Ischemic Stroke and Dietary Vitamin B12 Deficiency in Old-Aged Females: Impaired Motor Function, Increased Ischemic Damage Size, and Changed Metabolite Profiles in Brain and Cecum Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Diet
2.4. Photothrombosis Model
2.5. Behavioral Testing
2.5.1. Bederson Scale and Neurological Scoring Scale
2.5.2. Accelerating Rotarod
2.5.3. Forepaw Placement
2.5.4. Ladder Beam
2.6. Total Homocysteine and Choline Metabolite Measurements
2.7. Brain Tissue Processing
2.8. Immunofluorescence Experiments
2.9. Metabolomics
2.10. Data Analysis and Statistics
3. Results
3.1. Increased Levels of Homocysteine in Plasma and Liver and Changes in Other One-Carbon Metabolites in Vitamin B12-Deficient Diet Animals
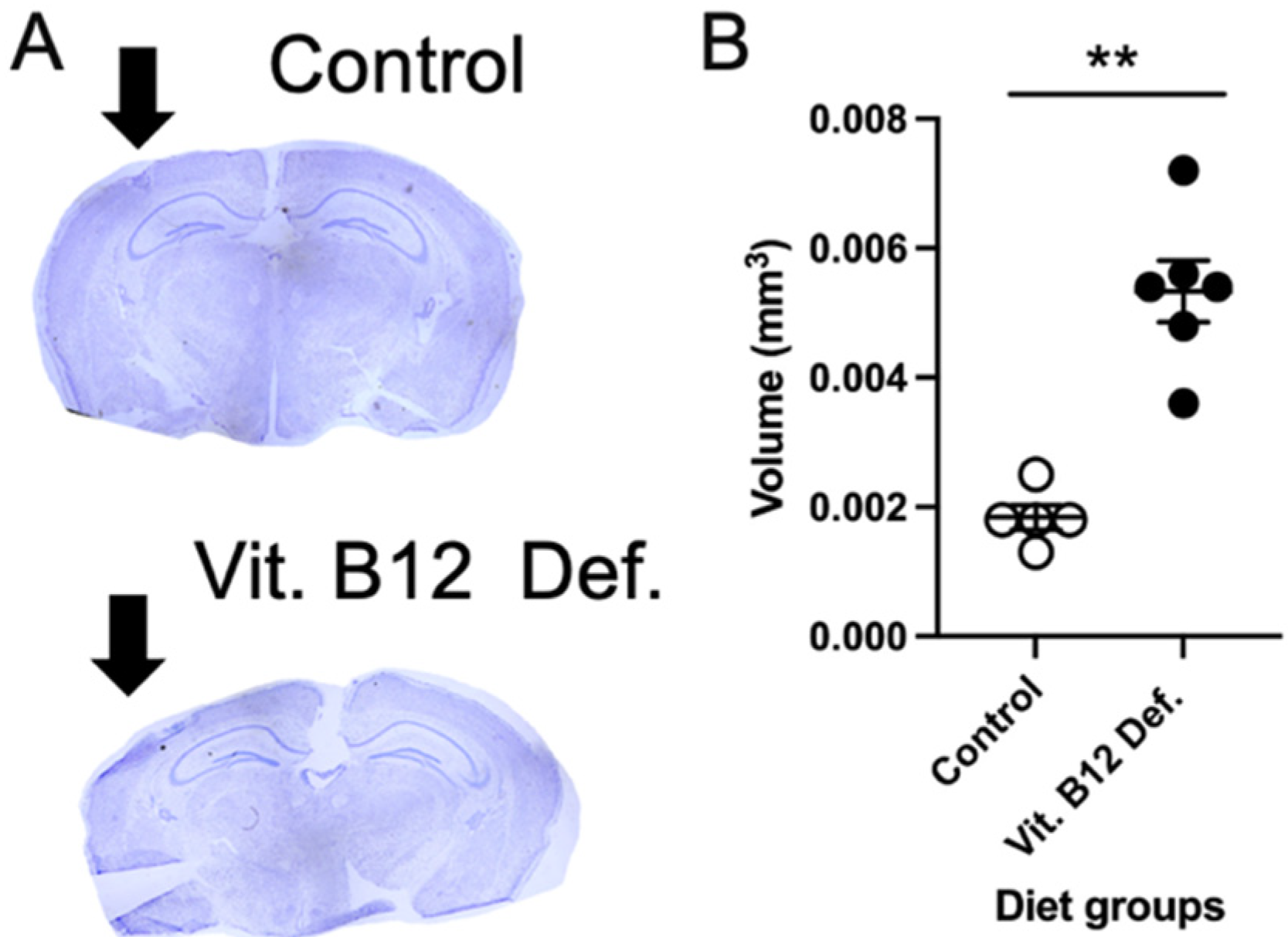
3.2. Increased Ischemic Damage Volume in Vitamin B12-Deficient Animals after Stroke
3.3. More Neuronal Apoptosis in Vitamin B12-Deficient Animals after Ischemic Stroke
3.4. Metabolomic Measurements
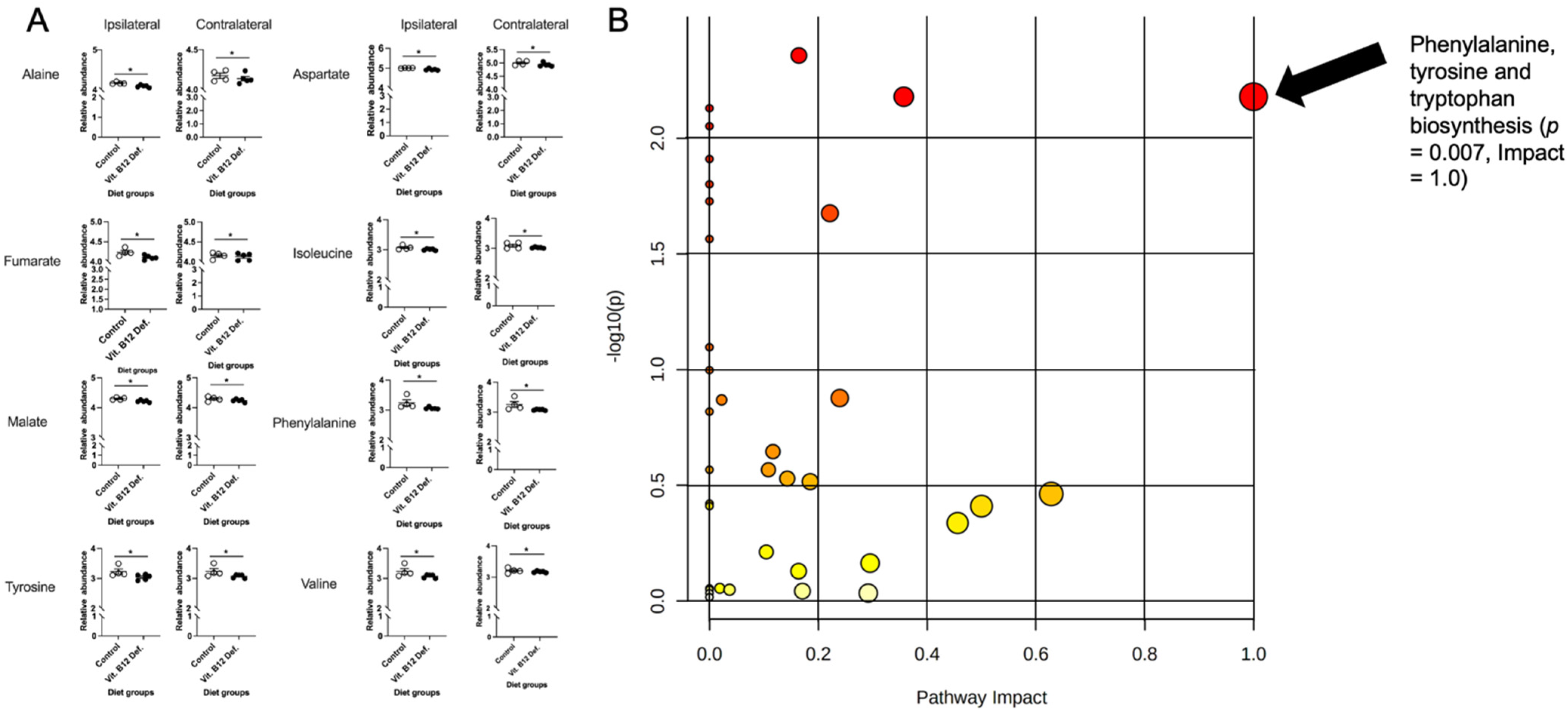
3.4.1. Reductions in TCA Cycle in Brain Tissue of Vitamin B12-Deficient Females after Ischemic Stroke
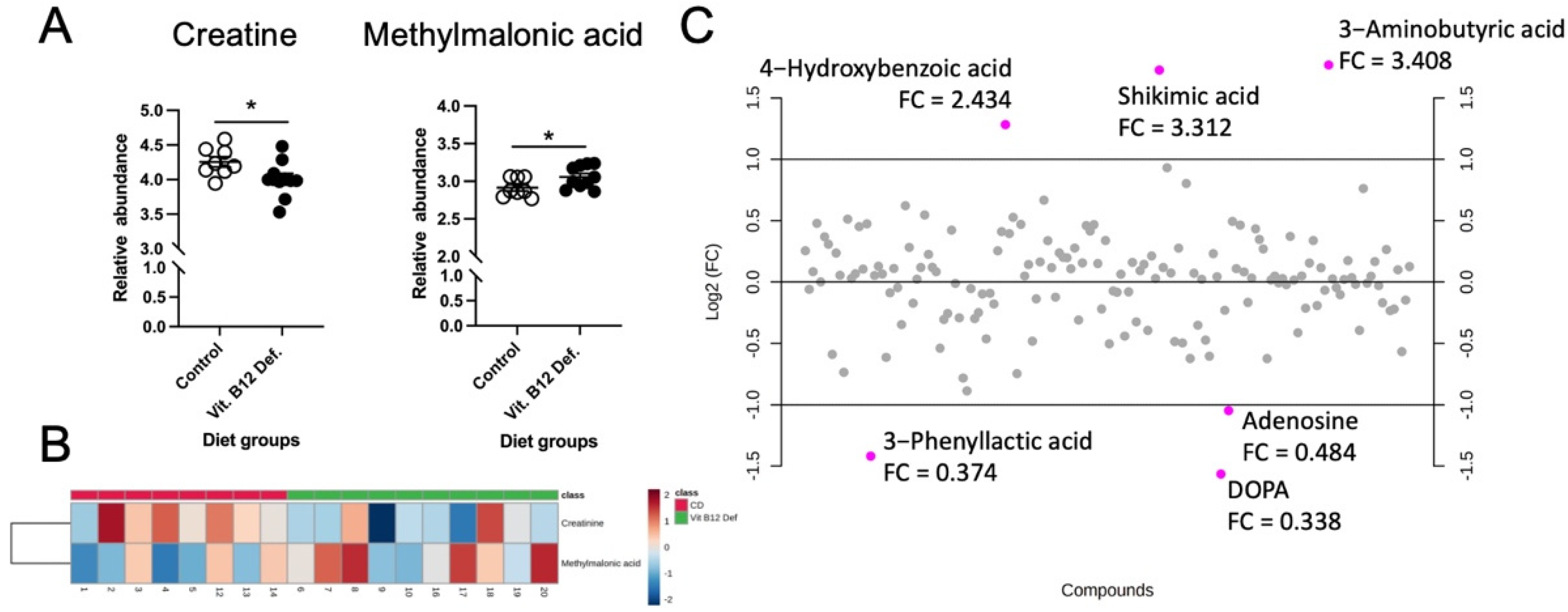
3.4.2. Reduced Levels of Creatine and Increased Levels of Methylmalonic Acid in Cecum of Vitamin B12-Deficient Animals after Ischemic Stroke
3.5. Reduced Stroke Outcome in Vitamin B12-Deficient Animals
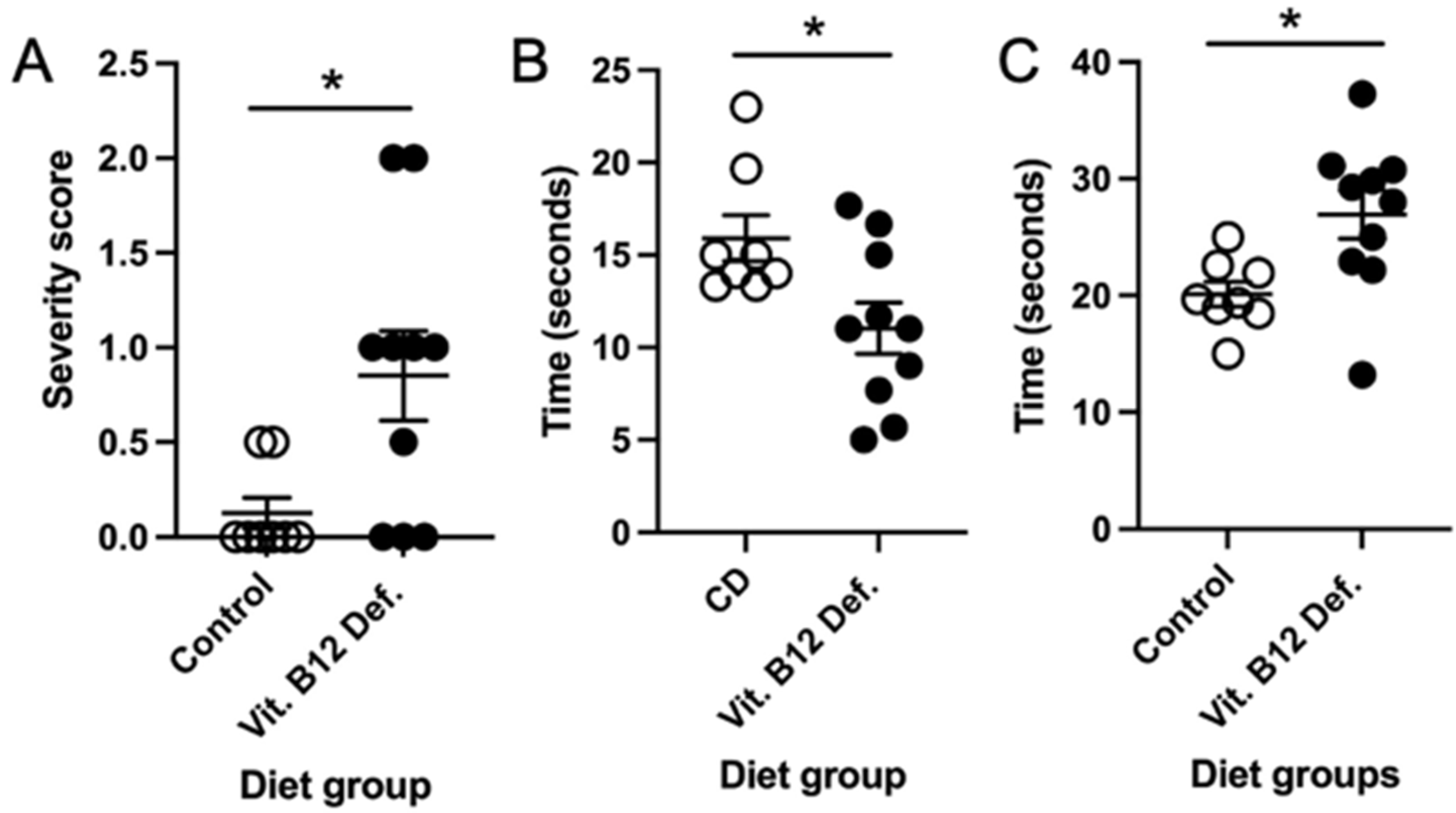
3.5.1. Higher Neuro Deficit Score in Vitamin B12-Deficient Animals after Ischemic Stroke
3.5.2. Impaired Balance and Coordination in Vitamin B12-Deficient Animals after Ischemic Stroke
3.5.3. No Difference in Forepaw Placement between Dietary Groups
3.5.4. No Difference in Skilled Motor Function between Dietary Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ovbiagele, B.; Goldstein, L.B.; Higashida, R.T.; Howard, V.J.; Johnston, S.C.; Khavjou, O.A.; Lackland, D.T.; Lichtman, J.H.; Mohl, S.; Sacco, R.L.; et al. Forecasting the Future of Stroke in