Protective Effect of Bilberry Anthocyanin Extracts on Dextran Sulfate Sodium-Induced Intestinal Damage in Drosophila melanogaster
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fly Stock, Culture, and Treatment
2.3. Lifespan Assays
2.4. Smurf Assays
2.5. Intestinal Morphology Assays
2.6. 7-AAD Assays
2.7. Intestinal Epithelial Cell ROS Assays
2.8. RNA Isolation and Real-Time Quantitative PCR
2.9. Antioxidant Capacity Assays
2.10. Intestinal Microbiome Assays
2.11. Statistical Analysis
3. Results
3.1. Effects of BANCs on the Lifespans of Normal and Axenic D. melanogaster
3.2. Effects of BANCs on the Intestinal Injury Index of Normal and Axenic D. melanogaster
3.2.1. Effect of BANCs on the Intestine Length in Normal and Axenic DSS-Treated Flies
3.2.2. Effects of BANCs on the Intestinal Integrity of Normal and Sterile Drosophila with DSS-Induced Inflammation
3.2.3. Protective Effect of BANCs on Epithelial Cells in the DSS-Induced Inflamed Intestines of Normal and Axenic D. melanogaster
3.2.4. Effects of BANCs on the ROS Content in Epithelial Cells of D. melanogaster
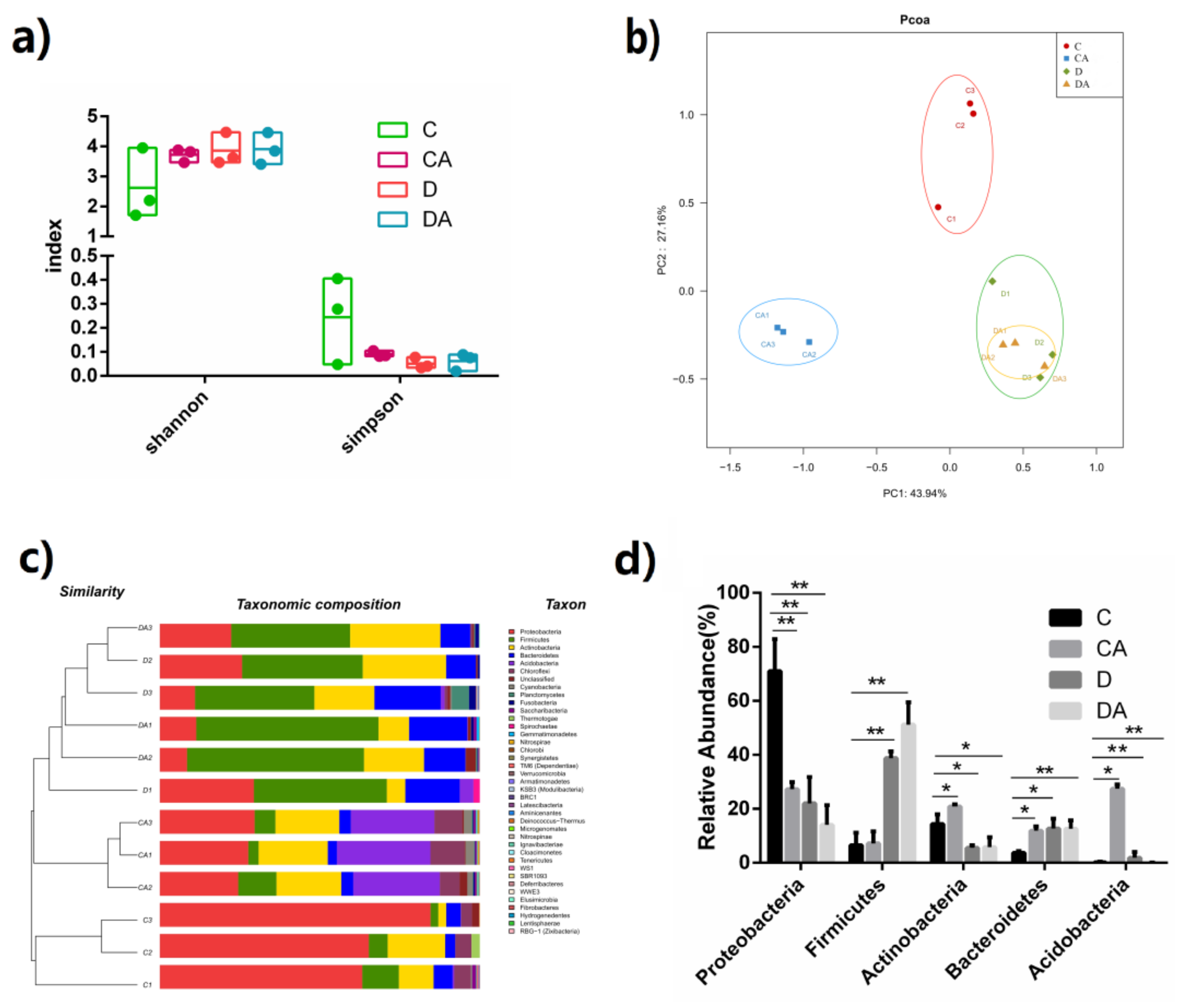
3.3. Effects of BANCs on Intestinal Microflora of Normal D. melanogaster
3.4. Effects of BANCs on the NRF2 Signaling Pathway and Antioxidant Capacity of Normal Female D. melanogaster Exposed to DSS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGuckin, M.A.; Eri, R.; Simms, L.A.; Florin, T.H.; Radford-Smith, G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm. Bowel Dis. 2009, 15, 100–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nenci, A.; Becker, C.; Wullaert, A.; Gareus, R.; van Loo, G.; Danese, S.; Huth, M.; Nikolaev, A.; Neufert, C.; Madison, B.; et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 2007, 446, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.D.; Lee, J.H.; Lee, Y.M.; Kim, D.K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.R.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef]
- Abdin, M.; Hamed, Y.S.; Akhtar, H.M.S.; Chen, D.; Chen, G.; Wan, P.; Zeng, X. Antioxidant and anti-inflammatory activities of target anthocyanins di-glucosides isolated from Syzygium cumini pulp by high speed counter-current chromatography. J. Food Biochem. 2020, 44, 1050–1062. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, rubus, and ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Liu, Y.X.; Xue, S.; Yong, H.; Feng, Z.; Di, Z. Identification of anthocyanin components of wild chinese blueberries and amelioration of light-induced retinal damage in pigmented rabbit using whole berries. J. Agric. Food Chem. 2011, 59, 356–363. [Google Scholar] [CrossRef]
