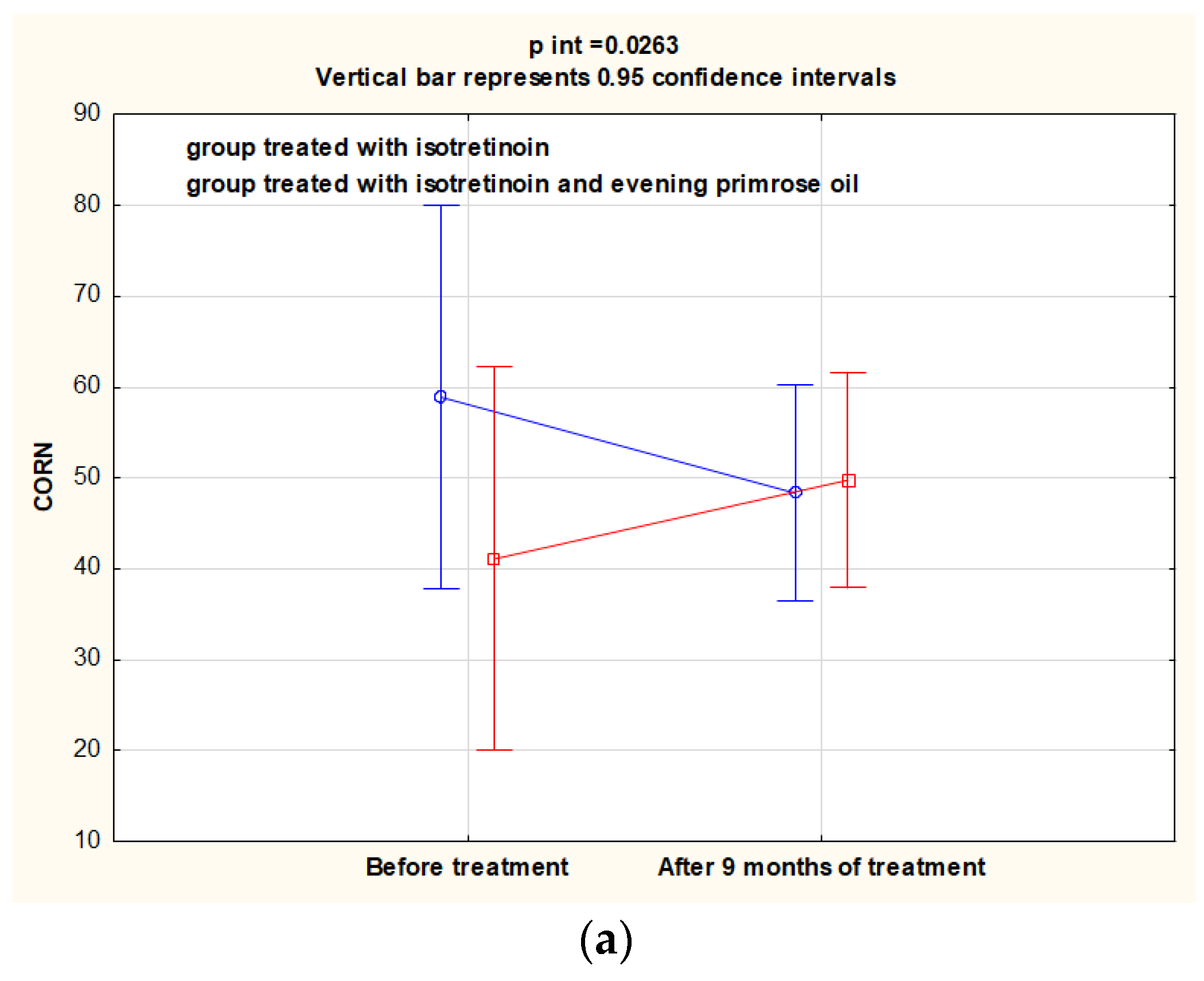
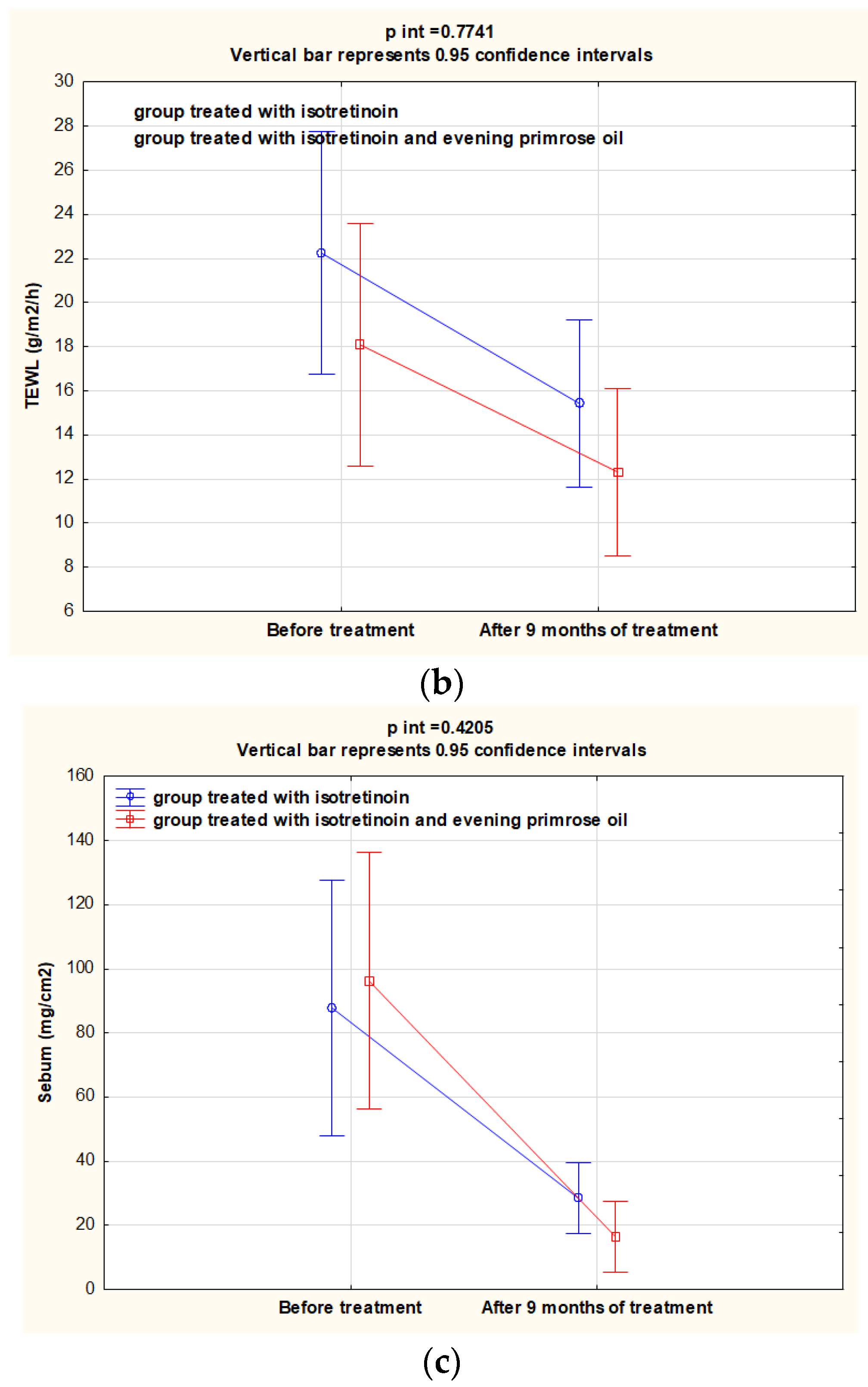
3.1. Evaluation of CORN, TEWL and Sebum Parameters before and after the Intervention
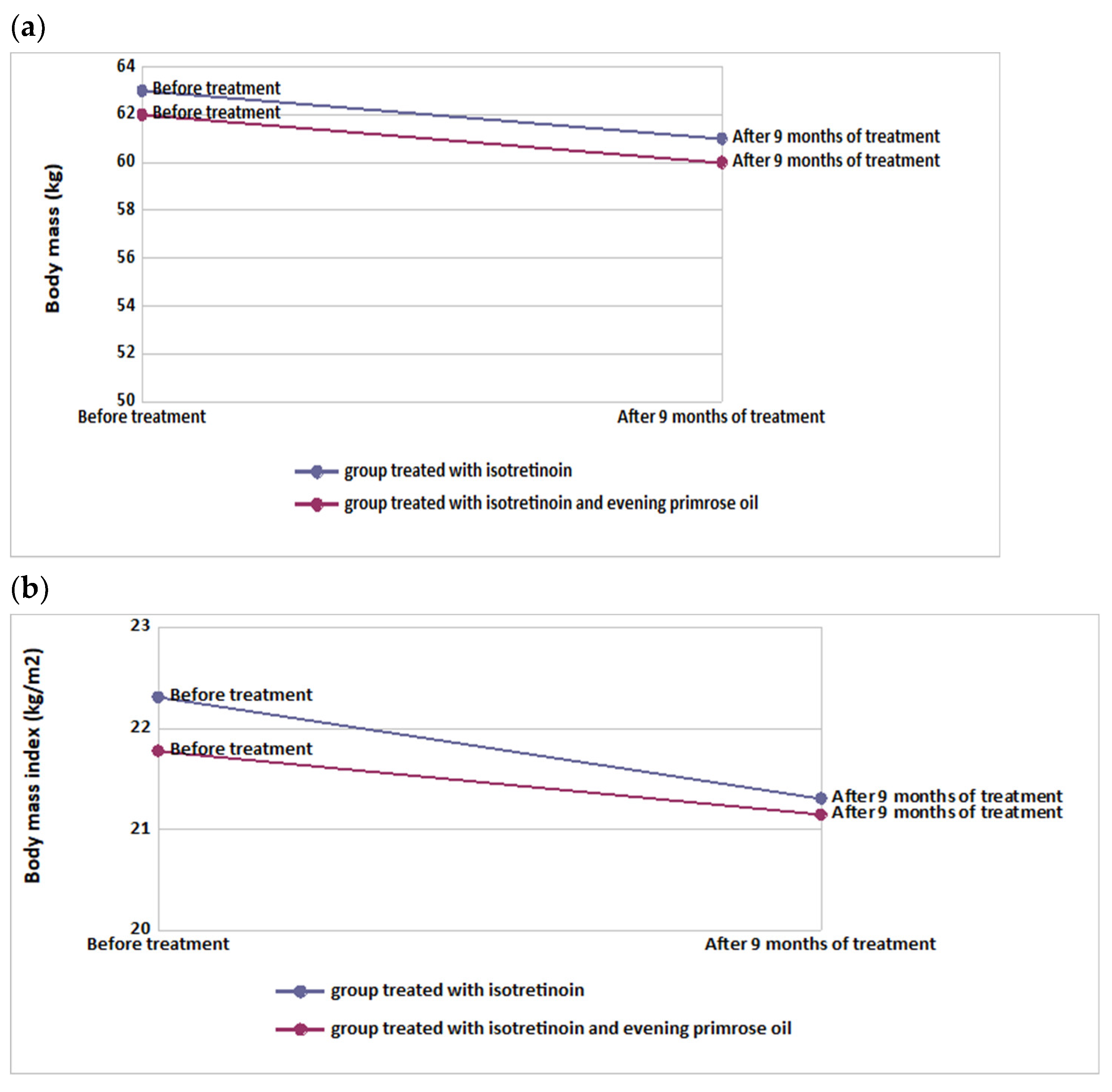
3.2. Assessment of Anthropometric Parameters before and after Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirsten, N.; Mohr, N.; Augustin, M. Prevalence and Cutaneous Comorbidity of Acne Vulgaris in the Working Population. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, D.Z.; Sprague, J.; Eichenfield, L.F. Management of Acne Vulgaris: A Review. JAMA 2021, 326, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, A.; Pandey, K.; Nimisha. Current Insights for the Management of Acne in the Modern Era. Recent Pat. Anti-Infect. Drug Discov. 2020, 15, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Tan, J. Beyond the Face: The Hidden Burden of Truncal Acne. Acta Derm. Venereol. 2021, 101, adv00495. [Google Scholar] [CrossRef]
- Daye, M.; Cihan, F.G.; Işık, B.; Hafızoğlu, B. Evaluation of Bowel Habits in Patients with Acne Vulgaris. Int. J. Clin. Pract. 2021, 75, e14903. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, Y.; Hu, H.; Liu, X.; Li, L.; Yang, B.; Wu, W.; Liang, Z.; Deng, D. The Analysis of Acne Increasing Suicide Risk. Medicine 2021, 100, e26035. [Google Scholar] [CrossRef]
- Barbieri, J.S.; Spaccarelli, N.; Margolis, D.J.; James, W.D. Approaches to Limit Systemic Antibiotic Use in Acne: Systemic Alternatives, Emerging Topical Therapies, Dietary Modification, and Laser and Light-Based Treatments. J. Am. Acad. Dermatol. 2019, 80, 538–549. [Google Scholar] [CrossRef]
