Inositol Nutritional Supplementation for the Prevention of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Study Selection
- RCTs evaluating the effects of inositol (MI and/or DCI) for preventing GDM during pregnancy.
- Pregnant women who were already diagnosed with type 1 diabetes (T1DM), type 2 diabetes mellitus (T2DM), or GDM.
- Interventions included supplementation other than inositol and folic acid.
- Did not assess incidence of GDM as outcome.
- Pilots, protocols, observational studies, reviews, case reports, trials, comments, letters, news, notes, editorial, or conference abstracts.
- Unable to access the full text of article.
2.4. Data Extraction
2.5. Outcome Measures
2.6. Risk of Bias and Quality Assessment
2.7. Data Analysis
3. Results
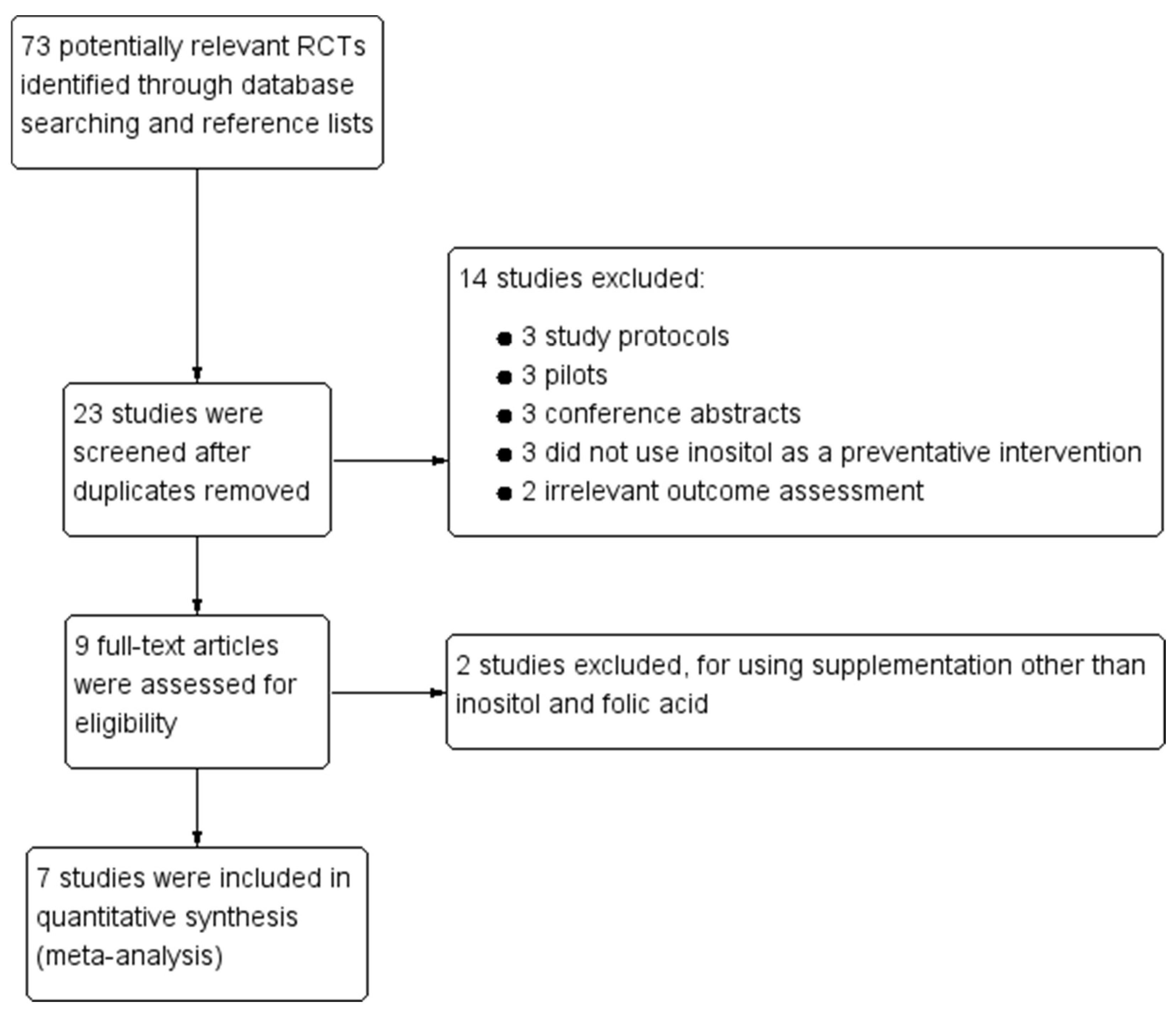
3.1. Literature Search
3.2. Characteristics of Included Studies
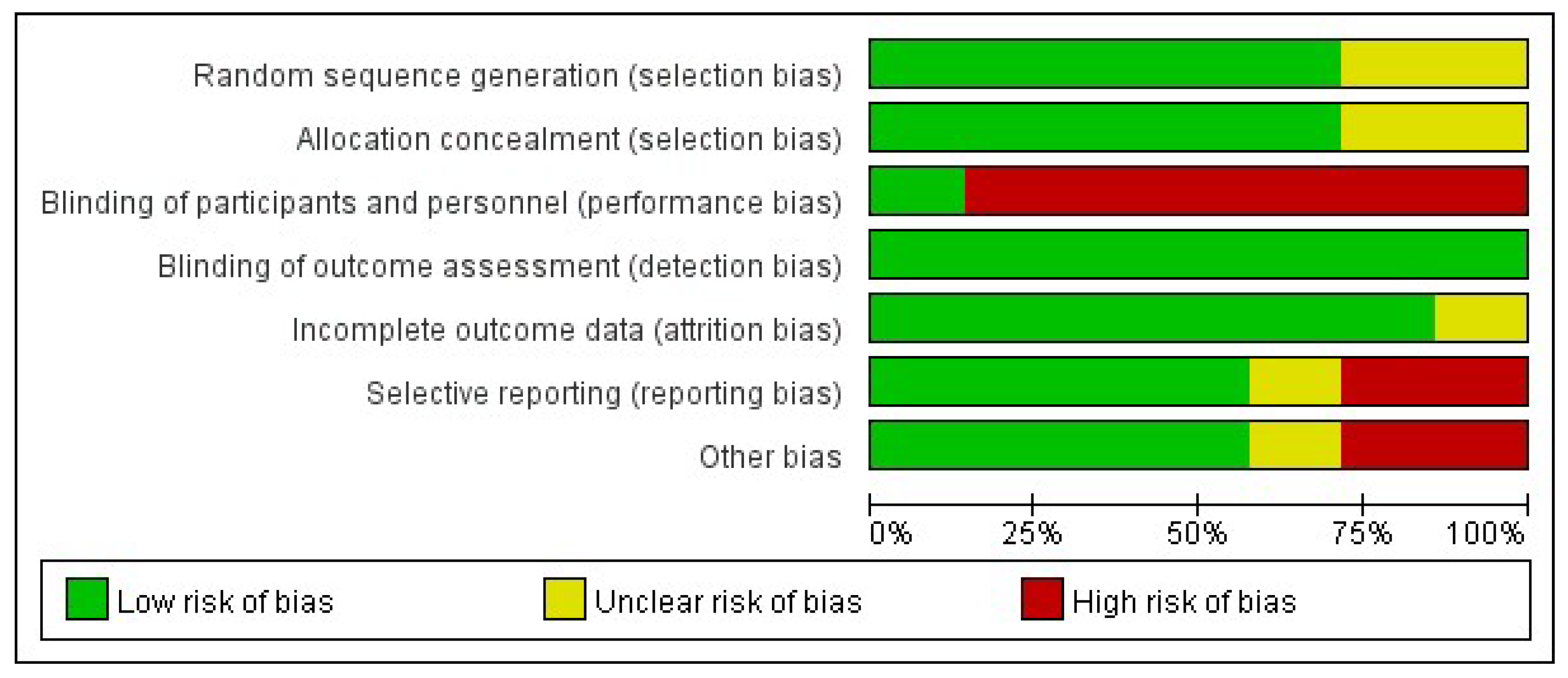
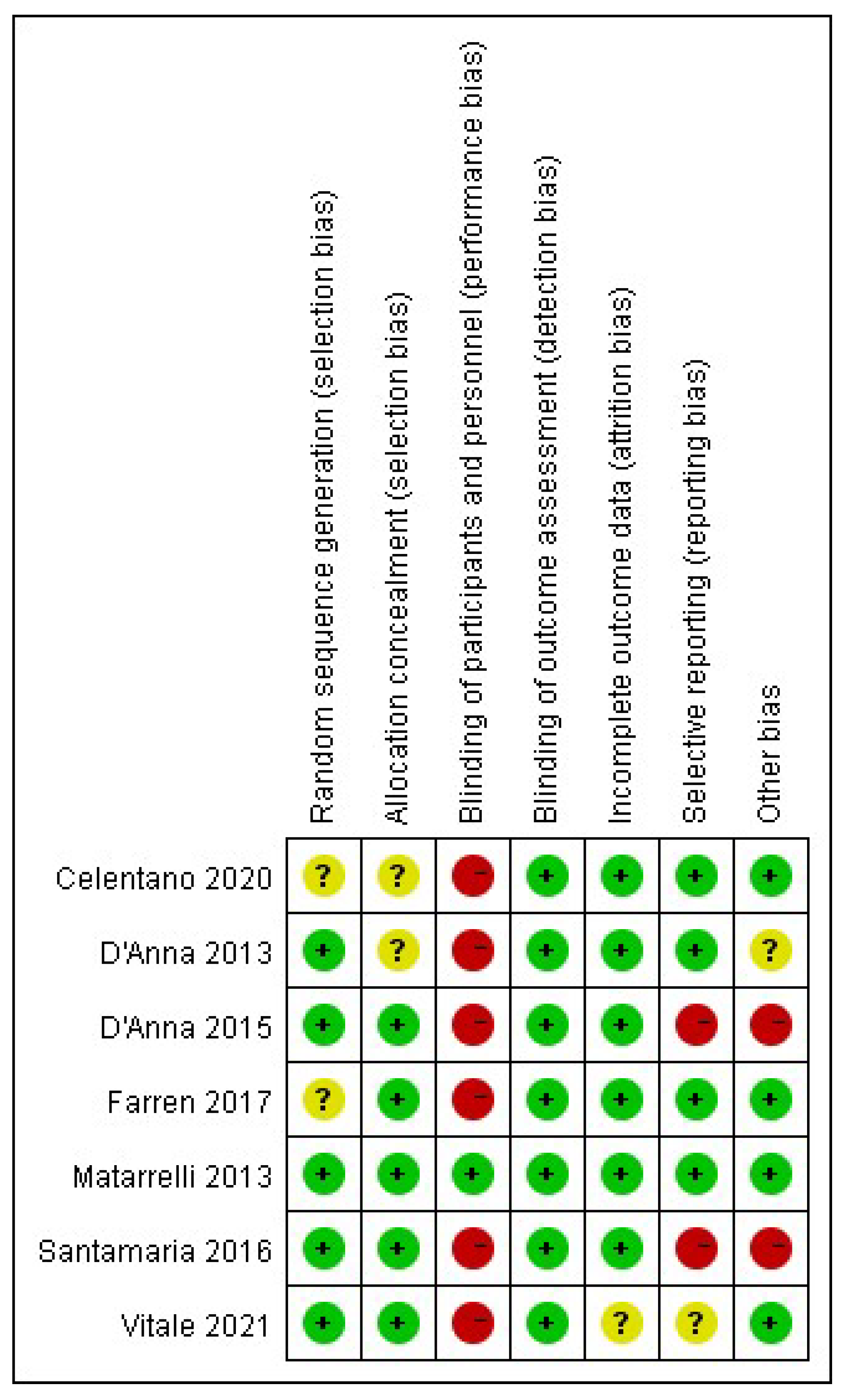
3.3. Risk of Bias of Included Studies
3.3.1. Selection Bias
3.3.2. Performance Bias
3.3.3. Detection Bias
3.3.4. Attrition Bias
3.3.5. Reporting Bias
3.3.6. Other Bias
3.4. Effects of Intervention
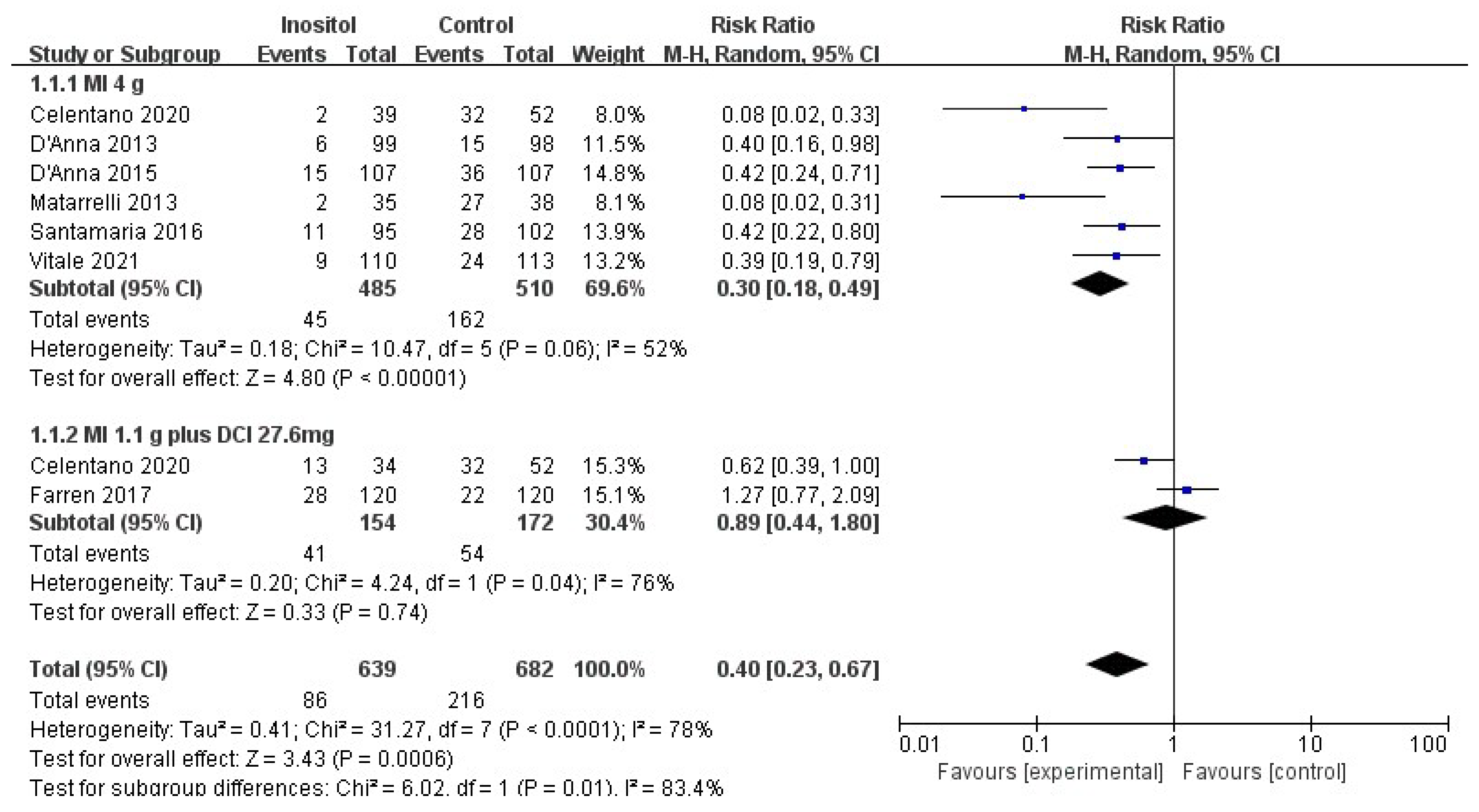
3.4.1. Primary Outcomes
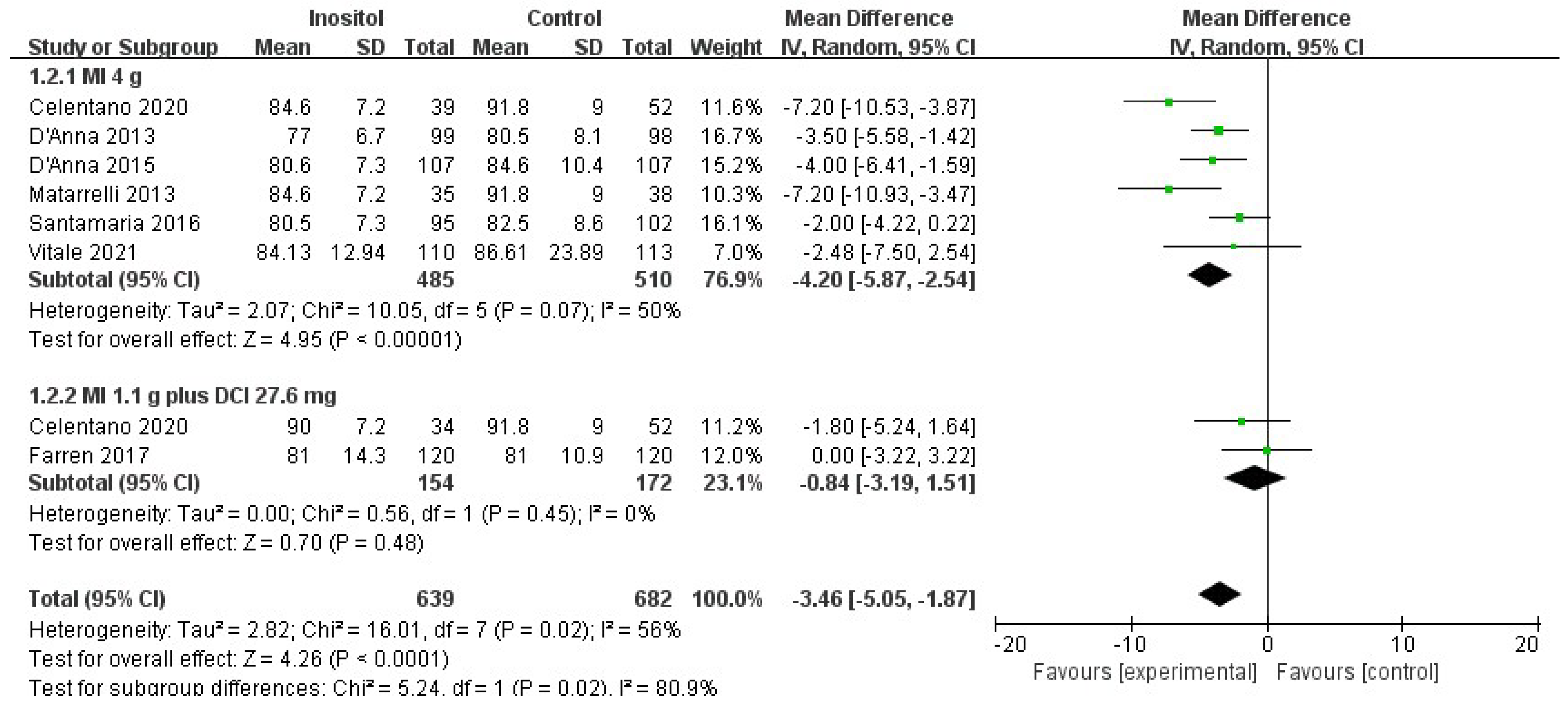
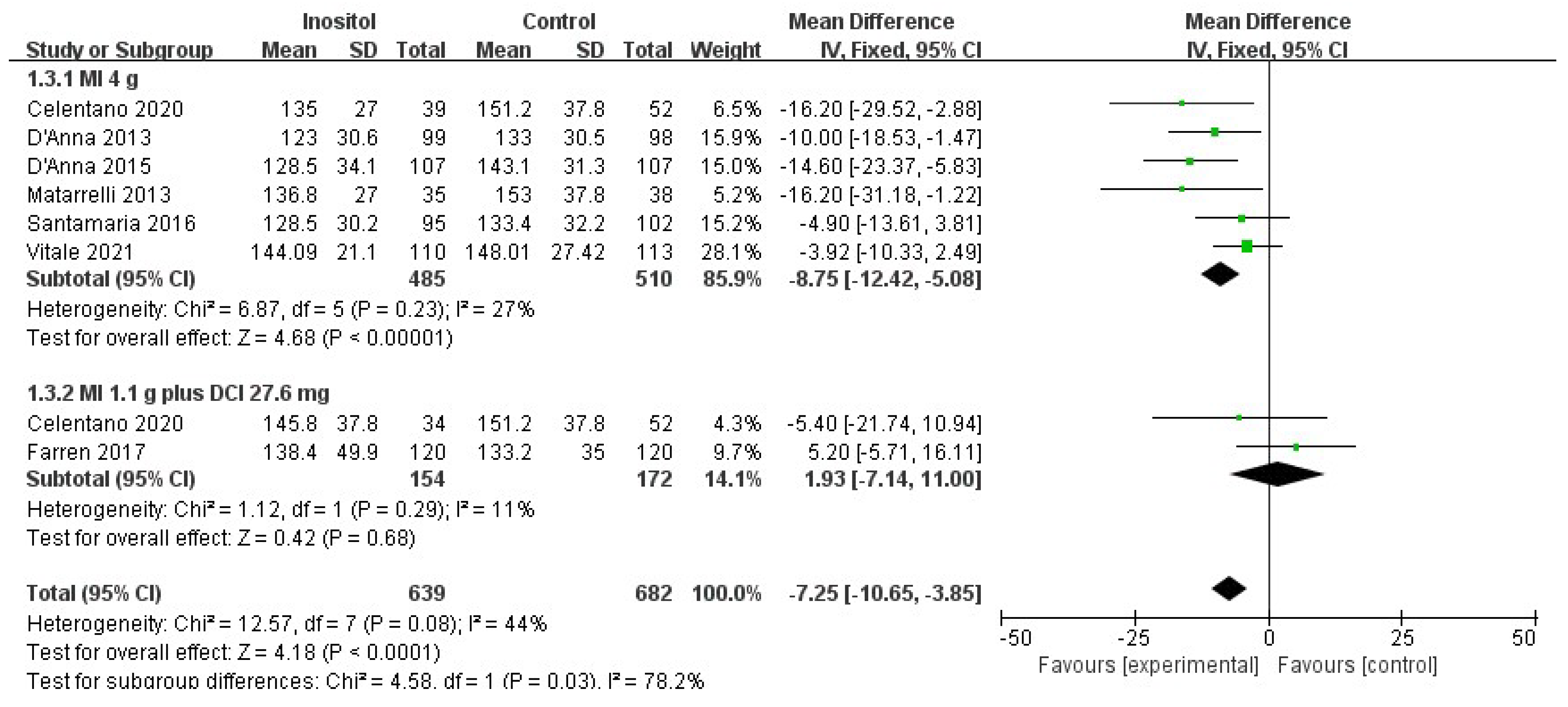
3.4.2. Secondary Outcomes