- Kuntz, S.; Kunz, C.; Herrmann, J.; Borsch, C.H.; Abel, G.; Fröhling, B.; Dietrich, H.; Rudloff, S. Anthocyanins from fruit juices improve the antioxidant status of healthy young female volunteers without affecting anti-inflammatory parameters: Results from the randomised, double-blind, placebo-controlled, cross-over anthonia(anthocyanins in nutrition investigation alliance) study. Br. J. Nutr. 2014, 112, 925–936. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S. Anthocyanins in the black soybean (Glycine max L.) protect U2OS cells from apoptosis by induced autophagy via the activation of adenosyl monophosphate-dependent protein kinase. Oncol. Rep. 2012, 28, 2049–2056. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Ann. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Di Gesso, J.L.; Kerr, J.S.; Zhang, Q.; Raheem, S.; Yalamanchili, S.K.; O’Hagan, D.; Kay, C.D.; O’Connell, M.A. Flavonoid metabolites reduce tumor necrosis factor-α secretion to a greater extent than their precursor compounds in human THP-1 monocytes. Mol. Nutr. Food Res. 2015, 59, 1143–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Adamo, A.M.; Mills, D.; Kalanetra, K.; Hester, S.N.; Wood, S.M.; Fraga, C.G.; Oteiza, P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol. 2019, 26, 101269. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, T.; Li, N.; Wang, X.; Chen, G.; Lyu, X. Bilberry anthocyanin extract promotes intestinal barrier function and inhibits digestive enzyme activity by regulating the gut microbiota in aging rats. Food Funct. 2019, 10, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Moze, S.; Polak, T.; Gasperlin, L.; Koron, D.; Vanzo, A.; Poklar Ulrih, N.; Abram, V. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef]
- Chan, S.W.; Tomlinson, B. Effects of Bilberry supplementation on metabolic and cardiovascular disease risk. Molecules 2020, 25, 1653. [Google Scholar] [CrossRef] [Green Version]
- Habanova, M.; Saraiva, J.A.; Haban, M.; Schwarzova, M.; Chlebo, P.; Predna, L.; Gažo, J.; Wyka, J. Intake of bilberries (Vaccinium myrtillus L.) reduced risk factors for cardiovascular disease by inducing favorable changes in lipoprotein profiles. Nutr. Res. 2016, 36, 1415–1422. [Google Scholar] [CrossRef]
- Lyles, J.T.; Luo, L.; Liu, K.; Jones, D.P.; Jones, R.M. Cruciferous vegetables (Brassica oleracea) confer cytoprotective effects in Drosophila intestines. Gut Microbes 2021, 13, 1921926. [Google Scholar] [CrossRef]
- Amcheslavsky, A.; Jiang, J.; Ip, Y.T. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 2009, 4, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Luo, Q.; Jin, L.H. Acanthopanax senticosus extracts have a protective effect on Drosophila gut immunity. J. Ethnopharmacol. 2013, 146, 257–263. [Google Scholar] [CrossRef]