- Titus, S.; Hodge, J. Diagnosis and Treatment of Acne. Am. Fam. Physician 2012, 86, 734–740. [Google Scholar]
- Fox, L.; Csongradi, C.; Aucamp, M.; du Plessis, J.; Gerber, M. Treatment Modalities for Acne. Molecules 2016, 21, 1063. [Google Scholar] [CrossRef] [Green Version]
- Han, J.J.; Faletsky, A.; Barbieri, J.S.; Mostaghimi, A. New Acne Therapies and Updates on Use of Spironolactone and Isotretinoin: A Narrative Review. Dermatol. Ther. 2021, 11, 79–91. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagatin, E.; Costa, C.S. The Use of Isotretinoin for Acne—An Update on Optimal Dosing, Surveillance, and Adverse Effects. Expert Rev. Clin. Pharmacol. 2020, 13, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Landis, M.N. Optimizing Isotretinoin Treatment of Acne: Update on Current Recommendations for Monitoring, Dosing, Safety, Adverse Effects, Compliance, and Outcomes. Am. J. Clin. Dermatol. 2020, 21, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Yoo, K.H.; Park, K.Y.; Han, T.Y.; Li, K.; Seo, S.J.; Hong, C.K. Effectiveness of Conventional, Low-Dose and Intermittent Oral Isotretinoin in the Treatment of Acne: A Randomized, Controlled Comparative Study. Br. J. Dermatol. 2011, 164, 1369–1375. [Google Scholar] [CrossRef]
- Borghi, A.; Mantovani, L.; Minghetti, S.; Giari, S.; Virgili, A.; Bettoli, V. Low-Cumulative Dose Isotretinoin Treatment in Mild-to-Moderate Acne: Efficacy in Achieving Stable Remission. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1094–1098. [Google Scholar] [CrossRef]
