Digested Human Colostrum Reduces Interleukin-8 Production in Induced Human Intestinal Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Digestion of Colostrum Samples
2.2. Cell Culture
2.3. Colostrum Treatment and Stimulation
2.4. Measurements of IL-8 and Cytotoxicity
2.5. Statistical Analysis
3. Results
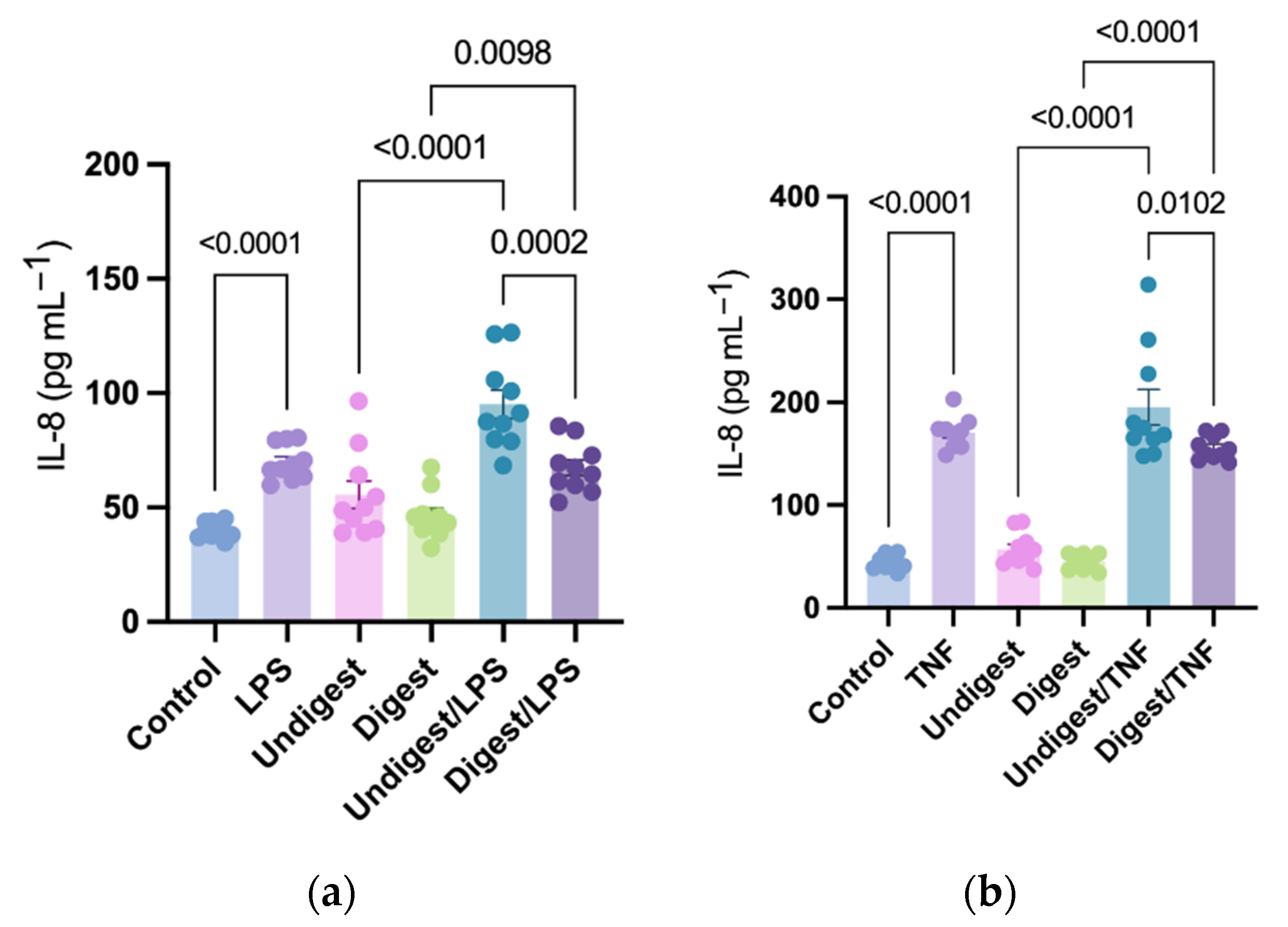
3.1. LPS and TNF Stimulation Induced IL-8 Production
3.2. Digested Colostrum Reduced IL-8 Production in Caco2BBe Cells under Both LPS and TNF Stimulation
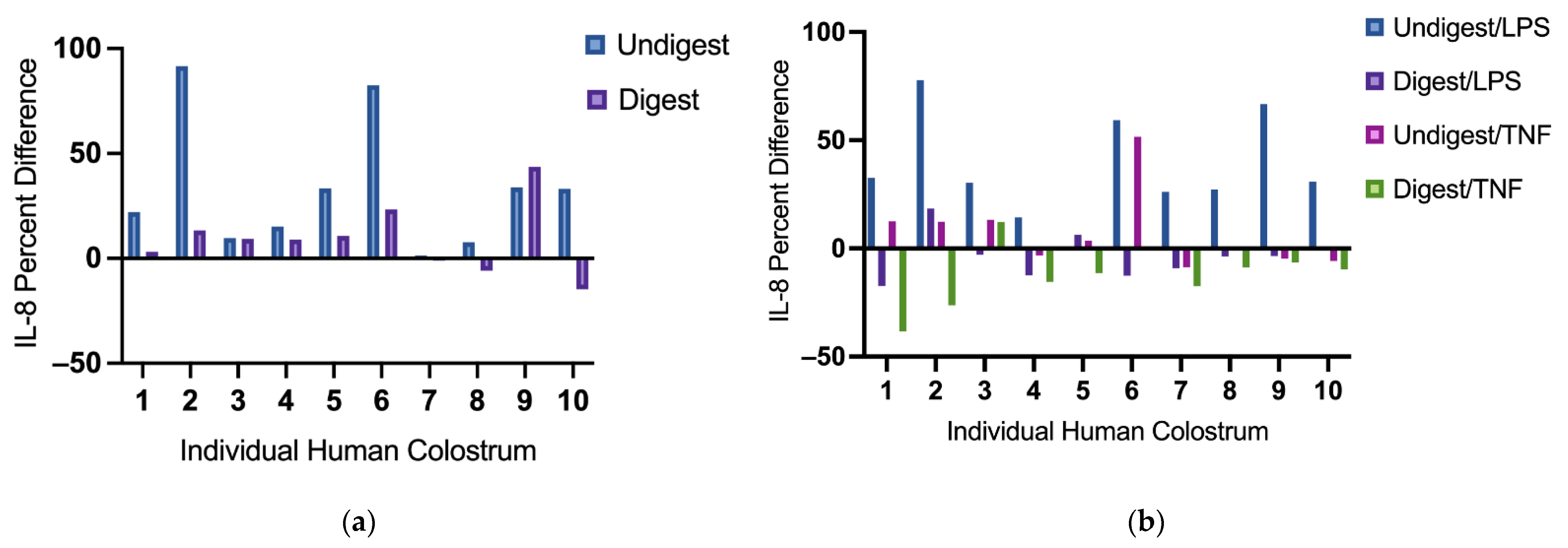
3.3. The Inhibition Effects on IL-8 Production Varies among Individual Human Colostrum
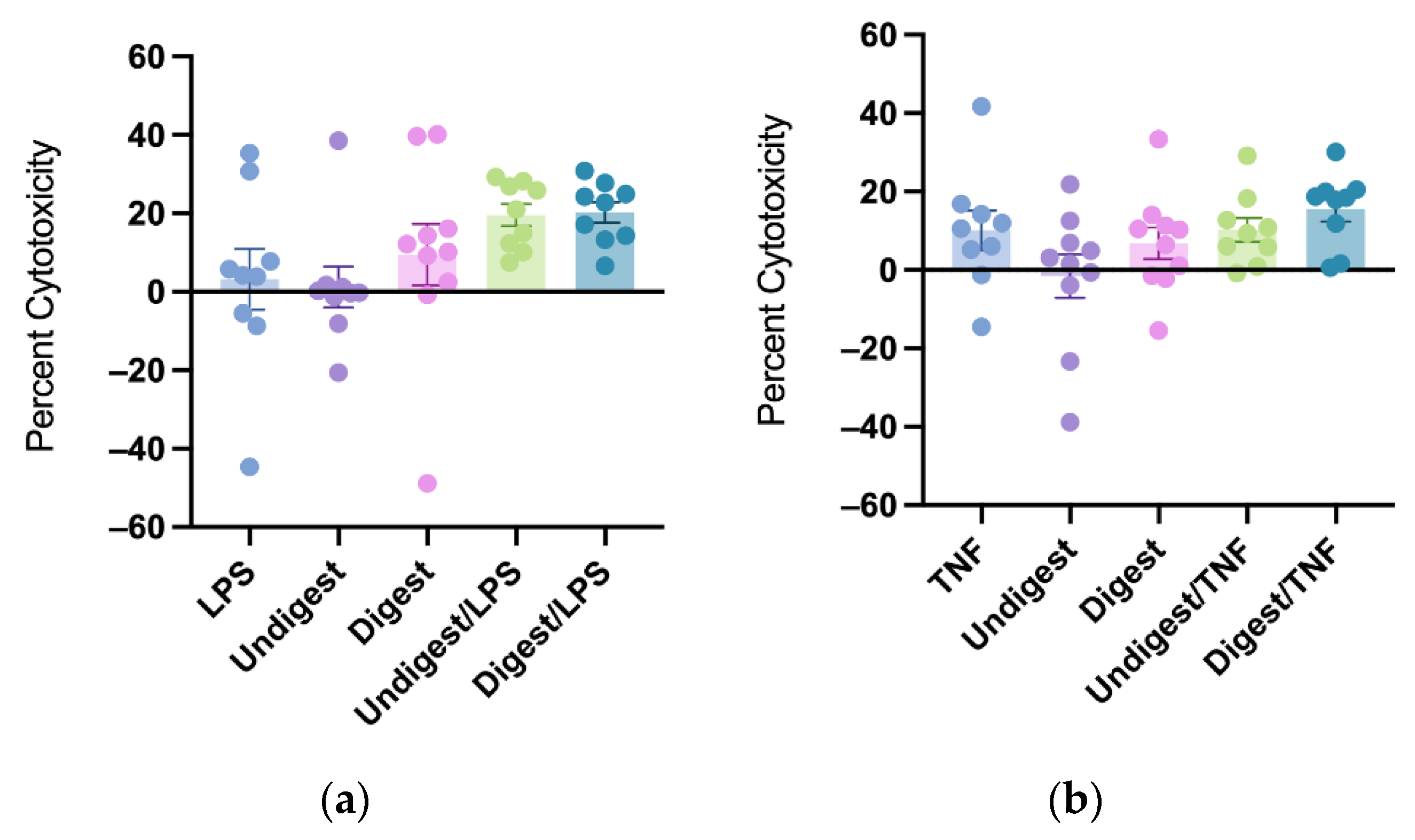
3.4. Digested and Undigested Colostrum Did Not Affect Cytotoxicity in Caco2BBe Cells Overall
3.5. Individual Colostrum Affected Cytotoxicity Differently
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neu, J.; Walker, W.A. Necrotizing Enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hull, M.A.; Fisher, J.G.; Gutierrez, I.M.; Jones, B.A.; Kang, K.H.; Kenny, M.; Zurakowski, D.; Modi, B.P.; Horbar, J.D.; Jaksic, T. Mortality and Management of Surgical Necrotizing Enterocolitis in Very Low Birth Weight Neonates: A Prospective Cohort Study. J. Am. Coll. Surg. 2013, 218, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.C.; Golden, J.M.; Ford, H.R. Pathogenesis of neonatal necrotizing enterocolitis. Pediatr. Surg. Int. 2015, 31, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Cortez, J.; Makker, K.; Kraemer, D.F.; Neu, J.; Sharma, R.; Hudak, M.L. Maternal milk feedings reduce sepsis, necrotizing enterocolitis and improve outcomes of premature infants. J. Perinatol. 2017, 38, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.L.; Kim, J.H. Human milk and necrotizing enterocolitis. Semin. Pediatr. Surg. 2018, 27, 34–38. [Google Scholar] [CrossRef]