3.5. Side Effects
3.6. Overall Quality of Evidence
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Belgium, Brussels, 2021. [Google Scholar]
- Tsakiridis, I.; Giouleka, S.; Mamopoulos, A.; Kourtis, A.; Athanasiadis, A.; Filopoulou, D.; Dagklis, T. Diagnosis and Management of Gestational Diabetes Mellitus: An Overview of National and International Guidelines. Obstet. Gynecol. Surv. 2021, 76, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, H. Gestational diabetes in China: Challenges and coping strategies. Lancet Diabetes Endocrinol. 2014, 2, 930–931. [Google Scholar] [CrossRef]
- Shou, C.; Wei, Y.M.; Wang, C.; Yang, H.X. Updates in Long-term Maternal and Fetal Adverse Effects of Gestational Diabetes Mellitus. Matern. Fetal Med. 2019, 1, 91–94. [Google Scholar]
- Griffith, R.J.; Alsweiler, J.; Moore, A.E.; Brown, S.; Middleton, P.; Shepherd, E.; Crowther, C.A. Interventions to prevent women from developing gestational diabetes mellitus: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2020, 6, CD012394. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Dinicola, S.; Unfer, V.; Facchinetti, F.; Soulage, C.O.; Greene, N.D.; Bizzarri, M.; Laganà, A.S.; Chan, S.Y.; Bevilacqua, A.; Pkhaladze, L.; et al. Inositols: From Established Knowledge to Novel Approaches. Int. J. Mol. Sci. 2021, 22, 10575. [Google Scholar] [CrossRef]
- Unfer, V.; Nestler, J.E.; Kamenov, Z.A.; Prapas, N.; Facchinetti, F. Effects of Inositol(s) in Women with PCOS: A Systematic Review of Randomized Controlled Trials. Int. J. Endocrinol. 2016, 2016, 1849162. [Google Scholar] [CrossRef] [Green Version]
- Crawford, T.J.; Crowther, C.A.; Alsweiler, J.; Brown, J. Antenatal dietary supplementation with myo-inositol in women during pregnancy for preventing gestational diabetes. Cochrane Database Syst. Rev. 2015, 2015, CD011507. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane. 2021. Available online: www.training.cochrane.org/handbook (accessed on 4 May 2022).