- Issa, A.R.; Sun, J.; Petitgas, C.; Mesquita, A.; Dulac, A.; Robin, M.; Mollereau, B.; Jenny, A.; Chérif-Zahar, B.; Birman, S. The lysosomal membrane protein LAMP2A promotes autophagic flux and prevents SNCA-induced Parkinson disease-like symptoms in the Drosophila brain. Autophagy 2018, 14, 1898–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diep, Q.N.; El Mabrouk, M.; Cohn, J.S.; Endemann, D.; Amiri, F.; Virdis, A.; Neves, M.F.; Schiffrin, E.L. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: Role of peroxisome proliferator-activated receptor-gamma. Circulation 2002, 105, 2296–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Adolph, T.E.; Tomczak, M.F.; Niederreiter, L.; Ko, H.J.; Bck, J.; Martinez-Naves, E.; Glickman, J.N.; Tschurtschenthaler, M.; Hartwig, J.; Hosomi, S. Paneth cells as a site of origin for intestinal inflammation. Nature 2013, 503, 272–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.Y.; Núñez, G. Inflammasomes in intestinal inflammation and cancer. Gastroenterology 2011, 141, 1986–1999. [Google Scholar] [CrossRef] [Green Version]
- Arshad, A.; Heinrich, J. Intestinal inflammation and stem cell homeostasis in aging Drosophila melanogaster. Front. Cell. Infection Microbiol. 2013, 3, 98. [Google Scholar] [CrossRef]
- Van de Velde, F.; Esposito, D.; Grace, M.H.; Pirovani, M.E.; Lila, M.A. Anti-inflammatory and wound healing properties of polyphenolic extracts from strawberry and blackberry fruits. Food Res. Int. 2019, 121, 453–462. [Google Scholar] [CrossRef]
- Tojo, R.; Suárez, A.; Clemente, M.G.; de los Reyes-Gavilán, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, X.K.; Cheng, M.L.; Yang, G.Z.; Wang, B.; Liu, H.J.; Hu, Y.X.; Zhu, L.L.; Zhang, S.; Xiao, Z.W.; et al. Saccharomyces boulardii administration changes gut microbiota and attenuates D-Galactosamine-induced liver injury. Sci. Rep. 2017, 7, 1359. [Google Scholar] [CrossRef] [Green Version]
- Keppler, K.; Humpf, H.U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorganic Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, J.; Zhang, M.; Chen, Z.; Ma, Q.; Liu, H.; Nie, C.; Zhang, Z.; An, W.; Li, J. Lycium ruthenicum anthocyanins attenuate high-fat diet-induced colonic barrier dysfunction and inflammation in mice by modulating the gut microbiota. Mol. Nutr. Food Res. 2021, 65, e2000745. [Google Scholar] [CrossRef] [PubMed]