- Uruakpa, F.; Ismond, M.; Akobundu, E. Colostrum and its benefits: A review. Nutr. Res. 2002, 22, 755–767. [Google Scholar] [CrossRef]
- Wada, Y.; Lönnerdal, B. Bioactive peptides derived from human milk proteins—Mechanisms of action. J. Nutr. Biochem. 2013, 25, 503–514. [Google Scholar] [CrossRef]
- Liang, N.; Beverly, R.L.; Scottoline, B.P.; Dallas, D.C. Peptides Derived from in Vitro and in Vivo Digestion of Human Milk Are Immunomodulatory in THP-1 Human Macrophages. J. Nutr. 2021, 152, 331–342. [Google Scholar] [CrossRef]
- Claud, E.C.; Savidge, T.; Walker, W.A. Modulation of Human Intestinal Epithelial Cell IL-8 Secretion by Human Milk Factors. Pediatr. Res. 2003, 53, 419–425. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Liu, S.; Kling, D.; Leone, S.; Lawlor, N.; Huang, Y.; Feinberg, S.B.; Hill, D.; Newburg, D.S. The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2014, 65, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Holscher, H.; Bode, L.; Tappenden, K. Human Milk Oligosaccharides Influence Intestinal Epithelial Cell Maturation in Vitro. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Patel, A.; Meier, P.P.; Fantuzzi, G. Digested Early Preterm Human Milk Suppresses Tumor Necrosis Factor–induced Inflammation and Cytotoxicity in Intestinal Epithelial Cells. J. Pediatr. Gastroenterol. Nutr. 2018, 66, e153–e157. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Liepke, C.; Adermann, K.; Raida, M.; Mägert, H.-J.; Forssmann, W.-G.; Zucht, H.-D. Human milk provides peptides highly stimulating the growth of bifidobacteria. JBIC J. Biol. Inorg. Chem. 2002, 269, 712–718. [Google Scholar] [CrossRef]