- Agarwal, U.S.; Besarwal, R.K.; Bhola, K. Oral Isotretinoin in Different Dose Regimens for Acne Vulgaris: A Randomized Comparative Trial. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 688–694. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E. Retinoids: Active Molecules Influencing Skin Structure Formation in Cosmetic and Dermatological Treatments. Postepy Dermatol. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Duester, G. Retinoic Acid Synthesis and Signaling during Early Organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef] [Green Version]
- Kraft, J.; Freiman, A. Management of Acne. Can. Med. Assoc. J. 2011, 183, E430–E435. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the Treatment of Skin Aging: An Overview of Clinical Efficacy and Safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef]
- Retinoids. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Szymański, Ł.; Skopek, R.; Palusińska, M.; Schenk, T.; Stengel, S.; Lewicki, S.; Kraj, L.; Kamiński, P.; Zelent, A. Retinoic Acid and Its Derivatives in Skin. Cells 2020, 9, 2660. [Google Scholar] [CrossRef] [PubMed]
- Adamski, Z.; Kaszuba, A. Dermatologia dla Kosmetologów; Edra Urban & Partner: Wrocław, Poland, 2019; ISBN 978-83-66310-14-8. [Google Scholar]
- Retinoid Antagonists and Use Thereof|Paper|Microsoft Academic. Available online: https://academic.microsoft.com/paper/1553050318/reference/search?q=Retinoid%20antagonists%20and%20use%20thereof&qe=Or(Id%253D2151553962%252CId%253D2161761876%252CId%253D2952734539%252CId%253D2252529015%252CId%253D1899570853%252CId%253D2077958288%252CId%253D1554794327%252CId%253D2271100279%252CId%253D1994250284)&f=&orderBy=0 (accessed on 7 October 2021).