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Matarrelli, B.; Vitacolonna, E.; D’Angelo, M.; Pavone, G.; Mattei, P.A.; Liberati, M.; Celentano, C. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: A randomized controlled trial. J. Matern. Fetal Neonatal Med. 2013, 26, 967–972. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, R.; Scilipoti, A.; Giordano, D.; Caruso, C.; Cannata, M.L.; Interdonato, M.L.; Corrado, F.; Di Benedetto, A. myo-Inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: A prospective, randomized, placebo-controlled study. Diabetes Care 2013, 36, 854–857. [Google Scholar] [CrossRef] [Green Version]
- D’Anna, R.; Di Benedetto, A.; Scilipoti, A.; Santamaria, A.; Interdonato, M.L.; Petrella, E.; Neri, I.; Pintaudi, B.; Corrado, F.; Facchinetti, F. Myo-inositol Supplementation for Prevention of Gestational Diabetes in Obese Pregnant Women: A Randomized Controlled Trial. Obstet. Gynecol. 2015, 126, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Santamaria, A.; Di Benedetto, A.; Petrella, E.; Pintaudi, B.; Corrado, F.; D’Anna, R.; Neri, I.; Facchinetti, F. Myo-inositol may prevent gestational diabetes onset in overweight women: A randomized, controlled trial. J. Matern. Fetal Neonatal Med. 2016, 29, 3234–3237. [Google Scholar] [CrossRef] [PubMed]
- Farren, M.; Daly, N.; McKeating, A.; Kinsley, B.; Turner, M.J.; Daly, S. The Prevention of Gestational Diabetes Mellitus with Antenatal Oral Inositol Supplementation: A Randomized Controlled Trial. Diabetes Care 2017, 40, 759–763. [Google Scholar] [CrossRef] [Green Version]
- Celentano, C.; Matarrelli, B.; Pavone, G.; Vitacolonna, E.; Mattei, P.A.; Berghella, V.; Liberati, M. The influence of different inositol stereoisomers supplementation in pregnancy on maternal gestational diabetes mellitus and fetal outcomes in high-risk patients: A randomized controlled trial. J. Matern. Fetal Neonatal Med. 2020, 33, 743–751. [Google Scholar] [CrossRef]
- Vitale, S.G.; Corrado, F.; Caruso, S.; Di Benedetto, A.; Giunta, L.; Cianci, A.; D’Anna, R. Myo-inositol supplementation to prevent gestational diabetes in overweight non-obese women: Bioelectrical impedance analysis, metabolic aspects, obstetric and neonatal outcomes—A randomized and open-label, placebo-controlled clinical trial. Int. J. Food Sci. Nutr. 2021, 72, 670–679. [Google Scholar] [CrossRef]
- HAPO Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with neonatal anthropometrics. Diabetes 2009, 58, 453–459. [Google Scholar] [CrossRef] [Green Version]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.D.; Hod, M.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wei, Y.; Zhang, X.; Zhang, Y.; Xu, Q.; Sun, Y.; Su, S.; Zhang, L.; Liu, C.; Feng, Y.; et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet. Gynecol. 2017, 216, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guelfi, K.J.; Yang, H.X. Exercise and its role in gestational diabetes mellitus. Chronic Dis. Transl. Med. 2016, 2, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Liang, W.; Wei, M.; Gou, X.; Han, S.; Bai, J. Effects of D-Chiro-Inositol on Glucose Metabolism in db/db Mice and the Associated Underlying Mechanisms. Front. Pharmacol. 2020, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Dell’Edera, D.; Sarlo, F.; Allegretti, A.; Simone, F.; Lupo, M.G.; Epifania, A.A. The influence of D-chiro-inositol and D-myo-inositol in pregnant women with glucose intolerance. Biomed. Rep. 2017, 7, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, K.M.; Barton, S.J.; El-Heis, S.; Kenealy, T.; Nield, H.; Baker, P.N.; Chong, Y.S.; Cutfield, W.; Chan, S.Y. Myo-Inositol, Probiotics, and Micronutrient Supplementation from Preconception for Glycemia in Pregnancy: NiPPeR International Multicenter Double-Blind Randomized Controlled Trial. Diabetes Care 2021, 44, 1091–1099. [Google Scholar] [CrossRef]
- Chan, K.Y.; Wong, M.; Pang, S. Lo, K.K.H. Dietary supplementation for gestational diabetes prevention and management: A meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 2021, 303, 1381–1391. [Google Scholar] [CrossRef]
- Davidson, S.J.; Barrett, H.L.; Price, S.A.; Callaway, L.K.; Dekker Nitert, M. Probiotics for preventing gestational diabetes. Cochrane Database Syst. Rev. 2021, 4, CD009951. [Google Scholar] [CrossRef]