the United States: A Policy Statement From the American Heart Association and American Stroke Association. Stroke 2013, 44, 2361–2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics—2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Mensah, G.A.; Norrving, B.; Murray, C.J.L.; Roth, G.A. GBD 2013 Stroke Panel Experts Group Atlas of the Global Burden of Stroke (1990–2013): The GBD 2013 Study. Neuroepidemiology 2015, 45, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Spence, J. Nutrition and Risk of Stroke. Nutrients 2019, 11, 647. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Bogiatzi, C.; Hackam, D.G.; Rutledge, A.C.; Sposato, L.A.; Khaw, A.; Mandzia, J.; Azarpazhoo, M.R.; Hachinski, V.; Spence, J.D. Vitamin B 12 Deficiency and Hyperhomocysteinaemia in Outpatients with Stroke or Transient Ischaemic Attack: A Cohort Study at an Academic Medical Centre. BMJ Open 2019, 9, e026564. [Google Scholar] [CrossRef] [Green Version]
- Yahn, G.; Abato, J.; Jadavji, N. Role of Vitamin B12 Deficiency in Ischemic Stroke Risk and Outcome. Neural Regen. Res. 2021, 16, 470–474. [Google Scholar] [CrossRef]
- Okunrintemi, V.; Valero-Elizondo, J.; Patrick, B.; Salami, J.; Tibuakuu, M.; Ahmad, S.; Ogunmoroti, O.; Mahajan, S.; Nasir, K.; Michos, E.D. Gender Differences in Patient-Reported Outcomes Among Adults With Atherosclerotic Cardiovascular Disease. J. Am. Heart Assoc. 2018, 7, e010498. [Google Scholar] [CrossRef] [Green Version]
- Persky, R.W.; Turtzo, L.C.; McCullough, L.D. Stroke in Women: Disparities and Outcomes. Curr. Cardiol. Rep. 2010, 12, 6–13. [Google Scholar] [CrossRef] [Green Version]
- The Women Initiative for Stroke in Europe (WISE) Group; Cordonnier, C.; Sprigg, N.; Sandset, E.C.; Pavlovic, A.; Sunnerhagen, K.S.; Caso, V.; Christensen, H. Stroke in Women—From Evidence to Inequalities. Nat. Rev. Neurol 2017, 13, 521–532. [Google Scholar] [CrossRef]