- Gollnick, H.P.M. From New Findings in Acne Pathogenesis to New Approaches in Treatment. J. Eur. Acad. Dermatol. Venereol. 2015, 29 (Suppl. S5), 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kmieć, M.L.; Pajor, A.; Broniarczyk-Dyła, G. Evaluation of Biophysical Skin Parameters and Assessment of Hair Growth in Patients with Acne Treated with Isotretinoin. Postepy Dermatol. Alergol. 2013, 30, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oprica, C.; Emtestam, L.; Hagströmer, L.; Nord, C.E. Clinical and Microbiological Comparisons of Isotretinoin vs. Tetracycline in Acne Vulgaris. Acta Derm. Venereol. 2007, 87, 246–254. [Google Scholar] [CrossRef]
- Vallerand, I.A.; Lewinson, R.T.; Farris, M.S.; Sibley, C.D.; Ramien, M.L.; Bulloch, A.G.M.; Patten, S.B. Efficacy and Adverse Events of Oral Isotretinoin for Acne: A Systematic Review. Br. J. Dermatol. 2018, 178, 76–85. [Google Scholar] [CrossRef]
- Żaba, R. Special Paper Safety of the Treatment with Retinoids. Adv. Dermatol. Allergol. 2006, 23, 161–174. [Google Scholar]
- Kızılyel, O.; Metin, M.S.; Elmas, Ö.F.; Çayır, Y.; Aktaş, A. Effects of Oral Isotretinoin on Lipids and Liver Enzymes in Acne Patients. Cutis 2014, 94, 234–238. [Google Scholar]
- Zane, L.T.; Leyden, W.A.; Marqueling, A.L.; Manos, M.M. A Population-Based Analysis of Laboratory Abnormalities during Isotretinoin Therapy for Acne Vulgaris. Arch. Dermatol. 2006, 142, 1016–1022. [Google Scholar] [CrossRef] [Green Version]
- Zech, L.A.; Gross, E.G.; Peck, G.L.; Brewer, H.B. Changes in Plasma Cholesterol and Triglyceride Levels after Treatment with Oral Isotretinoin. A Prospective Study. Arch. Dermatol. 1983, 119, 987–993. [Google Scholar] [CrossRef]
- De Marchi, M.A.; Maranhão, R.C.; Brandizzi, L.I.V.; Souza, D.R.S. Effects of Isotretinoin on the Metabolism of Triglyceride-Rich Lipoproteins and on the Lipid Profile in Patients with Acne. Arch. Dermatol. Res. 2006, 297, 403–408. [Google Scholar] [CrossRef]
- Pile, H.D.; Sadiq, N.M. Isotretinoin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Schulpis, K.H.; Georgala, S.; Papakonstantinou, E.D.; Michas, T.; Karikas, G.A. The Effect of Isotretinoin on Biotinidase Activity. Skin Pharmacol. Appl. Skin Physiol. 1999, 12, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Cetinözman, F.; Aksoy, D.Y.; Elçin, G.; Yıldız, B.O. Insulin Sensitivity, Androgens and Isotretinoin Therapy in Women with Severe Acne. J. Dermatolog. Treat. 2014, 25, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Aydin, K.; Çetinözman, F.; Elcin, G.; Aksoy, D.Y.; Ucar, F.; Yildiz, B.O. Suppressed Adiponectin Levels and Increased Adiponectin Response to Oral Glucose Load in Lean Women with Severe Acne Normalizes after Isotretinoin Treatment. Dermatology 2017, 233, 314–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahboubi, M. Evening Primrose (Oenothera biennis) Oil in Management of Female Ailments. J. Menopausal Med. 2019, 25, 74–82. [Google Scholar] [CrossRef]
- Dominguez, Z. Omega 6/Omega 3 Fatty Acid Ratio. Rev. Digit. Postgrado 2014, 3, 14–22. [Google Scholar]
- Muggli, R. Systemic Evening Primrose Oil Improves the Biophysical Skin Parameters of Healthy Adults. Int. J. Cosmet. Sci. 2005, 27, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Timoszuk, M.; Bielawska, K.; Skrzydlewska, E. Evening Primrose (Oenothera biennis) Biological Activity Dependent on Chemical Composition. Antioxidants 2018, 7, 108. [Google Scholar] [CrossRef] [Green Version]
- Kendall, A.C.; Kiezel-Tsugunova, M.; Brownbridge, L.C.; Harwood, J.L.; Nicolaou, A. Lipid Functions in Skin: Differential Effects of n-3 Polyunsaturated Fatty Acids on Cutaneous Ceramides, in a Human Skin Organ Culture Model. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1679–1689. [Google Scholar] [CrossRef]
- Senapati, S.; Banerjee, S.; Gangopadhyay, D.N. Evening Primrose Oil Is Effective in Atopic Dermatitis: A Randomized Placebo-Controlled Trial. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 447–452. [Google Scholar] [CrossRef]
- Białek, M.; Rutkowska, J. Importance of γ-linolenic acid in prevention and therapy. Postepy Hig. Med. Dosw. 2015, 69, 892–904. [Google Scholar] [CrossRef]