- Malvasi, A.; Casciaro, F.; Minervini, M.M.; Kosmas, I.; Mynbaev, O.A.; Pacella, E.; Monti Condesnitt, V.; Creanza, A.; Di Renzo, G.C.; Tinelli, A. Myo-inositol, D-chiro-inositol, folic acid and manganese in second trimester of pregnancy: A preliminary investigation. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 270–274. [Google Scholar]
- Malvasi, A.; Kosmas, I.; Mynbaev, O.A.; Sparic, R.; Gustapane, S.; Guido, M.; Tinelli, A. Can trans resveratrol plus d-chiro-inositol and myo-inositol improve maternal metabolic profile in overweight pregnant patients? Clin. Ter. 2017, 168, e240–e247. [Google Scholar] [CrossRef]
- Zhu, B.; Ge, X.; Huang, K.; Mao, L.; Yan, S.; Xu, Y.; Huang, S.; Hao, J.; Zhu, P.; Niu, Y.; et al. Folic Acid Supplement Intake in Early Pregnancy Increases Risk of Gestational Diabetes Mellitus: Evidence from a Prospective Cohort Study. Diabetes Care 2016, 39, e36–e37. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Yu, X.; Li, L.; Chen, Y.; Yang, Y.; Yang, Y.; Hu, Y.; Zhao, Y.; Tang, H.; Xu, D.; et al. Duration of periconceptional folic acid supplementation and risk of gestational diabetes mellitus. Asia Pac. J. Clin. Nutr. 2019, 28, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Appetecchia, M.; Aragona, C.; Bevilacqua, A.; Bezerra Espinola, M.S.; Bizzarri, M.; D’Anna, R.; Dewailly, D.; Diamanti-Kandarakis, E.; Hernández Marín, I.; et al. Experts’ opinion on inositols in treating polycystic ovary syndrome and non-insulin dependent diabetes mellitus: A further help for human reproduction and beyond. Expert. Opin. Drug Metab. Toxicol. 2020, 16, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Cavalli, P.; Copp, A.J.; D’Anna, R.; Kandaraki, E.; Greene, N.D.E.; Unfer, V. Experts Group on Inositol in Basic and Clinical Research. An update on the use of inositols in preventing gestational diabetes mellitus (GDM) and neural tube defects (NTDs). Expert. Opin. Drug Metab. Toxicol. 2020, 16, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Guo, S.; Miao, Z.; Li, Z.; Zhang, H. Myo-inositol lowers the risk of developing gestational diabetic mellitus in pregnancies: A systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. J. Diabetes Complicat. 2018, 32, 342–348. [Google Scholar] [CrossRef]
- Vitagliano, A.; Saccone, G.; Cosmi, E.; Visentin, S.; Dessole, F.; Ambrosini, G.; Berghella, V. Inositol for the prevention of gestational diabetes: A systematic review and meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 2019, 299, 55–68. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z. The efficacy of myo-inositol supplementation to reduce the incidence of gestational diabetes: A meta-analysis. Gynecol. Endocrinol. 2022, 38, 450–454. [Google Scholar] [CrossRef]
| Study | Country | Inclusion Criteria | Interventions | Inositol Group | Control Group |
|---|---|---|---|---|---|
| Matarrelli 2013 [14] | Italy | (1) Prepregnancy BMI < 35 kg/m2 (2) FPG ≥ 5.1 mmol/L and <7.0 mmol/L in the first or early second trimester (3) Single pregnancy | Group A: 2 g MI plus 200 μg folic acid twice a day Group B: 200 μg folic acid twice a day | Group A (n = 35) Age: 33.0 ± 4.9 BMI: 23.5 ± 3.4 FPG: 5.4 ± 0.3 | Group B (n = 38) Age: 33.8 ± 4.7 BMI: 24.7 ± 4.2 FPG: 5.4 ± 0.3 |
| D’Anna 2013 [15] | Italy | (1) GA: 12~13 w (2) First-degree relatives affected by T2DM (3) Prepregnancy BMI < 30 kg/m2 (4) FPG < 126 mg/dl, RG < 200 mg/dl (5) Single pregnancy (6) Caucasian race | Group A: 2 g MI plus 200 μg folic acid twice a day Group B: 200 μg folic acid twice a day | Group A (n = 99) Age: 31.0 ± 5.3 BMI: 22.8 ± 3.1 | Group B (n = 98) Age: 31.6 ± 5.6 BMI: 23.6 ± 3.1 |
| D’Anna 2015 [16] | Italy | (1) GA: 12~13 w (2) Prepregnancy BMI ≥ 30 kg/m2 (3) FPG < 126 mg/dl, RG < 200 mg/dl (4) Single pregnancy (5) Caucasian race | Group A: 2 g MI plus 200 μg folic acid twice a day Group B: 200 μg folic acid twice a day | Group A (n = 107) Age: 30.9 (18~44) BMI: 33.8 (30.0~46.9) FPG: 83.1 ± 8.5 | Group B (n = 107) Age: 31.7 (19~43) BMI: 33.8 (30.0~46.0) FPG: 82.3 ± 10.6 |
| Santamaria 2016 [17] | Italy | (1) GA: 12~13 w (2) Prepregnancy BMI ≥ 25 kg/m2, <30 kg/m2 (3) FPG < 126 mg/dl, RG < 200 mg/dl (4) Single pregnancy (5) Caucasian race | Group A: 2 g MI plus 200 μg folic acid twice a day Group B: 200 μg folic acid twice a day | Group A (n = 95) Age: 32.1 ± 4.8 BMI: 26.9 ± 1.3 FPG: 81.09 ± 8..03 | Group B (n = 102) Age: 32.7 ± 5.3 BMI: 27.1 ± 1.3 FPG: 78.63 ± 6.15 |