- Mohanty, D.; Mohapatra, S.; Misra, S.; Sahu, P. Milk derived bioactive peptides and their impact on human health—A review. Saudi J. Biol. Sci. 2015, 23, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef]
- Reyes-Díaz, A.; González-Córdova, A.F.; Hernández-Mendoza, A.; Reyes-Díaz, R.; Vallejo-Cordoba, B. Immunomodulation by hydrolysates and peptides derived from milk proteins. Int. J. Dairy Technol. 2017, 71, 1–9. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Purup, S.; Larsen, L.B. Effect of Casein Hydrolysates on Intestinal Cell Migration and Their Peptide Profiles by LC-ESI/MS/MS. Foods 2019, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Meisel, H. Biochemical properties of bioactive peptides derived from milk proteins: Potential nutraceuticals for food and pharmaceutical applications. Livest. Prod. Sci. 1997, 50, 125–138. [Google Scholar] [CrossRef]
- Hodzic, Z.; Bolock, A.M.; Good, M. The Role of Mucosal Immunity in the Pathogenesis of Necrotizing Enterocolitis. Front. Pediatr. 2017, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, A.L.W.; Juul-Madsen, H.R.; Stagsted, J. Colostrum and bioactive, colostral peptides differentially modulate the innate immune response of intestinal epithelial cells. J. Pept. Sci. 2009, 16, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Beverly, R.L.; Huston, R.K.; Markell, A.M.; McCulley, E.A.; Martin, R.L.; Dallas, D.C. Differences in human