- Haslett, C.; Douglas, J.G.; Chalmers, S.R.; Weighhill, A.; Munro, J.F. A Double-Blind Evaluation of Evening Primrose Oil as an Antiobesity Agent. Int. J. Obes. 1983, 7, 549–553. [Google Scholar] [PubMed]
- Wilson, J.H.; Rietveld, T.; Van den Berg, J.W.; Jansen, H.; Swart, G.R.; Lamberts, S.W. The Effect of Very Low Energy Diets on the Fatty Acid Composition of Serum Lipids. Int. J. Obes. 1989, 13 (Suppl. S2), 51–60. [Google Scholar] [PubMed]
- Xiaoting, T.; Yu, X.; Laihui, D.; Li, N.; Yanling, N.; Junli, Z. Effects of evening primrose oil on the metabolism and intestinal flora of obese infertile women. J. Shandong Univ. (Health Sci.) 2021, 59, 48–54. [Google Scholar] [CrossRef]
- Mert, H.; İrak, K.; Çibuk, S.; Yıldırım, S.; Mert, N. The Effect of Evening Primrose Oil (Oenothera biennis) on the Level of Adiponectin and Some Biochemical Parameters in Rats with Fructose Induced Metabolic Syndrome. Arch. Physiol. Biochem. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- CONSORT-SPI 2018 Explanation and Elaboration: Guidance for Reporting Social and Psychological Intervention Trials|Trials|Full Text. Available online: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-018-2735-z (accessed on 5 March 2022).
- MacPherson, H.; Altman, D.G.; Hammerschlag, R.; Youping, L.; Taixiang, W.; White, A.; Moher, D.; STRICTA Revision Group. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT Statement. PLoS Med. 2010, 7, e1000261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaźmierska, A.; Bolesławska, I.; Jagielski, P.; Polańska, A.; Dańczak-Pazdrowska, A.; Kosewski, G.; Adamski, Z.; Przysławski, J. Effect of Evening Primrose Oil Supplementation on Biochemical Parameters and Nutrition of Patients Treated with Isotretinoin for Acne Vulgaris: A Randomized Double-Blind Trial. Nutrients 2022, 14, 1342. [Google Scholar] [CrossRef]
- Acmaz, G.; Cınar, L.; Acmaz, B.; Aksoy, H.; Kafadar, Y.T.; Madendag, Y.; Ozdemir, F.; Sahin, E.; Muderris, I. The Effects of Oral Isotretinoin in Women with Acne and Polycystic Ovary Syndrome. Biomed. Res. Int. 2019, 2019, 2513067. [Google Scholar] [CrossRef]
- Mohd Ariffin, N.H.; Hasham, R. Assessment of Non-Invasive Techniques and Herbal-Based Products on Dermatological Physiology and Intercellular Lipid Properties. Heliyon 2020, 6, e03955. [Google Scholar] [CrossRef]
- Barel, A.; Clarys, P. Measurement of Epidermal Capacitance. In Handbook of Non-Invasive Methods and the Skin, 2nd ed.; Serup, J., Jemec, G.B.E., Grove, G.L., Eds.; Informa Healthcare: London, UK, 2007; Volume 39, pp. 337–344. ISBN 978-0-8493-1437-7. [Google Scholar]
- Rogiers, V.; Balls, M.; Basketter, D.; Berardesca, E.; Edwards, C.; Elsner, P.; Ennen, J.; Lévêque, J.L.; Lóden, M.; Masson, P.; et al. The Potential Use of Non-Invasive Methods in the Safety Assessment of Cosmetic Products: The Report and Recommendations of an ECVAM/EEMCO Workshop (ECVAM. Workshop 36). Altern. Lab. Anim. 1999, 27, 515–537. Available online: https://journals.sagepub.com/doi/abs/10.1177/026119299902700404 (accessed on 7 October 2021). [CrossRef]
- Chu, S.; Michelle, L.; Ekelem, C.; Sung, C.T.; Rojek, N.; Mesinkovska, N.A. Oral Isotretinoin for the Treatment of Dermatologic Conditions Other than Acne: A Systematic Review and Discussion of Future Directions. Arch. Dermatol. Res. 2021, 313, 391–430. [Google Scholar] [CrossRef]
- Layton, A. The Use of Isotretinoin in Acne. Dermato-Endocrinology 2009, 1, 162–169. [Google Scholar] [CrossRef]
- Leung, A.K.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Dermatology: How to Manage Acne Vulgaris. Drugs Context 2021, 10, 2021-8-6. [Google Scholar] [CrossRef] [PubMed]
- Roenigk, H.H. Liver Toxicity of Retinoid Therapy. Pharmacol. Ther. 1989, 40, 145–155. [Google Scholar] [CrossRef]
- Yaldiz, M.; Kara, A.; Güven, M.; Solak, B.; Kara, R.; Erdem, M.T. Assessment of Auditory Function and Lipid Levels in Patients Receiving Oral Isotretinoin (13-Cis Retinoid) Therapy for Acne Vulgaris. Postepy Dermatol. Alergol. 2020, 37, 360–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, C.S.; Bagatin, E.; Martimbianco, A.L.C.; da Silva, E.M.; Lúcio, M.M.; Magin, P.; Riera, R. Oral Isotretinoin for Acne. Cochrane Database Syst. Rev. 2018, 11, CD009435. [Google Scholar] [CrossRef] [PubMed]
- Isotretinoin and Intestinal Damage. Prescrire Int. 2008, 17, 154–156.