- Macrae, I. Preclinical Stroke Research—Advantages and Disadvantages of the Most Common Rodent Models of Focal Ischaemia: Animal Models of Focal Cerebral Ischaemia. Br. J. Pharmacol. 2011, 164, 1062–1078. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Wasan, H.; Reeta, K.H. Preclinical Stroke Research and Translational Failure: A Bird’s Eye View on Preventable Variables. Cell Mol. Neurobiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.; Emmerson, J.; Jadavji, N.M. Roles of Folate in Neurological Function. In Folic Acid: Sources, Health Effects and Role in Disease; Nova Publishers Science Inc.: New York, NY, USA, 2017. [Google Scholar]
- Pieters, B.; Staals, J.; Knottnerus, I.; Rouhl, R.; Menheere, P.; Kessels, A.F.; Lodder, J. Periventricular White Matter Lucencies Relate to Low Vitamin B12 Levels in Patients with Small Vessel Stroke. Stroke 2009, 40, 1623–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zacharia, G.; Shani, D.; Ortiz, R.A. Recurrent Stroke in a Patient with Vitamin B 12 Deficiency and MTHFR Mutation. Neurol. Clin. Pract. 2017, 7, e1–e4. [Google Scholar] [CrossRef] [Green Version]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial Function and Toxicity: Role of B Vitamins on the One-Carbon Transfer Pathways. Chem-Biol. Interact. 2006, 163, 113–132. [Google Scholar] [CrossRef]
- Nguyen, H.; Zarriello, S.; Rajani, M.; Tuazon, J.; Napoli, E.; Borlongan, C.V. Understanding the Role of Dysfunctional and Healthy Mitochondria in Stroke Pathology and Its Treatment. Int. J. Mol. Sci. 2018, 19, 2127. [Google Scholar] [CrossRef] [Green Version]
- Abato, J.E.; Moftah, M.; Cron, G.O.; Smith, P.D.; Jadavji, N.M. Methylenetetrahydrofolate Reductase Deficiency Alters Cellular Response after Ischemic Stroke in Male Mice. Nutr. Neurosci. 2020, 25, 558–566. [Google Scholar] [CrossRef]
- Jadavji, N.; Emmerson, J.T.; Shanmugalingam, U.; Willmore, W.G.; Macfarlane, A.J.; Smith, P.D. A Genetic Deficiency in Folic Acid Metabolism Impairs Recovery after Ischemic Stroke. Exp. Neurol. 2018, 309, 14–22. [Google Scholar] [CrossRef]