- Le Bras, A. Gut microbiota drives disease variability in the DSS colitis mouse model. Lab. Anim. 2022, 51, 131. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Gao, X.; Nie, L.; Xie, J.; Dai, T.; Shi, C.; Tao, L.; Wang, Y.; Tian, Y.; Sheng, J. Astragalin attenuates Dextran Sulfate Sodium (DSS)-induced acute experimental colitis by alleviating gut microbiota dysbiosis and inhibiting NF-κB activation in mice. Front. Immunol. 2020, 11, 2058. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; de la Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in disease: Timing is everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef]
- Jaiswal, V.; Park, M.; Lee, H.J. Comparative transcriptome analysis of the expression of antioxidant and immunity genes in the spleen of a Cyanidin 3-O-Glucoside-treated alzheimer’s mouse model. Antioxidants 2021, 10, 1435. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, J.; Zhi, Q.; Yuan, T.; Lei, X.; Zeng, K.; Ming, J. Anti-aging effects of the fermented anthocyanin extracts of purple sweet potato on Caenorhabditis elegans. Food Funct. 2021, 12, 12647–12658. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Back, P.; Braeckman, B.P.; Matthijssens, F. ROS in aging caenorhabditis elegans: Damage or signaling? Oxid. Med. Cell. Longev. 2012, 3, 608478. [Google Scholar] [CrossRef] [Green Version]






| Gene Name | Sequence (5′–3′) | Annealing Temperature | Gene Accession Number |
|---|---|---|---|
| NRF2 | F: AGCTTCTGTCGCATGGTTGA R: AGCCGTTGCTAACATGTCCA | 60 °C | NM_170055.2 |
| GCL | F: GACACCGATACGCATTGCAC R: CTCACCACGGAATCCTGCTT | 60 °C | NM_001298073.1 |
| GSTS | F: CAGACCGTCAAGGACAACGA R: TCGCGCTTGACCATGTAGTT | 60 °C | NM_166216.2 |
| SOD | F: ACCGACTCCAAGATTACGCTCT R: GTTGCCCGTTGACTTGCTC | 60 °C | NM_057387.5 |
| Rp49 | F:AGGGTATCGACAACAGAGTG R:CACCAGGAACTTCTTGAATC | 60 °C | NM_079843.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Gu, Y.; Dai, X. Protective Effect of Bilberry Anthocyanin Extracts on Dextran Sulfate Sodium-Induced Intestinal Damage in Drosophila melanogaster. Nutrients 2022, 14, 2875. https://doi.org/10.3390/nu14142875
Zhang G, Gu Y, Dai X. Protective Effect of Bilberry Anthocyanin Extracts on Dextran Sulfate Sodium-Induced Intestinal Damage in Drosophila melanogaster. Nutrients. 2022; 14(14):2875. https://doi.org/10.3390/nu14142875
Chicago/Turabian StyleZhang, Guocai, Yunyao Gu, and Xianjun Dai. 2022. "Protective Effect of Bilberry Anthocyanin Extracts on Dextran Sulfate Sodium-Induced Intestinal Damage in Drosophila melanogaster" Nutrients 14, no. 14: 2875. https://doi.org/10.3390/nu14142875
APA StyleZhang, G., Gu, Y., & Dai, X. (2022). Protective Effect of Bilberry Anthocyanin Extracts on Dextran Sulfate Sodium-Induced Intestinal Damage in Drosophila melanogaster. Nutrients, 14(14), 2875. https://doi.org/10.3390/nu14142875