milk peptide release along the gastrointestinal tract between preterm and term infants. Clin. Nutr. 2020, 40, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Leone, S.; Newburg, D.S. Human colostrum oligosaccharides modulate major immunologic pathways of immature human intestine. Mucosal Immunol. 2014, 7, 1326–1339. [Google Scholar] [CrossRef]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.D.; Beverly, R.L.; Dallas, D.C. Milk Proteins Are Predigested Within the Human Mammary Gland. J. Mammary Gland Biol. Neoplasia 2017, 22, 251–261. [Google Scholar] [CrossRef]
- Lönnerdal, B. Bioactive Proteins in Human Milk: Mechanisms of Action. J. Pediatr. 2010, 156, S26–S30. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Underwood, M.A.; Beverly, R.L.; Nielsen, S.D.; Dallas, D.C. Comparison of Human Milk Immunoglobulin Survival during Gastric Digestion between Preterm and Term Infants. Nutrients 2018, 10, 631. [Google Scholar] [CrossRef] [Green Version]
- Demers-Mathieu, V.; Qu, Y.; Underwood, M.A.; Borghese, R.; Dallas, D.C. Premature Infants have Lower Gastric Digestion Capacity for Human Milk Proteins than Term Infants. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 816–821. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Le-Doare, K.M. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Zhou, Q.; Giuffrida, F.; Munblit, D.; Verhasselt, V.; Thakkar, S.K. Nutritional and Non-nutritional Composition of Human Milk Is Modulated by Maternal, Infant, and Methodological Factors. Front. Nutr. 2020, 7, 576133. [Google Scholar] [CrossRef] [PubMed]