- Jadavji, N.M.; Emmerson, J.; Willmore, W.G.; MacFarlane, A.J.; Smith, P. B-Vitamin and Choline Supplementation Increases Neuroplasticity and Recovery after Stroke. Neurobiol. Dis. 2017, 103, 89–100. [Google Scholar] [CrossRef]
- Labat-gest, V.; Tomasi, S. Photothrombotic Ischemia: A Minimally Invasive and Reproducible Photochemical Cortical Lesion Model for Mouse Stroke Studies. J. Vis. Exp. JoVE 2013, 76, e50370. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-K.; Kim, J.-E.; Sivula, M.; Strittmatter, S.M. Nogo Receptor Antagonism Promotes Stroke Recovery by Enhancing Axonal Plasticity. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 6209–6217. [Google Scholar] [CrossRef]
- Flurkey, K.; Currer, J.M.; Harrison, D.E. Mouse Models in Aging Research. In The-Mouse-in-Biomedical-Research, Normative Biology, Husbandry, and Models; Fox, J., Barthold, S., Davisson, M., Newcommer, C., Quimby, F., Smith, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 3, pp. 637–672. ISBN 978-0-12-369454-6. [Google Scholar]
- Reeves, P.G. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef] [PubMed]
- Bernard, D.J.; Pangilinan, F.J.; Cheng, J.; Molloy, A.M.; Brody, L.C. Mice Lacking the Transcobalamin-Vitamin B12 Receptor, CD320, Suffer from Anemia and Reproductive Deficits When Fed Vitamin B12-Deficient Diet. Hum. Mol. Genet. 2018, 27, 3627–3640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bederson, J.B.; Pitts, L.H.; Tsuji, M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H. Rat Middle Cerebral Artery Occlusion: Evaluation of the Model and Development of a Neurologic Examination. Stroke J. Cereb. Circ. 1986, 17, 472–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balkaya, M.; Kröber, J.; Gertz, K.; Peruzzaro, S.; Endres, M. Characterization of Long-Term Functional Outcome in a Murine Model of Mild Brain Ischemia. J. Neurosci. Methods 2013, 213, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Jadavji, N.M.; Farr, T.; Khalil, A.; Boehm-Sturm, P.; Foddis, M.; Harms, C.; Füchtemeier, M.; Dirnagl, U. Elevated Levels of Plasma Homocysteine, Deficiencies in Dietary Folic Acid and Uracil-DNA Glycosylase Impair Learning in a Mouse Model of Vascular Cognitive Impairment. Behav. Brain Res. 2015, 283, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Theoret, J.K.; Jadavji, N.M.; Zhang, M.; Smith, P.D. Granulocyte Macrophage Colony-Stimulating Factor Treatment Results in Recovery of Motor Function after White Matter Damage in Mice. Eur. J. Neurosci. 2016, 43, 17–24. [Google Scholar] [CrossRef]
- Farr, T.D.; Liu, L.; Colwell, K.L.; Whishaw, I.Q.; Metz, G.A. Bilateral Alteration in Stepping Pattern after Unilateral Motor Cortex Injury: A New Test Strategy for Analysis of Skilled Limb Movements in Neurological Mouse Models. J. Neurosci. Methods 2006, 153, 104–113. [Google Scholar] [CrossRef]
- Ducros, V.; Belva-Besnet, H.; Casetta, B.; Favier, A. A Robust Liquid Chromatography Tandem Mass Spectrometry Method for Total Plasma Homocysteine Determination in Clinical Practice. Clin. Chem. Lab. Med. (CCLM) 2006, 44, 987–990. [Google Scholar] [CrossRef]
- Jensen, E.C. Quantitative Analysis of Histological Staining and Fluorescence Using ImageJ. Anat. Rec. 2013, 296, 378–381. [Google Scholar] [CrossRef]
- Bapat, A.; Schippel, N.; Shi, X.; Jasbi, P.; Gu, H.; Kala, M.; Sertil, A.; Sharma, S. Hypoxia Promotes Erythroid Differentiation through the Development of Progenitors and Proerythroblasts. Exp. Hematol 2021, 97, 32–46.e35. [Google Scholar] [CrossRef]
- Jasbi, P.; Baker, O.; Shi, X.; Gonzalez, L.A.; Wang, S.; Anderson, S.; Xi, B.; Gu, H.; Johnston, C.S. Daily Red Wine Vinegar Ingestion for Eight Weeks Improves Glucose Homeostasis and Affects the Metabolome but Does Not Reduce Adiposity in Adults. Food Funct. 2019, 10, 7343–7355. [Google Scholar] [CrossRef] [PubMed]