- Alataş, Ö.D.; Alataş, E.T. Isotretinoin-Induced Pruritic Erythematous Lesions and Acute Chest Pain in a 15-Year-Old Girl. Am. J. Emerg Med. 2020, 38, 1043.e1–1043.e3. [Google Scholar] [CrossRef]
- Aksac, S.E.; Bilgili, S.G.; Yavuz, G.O.; Yavuz, I.H.; Aksac, M.; Karadag, A.S. Evaluation of Biophysical Skin Parameters and Hair Changes in Patients with Acne Vulgaris Treated with Isotretinoin, and the Effect of Biotin Use on These Parameters. Int. J. Dermatol. 2021, 60, 980–985. [Google Scholar] [CrossRef]
- Evening Primrose. In Drugs and Lactation Database (LactMed); National Library of Medicine (US): Bethesda, MD, USA, 2006.
- Chemical Information Review Document for Evening Primrose Oil (Oenothera biennis L.) [CAS No. 90028-66-3] Supporting Nomination for Toxicological Evaluation by the National Toxicology ProgrAm. 2009. Available online: https://ntp.niehs.nih.gov/ntp/noms/support_docs/evening_primrose_nov2009.pdf (accessed on 13 July 2022).
- Evening Primrose Oil Interactions. Available online: https://www.drugs.com/drug-interactions/evening-primrose,evening-primrose-oil.html (accessed on 13 July 2022).
- Agbabiaka, T.B.; Spencer, N.H.; Khanom, S.; Goodman, C. Prevalence of Drug-Herb and Drug-Supplement Interactions in Older Adults: A Cross-Sectional Survey. Br. J. Gen. Pract. 2018, 68, e711–e717. [Google Scholar] [CrossRef] [Green Version]
- Bayles, B.; Usatine, R. Evening Primrose Oil. Am. Fam. Physician 2009, 80, 1405–1408. [Google Scholar]
- Miller, L.G. Herbal Medicinals: Selected Clinical Considerations Focusing on Known or Potential Drug-Herb Interactions. Arch. Intern. Med. 1998, 158, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A. Interactions between Herbs and Conventional Drugs: Overview of the Clinical Data. Med. Princ. Pract. 2012, 21, 404–428. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Iurian, S.; Tomuta, I.; Moldovan, M. Improvement of Skin Condition in Striae Distensae: Development, Characterization and Clinical Efficacy of a Cosmetic Product Containing Punica Granatum Seed Oil and Croton Lechleri Resin Extract. Drug Des. Devel. Ther. 2017, 11, 521–531. [Google Scholar] [CrossRef] [Green Version]
- Caglar, S.; Yildiz, G.K.; Bakoglu, I.; Salihoglu, O. The Effect of Sunflower Seed and Almond Oil on Preterm Infant Skin: A Randomized Controlled Trial. Adv. Skin Wound Care 2020, 33, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Danby, S.G.; AlEnezi, T.; Sultan, A.; Lavender, T.; Chittock, J.; Brown, K.; Cork, M.J. Effect of Olive and Sunflower Seed Oil on the Adult Skin Barrier: Implications for Neonatal Skin Care. Pediatr. Dermatol. 2013, 30, 42–50. [Google Scholar] [CrossRef]
- Jeong, S.K.; Park, H.J.; Park, B.D.; Kim, I.-H. Effectiveness of Topical Chia Seed Oil on Pruritus of End-Stage Renal Disease (ESRD) Patients and Healthy Volunteers. Ann. Dermatol. 2010, 22, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Jia, T.; Qiao, W.; Gu, H.; Kaku, K. Unsaturated Fatty Acid-Enriched Extract from Hippophae Rhamnoides Seed Reduces Skin Dryness through up-Regulating Aquaporins 3 and Hyaluronan Synthetases 2 Expressions. J. Cosmet. Dermatol. 2021, 20, 321–329. [Google Scholar] [CrossRef]
- Blaak, J.; Staib, P. An Updated Review on Efficacy and Benefits of Sweet Almond, Evening Primrose and Jojoba Oils in Skin Care Applications. Int. J. Cosmet. Sci. 2022, 44, 1–9. [Google Scholar] [CrossRef]
- Krysiak, Z.J.; Knapczyk-Korczak, J.; Maniak, G.; Stachewicz, U. Moisturizing Effect of Skin Patches with Hydrophobic and Hydrophilic Electrospun Fibers for Atopic Dermatitis. Colloids Surf. B Biointerfaces 2021, 199, 111554. [Google Scholar] [CrossRef]
- Chung, B.Y.; Park, S.Y.; Jung, M.J.; Kim, H.O.; Park, C.W. Effect of Evening Primrose Oil on Korean Patients with Mild Atopic Dermatitis: A Randomized, Double-Blinded, Placebo-Controlled Clinical Study. Ann. Dermatol. 2018, 30, 409–416. [Google Scholar] [CrossRef]