- Munblit, D.; Treneva, M.; Peroni, D.G.; Colicino, S.; Chow, L.; Dissanayeke, S.; Abrol, P.; Sheth, S.; Pampura, A.; Boner, A.L.; et al. Colostrum and Mature Human Milk of Women from London, Moscow, and Verona: Determinants of Immune Composition. Nutrients 2016, 8, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villamil, E.; Rodríguez-Camejo, C.; Puyol, A.; Fazio, L.; Colistro, V.; Hernández, A. Immune profiling of breast milk from mothers with treated celiac disease. Pediatr. Res. 2020, 89, 488–495. [Google Scholar] [CrossRef]
- Lucas, A.; Cole, T. Breast milk and neonatal necrotising enterocolitis. Lancet 1990, 336, 1519–1523. [Google Scholar] [CrossRef]
- Penn, A.H.; Altshuler, A.E.; Small, J.W.; Taylor, S.F.; Dobkins, K.R.; Schmid-Schönbein, G.W. Effect of Digestion and Storage of Human Milk on Free Fatty Acid Concentration and Cytotoxicity. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Clevers, H. Growing Self-Organizing Mini-Guts from a Single Intestinal Stem Cell: Mechanism and Applications. Science 2013, 340, 1190–1194. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, Y.; Chen, Y. Digested Human Colostrum Reduces Interleukin-8 Production in Induced Human Intestinal Epithelial Cells. Nutrients 2022, 14, 2787. https://doi.org/10.3390/nu14142787
Lyu Y, Chen Y. Digested Human Colostrum Reduces Interleukin-8 Production in Induced Human Intestinal Epithelial Cells. Nutrients. 2022; 14(14):2787. https://doi.org/10.3390/nu14142787
Chicago/Turabian StyleLyu, Yang, and Yimin Chen. 2022. "Digested Human Colostrum Reduces Interleukin-8 Production in Induced Human Intestinal Epithelial Cells" Nutrients 14, no. 14: 2787. https://doi.org/10.3390/nu14142787