- Jasbi, P.; Shi, X.; Chu, P.; Elliott, N.; Hudson, H.; Jones, D.; Serrano, G.; Chow, B.; Beach, T.G.; Liu, L.; et al. Metabolic Profiling of Neocortical Tissue Discriminates Alzheimer’s Disease from Mild Cognitive Impairment, High Pathology Controls, and Normal Controls. J. Proteome Res. 2021, 20, 4303–4317. [Google Scholar] [CrossRef]
- Jasbi, P.; Mitchell, N.M.; Shi, X.; Grys, T.E.; Wei, Y.; Liu, L.; Lake, D.F.; Gu, H. Coccidioidomycosis Detection Using Targeted Plasma and Urine Metabolic Profiling. J. Proteome Res. 2019, 18, 2791–2802. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, H.; Palma-Duran, S.A.; Fierro, A.; Jasbi, P.; Shi, X.; Bresette, W.; Tasevska, N. Influence of Storage Conditions and Preservatives on Metabolite Fingerprints in Urine. Metabolites 2019, 9, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Langan, R.C.; Goodbred, A.J. Vitamin B12 Deficiency: Recognition and Management. Am. Fam. Physician 2017, 96, 384–389. [Google Scholar] [PubMed]
- Fluri, F.; Schuhmann, M.K.; Kleinschnitz, C. Animal Models of Ischemic Stroke and Their Application in Clinical Research. Drug Des. Dev. 2015, 9, 3445–3454. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA Cycle Metabolites Control Physiology and Disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.N.; Agharkar, A.S.; Gonzales, E.B. A Review of Creatine Supplementation in Age-Related Diseases: More than a Supplement for Athletes. F1000Research 2014, 3, 222. [Google Scholar] [CrossRef] [Green Version]
- van de Lagemaat, E.; de Groot, L.; van den Heuvel, E. Vitamin B12 in Relation to Oxidative Stress: A Systematic Review. Nutrients 2019, 11, 482. [Google Scholar] [CrossRef] [Green Version]
- An, R.; Li, D.; Dong, Y.; She, Q.; Zhou, T.; Nie, X.; Pan, R.; Deng, Y. Methylcobalamin Protects Melanocytes from H2O2-Induced Oxidative Stress by Activating the Nrf2/HO-1 Pathway. DDDT 2021, 15, 4837–4848. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Vieira, L.E.; Buttari, B.; Profumo, E.; Saso, L. The Nrf2 Pathway in Ischemic Stroke: A Review. Molecules 2021, 26, 5001. [Google Scholar] [CrossRef]