- Gehring, W.; Bopp, R.; Rippke, F.; Gloor, M. Effect of Topically Applied Evening Primrose Oil on Epidermal Barrier Function in Atopic Dermatitis as a Function of Vehicle. Arzneimittelforschung 1999, 49, 635–642. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Feingold, K.R.; Elias, P.M. Transepidermal Water Loss Reflects Permeability Barrier Status: Validation in Human and Rodent in Vivo and Ex Vivo Models. Exp. Dermatol. 2006, 15, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Chung, W.-Y. Monitoring Transepidermal Water Loss and Skin Wettedness Factor with Battery-Free NFC Sensor. Sensors 2020, 20, 5549. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damien, F.; Boncheva, M. The Extent of Orthorhombic Lipid Phases in the Stratum Corneum Determines the Barrier Efficiency of Human Skin in Vivo. J. Investig. Dermatol. 2010, 130, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Moon, I.J.; Lee, H.W.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Lee, W.J. The Efficacy and Safety of Dual-Frequency Ultrasound for Improving Skin Hydration and Erythema in Patients with Rosacea and Acne. J. Clin. Med. 2021, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Çölgeçen, E.; Özyurt, K.; Ferahbaş Kesikoğlu, A. The Effect of Systemic Isotretinoin Treatment on Skin Biophysicalparameters among Patients with Acne Vulgaris. Turk. J. Med. Sci. 2016, 46, 1641–1644. [Google Scholar] [CrossRef]
- Zouboulis, C.C. Acne and Sebaceous Gland Function. Clin. Dermatol. 2004, 22, 360–366. [Google Scholar] [CrossRef]
- Janiczek-Dolphin, N.; Cook, J.; Thiboutot, D.; Harness, J.; Clucas, A. Can Sebum Reduction Predict Acne Outcome? Br. J. Dermatol. 2010, 163, 683–688. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Matsubara, A.; Smiles, K. The Effect of 2% Niacinamide on Facial Sebum Production. J. Cosmet. Laser Ther. 2006, 8, 96–101. [Google Scholar] [CrossRef]
- Javadi, M.; Everts, H.; Hovenier, R.; Kocsis, S.; Lankhorst, A.E.; Lemmens, A.G.; Schonewille, J.T.; Terpstra, A.H.M.; Beynen, A.C. The Effect of Six Different C18 Fatty Acids on Body Fat and Energy Metabolism in Mice. Br. J. Nutr. 2004, 92, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, Y.; Ide, T.; Fujita, H. Dietary Gamma-Linolenic Acid in the Form of Borage Oil Causes Less Body Fat Accumulation Accompanying an Increase in Uncoupling Protein 1 MRNA Level in Brown Adipose Tissue. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 127, 213–222. [Google Scholar] [CrossRef]
| Analyzed Parameters | Isotretinoin (I) n = 25 | Isotretinoin with Evening Primrose Oil (IOW) n = 25 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex of Respondents | Women n, % | Men n, % | Women n, % | Men n, % | p (between Groups) | ||||
| 17; 68% | 8; 32% | 16; 64% | 9; 36% | 0.7650 | |||||
| Severity of acne before the study | No change n; % | Light n; % | Medium n; % | Hard n; % | No change n; % | Light n; % | Medium n; % | Hard n; % | |
| 0; 0% | 0; 0% | 13; 52% | 12; 48% | 0; 0% | 0; 0% | 13; 52% | 12; 48% | 1.000 | |
| Severity of acne after the study | No change n; % | Light n; % | Medium n; % | Hard n; % | No change n; % | Light n; % | Medium n; % | Hard n; % | |
| 23; 92% | 2; 8% | 0; 0% | 0; 0% | 24; 96% | 1; 4% | 0; 0% | 0; 0% | 0.5520 | |
| Analyzed Parameters | Total Researched n = 50 | Isotretinoin (I) n = 25 | Isotretinoin with Evening Primrose Oil (IOW) n = 25 | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| X ± SD | Me ± Q | Min | Max | X ± SD | Me ± Q | X ± SD | Me ± Q | ||
| Skin Condition Parameters | |||||||||
| CORN arbitrary unit | 50.5 ± 32.7 | 44.1 ± 11.3 | 6.70 | 391 | 58.9 ± 32.4 | 46.4 ± 10.8 | 41.1 ± 16.2 | 42.0 ± 9.70 | 0.367 |