| Farren 2017 [18] | Ireland | (1) GA: 10~16 w (2) First-degree relatives affected by T1DM/T2DM (3) FPG < 126 mg/dl, RG < 200 mg/dl (4) Single pregnancy | Group A: 1.1 g MI, 27.6 mg DCI plus 400 μg folic acid per day Group B: 400 μg folic acid per day | Group A (n = 120) Age: 31.1 ± 5.1 BMI: 26.0 ± 5.3 | Group B (n = 120) Age: 31.5 ± 5.0 BMI: 26.2 ± 5.5 |
| Celentano 2020 [19] | Italy | (1) Prepregnancy BMI < 35 kg/m2 (2) FPG ≥ 5.1 mmol/L and <7.0 mmol/L in the first trimester (3) Single pregnancy | Group A: 2 g MI plus 200 μg folic acid twice a day Group B: 500 mg DCI plus 400 μg folic acid per day Group C: 0.55 g MI, 13.8 mg DCI plus 200 μg folic acid twice a day Group D: 400 μg folic acid per day | Group A (n = 39) Age: 33.1 ± 4.9 BMI: 23.5 ± 3.4 FPG: 5.4 ± 0.3 Group B (n = 32) Age: 34.4 ± 3.7 BMI: 24.4 ± 4.9 FPG: 5.3 ± 0.2 Group C (n = 34) Age: 34.1 ± 4.2 BMI: 23.5 ± 4.6 FPG: 5.4 ± 0.3 | Group D (n = 52) Age: 33.9 ± 4.9 BMI: 24.4 ± 4.1 FPG: 5.4 ± 0.3 |
| Vitale 2021 [20] | Italy | (1) GA: 12~13 w (2) Prepregnancy BMI ≥ 25 kg/m2, <30 kg/m2 (3) FPG < 126 mg/dl, RG < 200 mg/dl (4) Single pregnancy (5) Caucasian race | Group A: 2 g MI plus 200 μg folic acid twice a day Group B: 200 μg folic acid twice a day | Group A (n = 110) Age: 27.18 ± 6.03 BMI: 27.00 ± 1.49 FPG: 82.20 ± 12.12 | Group B (n = 113) Age: 27.95 ± 4.90 BMI: 26.68 ± 1.56 FPG: 83.10 ± 14.10 |
| Inositol for the Prevention of GDM | ||||||
|---|---|---|---|---|---|---|
| Patient or Population: Women in Early Pregnancy Who Were at Risk of GDM (Those with Pre-Existing T1DM/T2DM Excluded) Intervention: MI 4 g/MI 1.1 g Plus DCI 27.6 mg | ||||||
| Outcomes | Illustrative comparative risks * (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Inositol | |||||
| GDM rate | Study population | RR 0.4 (0.23 to 0.67) | 1321 (7 studies) | ⊕⊖⊖⊖ very low 1,2,3,4 | ||
| 317 per 1000 | 127 per 1000 (73 to 212) | |||||
| Moderate | ||||||
| 306 per 1000 | 122 per 1000 (70 to 205) | |||||
| Insulin treatment | Study population | RR 0.27 (0.11 to 0.66) | 562 (4 studies) | ⊕⊕⊖⊖ low 1,2,4,5 | ||
| 84 per 1000 | 23 per 1000 (9 to 56) | |||||
| Moderate | ||||||
| 106 per 1000 | 29 per 1000 (12 to 70) | |||||
| Hypertensive disorders | Study population | RR 0.43 (0.2 to 0.91) | 1006 (5 studies) | ⊕⊖⊖⊖ very low 1,2,4 | ||
| 42 per 1000 | 18 per 1000 (8 to 38) | |||||
| Moderate | ||||||
| 30 per 1000 | 13 per 1000 (6 to 27) | |||||
| Preterm delivery | Study population | RR 0.4 (0.22 to 0.74) | 1006 (5 studies) | ⊕⊖⊖⊖ very low 1,2,4 | ||
| 69 per 1000 | 27 per 1000 (15 to 51) | |||||
| Moderate | ||||||
| 63 per 1000 | 25 per 1000 (14 to 47) | |||||
| Cesarean section | Study population | RR 0.89 (0.77 to 1.03) | 1006 (5 studies) | ⊕⊖⊖⊖ very low 1,2,4,6 | ||
| 448 per 1000 | 398 per 1000 (345 to 461) | |||||
| Moderate | ||||||
| 471 per 1000 | 419 per 1000 (363 to 485) | |||||
| Shoulder dystocia | Study population | RR 0.58 (0.12 to 2.68) | 595 (3 studies) | ⊕⊖⊖⊖ very low 1,2,4,6 | ||
| 13 per 1000 | 8 per 1000 (2 to 35) | |||||
| Moderate | ||||||
| 10 per 1000 | 6 per 1000 (1 to 27) | |||||
| Macrosomia | Study population | RR 0.35 (0.06 to 1.92) | 595 (3 studies) | ⊕⊖⊖⊖ very low 1,2,3,4,6 | ||
| 56 per 1000 | 20 per 1000 (3 to 107) | |||||
| Moderate | ||||||
| 49 per 1000 | 17 per 1000 (3 to 94) | |||||
| Neonatal hypoglycemia | Study population | RR 0.62 (0.32 to 1.18) | 1079 (6 studies) | ⊕⊖⊖⊖ very low 1,4,6 | ||
| 46 per 1000 | 29 per 1000 (15 to 54) | |||||
| Moderate | ||||||
| 10 per 1000 | 6 per 1000 (3 to 12) | |||||
| NICU admission | Study population | RR 0.53 (0.23 to 1.21) | 809 (4 studies) | ⊕⊖⊖⊖ very low 1,2,4,6 | ||
| 37 per 1000 | 20 per 1000 (9 to 45) | |||||
| Moderate | ||||||
| 39 per 1000 | 21 per 1000 (9 to 47) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Yan, J.; Yang, H. Inositol Nutritional Supplementation for the Prevention of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 2831. https://doi.org/10.3390/nu14142831
Wei J, Yan J, Yang H. Inositol Nutritional Supplementation for the Prevention of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2022; 14(14):2831. https://doi.org/10.3390/nu14142831
Chicago/Turabian StyleWei, Jingshu, Jie Yan, and Huixia Yang. 2022. "Inositol Nutritional Supplementation for the Prevention of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 14, no. 14: 2831. https://doi.org/10.3390/nu14142831
APA StyleWei, J., Yan, J., & Yang, H. (2022). Inositol Nutritional Supplementation for the Prevention of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 14(14), 2831. https://doi.org/10.3390/nu14142831