- Li, F. The Beneficial Role of Vitamin B12 in Injury Induced by Ischemia-Reperfusion: Beyond Scavenging Superoxide? J. Exp. Nephrol. 2021, 2, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Jadavji, N.M.; Mosnier, H.; Kelly, E.; Lawrence, K.; Cruickshank, S.; Stacey, S.; McCall, A.; Dhatt, S.; Arning, E.; Bottiglieri, T.; et al. One-Carbon Metabolism Supplementation Improves Outcome after Stroke in Aged Male MTHFR-Deficient Mice. Neurobiol. Dis. 2019, 132, 104613. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-S.; Li, X.; Wang, L.; Wang, J.-Z.; Ma, J.-P.; Wu, C.-J. Supplementation of Folic Acid and Vitamin B12 Reduces Plasma Levels of Asymmetric Dimethylarginine in Patients with Acute Ischemic Stroke. J. Clin. Neurosci. 2014, 21, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Schwammenthal, Y.; Tanne, D. Homocysteine, B-Vitamin Supplementation, and Stroke Prevention: From Observational to Interventional Trials. Lancet Neurol. 2004, 3, 493–495. [Google Scholar] [CrossRef]
- Spence, J.D. Stroke Nutrition in Stroke Prevention. Skull Base 2017, 37, 259–266. [Google Scholar] [CrossRef]
- Margalit, I.; Cohen, E.; Goldberg, E.; Krause, I. Vitamin B12 Deficiency and the Role of Gender: A Cross-Sectional Study of a Large Cohort. Ann. Nutr. Metab. 2018, 72, 265–271. [Google Scholar] [CrossRef]



| Concentration of Metabolites µM | CD | Vit. B12 Def. | Diet |
|---|---|---|---|
| Homocysteine | 8.25 ± 0.41 | 14.2 ± 1.15 | p = 0.0008 |
| S-adenosylmethionine | 0.34 ± 0.027 | 0.41 ± 0.059 | p = 0.29 |
| S-adenosylhomocysteine | 0.10 ± 0.017 | 0.16 ± 0.043 | p = 0.28 |
| Methionine | 85.0 ± 4.33 | 90.9 ± 7.41 | p = 0.53 |
| Cystathionine | 1.01 ± 88.4 | 0.95 ± 0.072 | p = 0.62 |
| Betaine | 51.1 ± 4.11 | 49.8 ± 4.98 | p = 0.85 |
| Choline | P = 24.6 ± 2.25 | 29.0 ± 6.01 | p = 0.55 |
| Concentration of Metabolites µmol/g | CD | Vit. B12 Def. | Diet |