| TEWL (g/m2/h) | 20.2 ± 13.7 | 16.0 ± 6.05 | 6.00 | 71.3 | 22.2 ± 16.5 | 15.7 ± 9.10 | 18.1 ± 10.0 | 17.1 ± 3.20 | 0.786 |
| Sebum (ug/cm2) | 92.0 ± 68.5 | 55.8 ± 30.5 | 0.00 | 383 | 87.8 ± 54.3 | 65.0 ± 34.5 | 96.2 ± 64.2 | 42.0 ± 30.8 | 0.705 |
| Anthropometric parameters | |||||||||
| Body height (cm) | 171 ± 5.79 | 169 ± 5.50 | 163 | 183 | 170 ± 5.79 | 169 ± 5.50 | 171 ± 5.79 | 169 ± 5.50 | 0.231 |
| Body weight (kg) | 65.0 ± 7.53 | 62.5 ± 4.50 | 55.0 | 86.0 | 64.6 ± 6.61 | 63.0 ± 4.00 | 65.4 ± 8.46 | 62.0 ± 5.00 | 0.953 |
| BMI (kg/m2) | 22.2 ± 1.42 | 22.0 ± 0.86 | 20.2 | 27.8 | 22.3 ± 1.15 | 22.3 ± 0.39 | 22.1 ± 1.67 | 21.8 ± 1.12 | 0.367 |
| Analyzed Parameters | Isotretinoin (I) n = 25 | Wil coxon Test | Isotretinoin with Evening Primrose Oil (IOW) n = 25 | Wilcoxon Test p | U Mann–Whitney Test (Between Groups) p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Treatment | After 9 Months of Treatment | Δ | Before Treatment | After 9 Months of Treatment | Δ | ||||||
| Me ± Q | Me ± Q | Me ± Q | Me ± Q | Me ± Q | Me ± Q | Before Treatment | After Treatment | Δ Before and after Treatment | |||
| Skin Condition Parameters | |||||||||||
| CORN arbitrary unit | 46.4 ± 10.8 | 39.8 ± 12.4 | −10.5 ± 38.0 | 0.015 | 42.0 ± 9.70 | 50.9 ± 10.4 | 8.70 ± 17.6 | 0.017 | 0.367 | 0.190 | 0.002 |
| TEWL (g/m2/h) | 15.7 ± 9.10 | 12.0 ± 6.10 | −6.80 ± 14.7 | 0.004 | 17.1 ± 3.20 | 11.5 ± 2.75 | −5.80 ± 10.4 | 0.001 | 0.786 | 0.760 | 0.946 |
| Sebum (ug/cm2) | 65.0 ± 34.5 | 14.0 ± 24.0 | −59.4 ± 73.8 | <0.001 | 42.0 ± 30.8 | 5.00 ± 11.5 | −79.8 ± 102 | <0.001 | 0.705 | 0.110 | 0.554 |
| Analyzed Parameters | Isotretinoin (I) n = 25 | Wilcoxon Test p | Isotretinoin with Evening Primrose oil (IOW) n = 25 | Wilcoxon Test p | U Mann–Whitney Test (Between Grups) p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Before Treatment | After 9 Months of Treatment | Δ | Before Treatment | After 9 Months of Treatment | Δ | ||||
| Me ± Q | Me ± Q | Me ± Q | Me ± Q | Me ± Q | Me ± Q | ||||
| Body weight (kg) | 63.0 ± 4.00 | 61.0 ± 4.00 | −2.00 ± 0.00 | <0.001 | 62.0 ± 5.00 | 60.0 ± 5.00 | −2.00 ± 0.00 | <0.001 | 0.5250 |
| BMI (kg/m2) | 22.3 ± 0.39 | 21.3 ± 0.49 | −0.72 ± 0.05 | <0.001 | 21.8 ± 1.12 | 21.2 ± 1.11 | −0.71 ± 0.05 | <0.001 | 0.6686 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaźmierska, A.; Bolesławska, I.; Polańska, A.; Dańczak-Pazdrowska, A.; Jagielski, P.; Drzymała-Czyż, S.; Adamski, Z.; Przysławski, J. Effect of Evening Primrose Oil Supplementation on Selected Parameters of Skin Condition in a Group of Patients Treated with Isotretinoin—A Randomized Double-Blind Trial. Nutrients 2022, 14, 2980. https://doi.org/10.3390/nu14142980
Kaźmierska A, Bolesławska I, Polańska A, Dańczak-Pazdrowska A, Jagielski P, Drzymała-Czyż S, Adamski Z, Przysławski J. Effect of Evening Primrose Oil Supplementation on Selected Parameters of Skin Condition in a Group of Patients Treated with Isotretinoin—A Randomized Double-Blind Trial. Nutrients. 2022; 14(14):2980. https://doi.org/10.3390/nu14142980
Chicago/Turabian StyleKaźmierska, Agnieszka, Izabela Bolesławska, Adriana Polańska, Aleksandra Dańczak-Pazdrowska, Paweł Jagielski, Sławomira Drzymała-Czyż, Zygmunt Adamski, and Juliusz Przysławski. 2022. "Effect of Evening Primrose Oil Supplementation on Selected Parameters of Skin Condition in a Group of Patients Treated with Isotretinoin—A Randomized Double-Blind Trial" Nutrients 14, no. 14: 2980. https://doi.org/10.3390/nu14142980
APA StyleKaźmierska, A., Bolesławska, I., Polańska, A., Dańczak-Pazdrowska, A., Jagielski, P., Drzymała-Czyż, S., Adamski, Z., & Przysławski, J. (2022). Effect of Evening Primrose Oil Supplementation on Selected Parameters of Skin Condition in a Group of Patients Treated with Isotretinoin—A Randomized Double-Blind Trial. Nutrients, 14(14), 2980. https://doi.org/10.3390/nu14142980