|---|---|---|---|
| Homocysteine | 6.39 ± 0.34 | 8.98 ± 0.40 | p = 0.001 |
| S-adenosylmethionine | 547 ± 94.1 | 797 ± 213 | p = 0.37 |
| S-adenosylhomocysteine | 425 ± 21.7 | 454 ± 37.1 | p = 0.53 |
| Methionine | 5033 ± 442 | 4746 ± 362 | p = 0.62 |
| Cystathionine | 256 ± 20.5 | 249 ± 24.9 | p = 0.85 |
| Betaine | 123 ± 11.2 | 117 ± 12.8 | p = 0.73 |
| Choline | 257 ± 35.2 | 373 ± 21.6 | p = 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poole, J.; Jasbi, P.; Pascual, A.S.; North, S.; Kwatra, N.; Weissig, V.; Gu, H.; Bottiglieri, T.; Jadavji, N.M. Ischemic Stroke and Dietary Vitamin B12 Deficiency in Old-Aged Females: Impaired Motor Function, Increased Ischemic Damage Size, and Changed Metabolite Profiles in Brain and Cecum Tissue. Nutrients 2022, 14, 2960. https://doi.org/10.3390/nu14142960
Poole J, Jasbi P, Pascual AS, North S, Kwatra N, Weissig V, Gu H, Bottiglieri T, Jadavji NM. Ischemic Stroke and Dietary Vitamin B12 Deficiency in Old-Aged Females: Impaired Motor Function, Increased Ischemic Damage Size, and Changed Metabolite Profiles in Brain and Cecum Tissue. Nutrients. 2022; 14(14):2960. https://doi.org/10.3390/nu14142960
Chicago/Turabian StylePoole, Joshua, Paniz Jasbi, Agnes S. Pascual, Sean North, Neha Kwatra, Volkmar Weissig, Haiwei Gu, Teodoro Bottiglieri, and Nafisa M. Jadavji. 2022. "Ischemic Stroke and Dietary Vitamin B12 Deficiency in Old-Aged Females: Impaired Motor Function, Increased Ischemic Damage Size, and Changed Metabolite Profiles in Brain and Cecum Tissue" Nutrients 14, no. 14: 2960. https://doi.org/10.3390/nu14142960
APA StylePoole, J., Jasbi, P., Pascual, A. S., North, S., Kwatra, N., Weissig, V., Gu, H., Bottiglieri, T., & Jadavji, N. M. (2022). Ischemic Stroke and Dietary Vitamin B12 Deficiency in Old-Aged Females: Impaired Motor Function, Increased Ischemic Damage Size, and Changed Metabolite Profiles in Brain and Cecum Tissue. Nutrients, 14(14), 2960. https://doi.org/10.3390/nu14142960









