GOS Ameliorates Nonalcoholic Fatty Liver Disease Induced by High Fat and High Sugar Diet through Lipid Metabolism and Intestinal Microbes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.1.1. Animals
2.1.2. Establishment and Treatment of the NAFLD Mouse Model
2.1.3. Biochemical Analysis
2.1.4. Glucose Tolerance Test
2.1.5. Histopathological Examination of Liver
2.1.6. Liver Metabolomics
2.1.7. Fecal Flora 16S rRNA Sequencing
2.1.8. Analysis of Short-Chain Fatty Acids (SCFAs) in Feces
2.1.9. Fluorescence Quantitative PCR
2.2. Cell Experiment
2.2.1. Model of Hepatocyte Steatosis
2.2.2. Cell Oil Red O Staining
2.2.3. Media and Biochemical Analysis of Hepatocytes
2.2.4. Fluorescence Quantitative PCR
2.3. Statistics and Analysis
3. Results
3.1. The Effect of GOS on NAFLD Mice
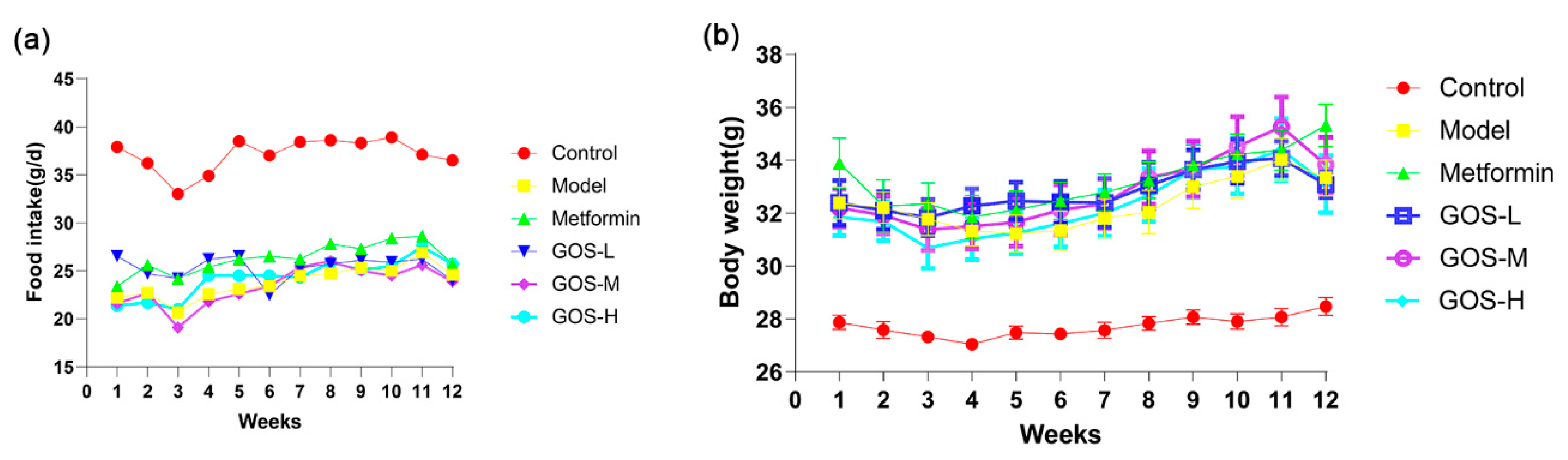
3.1.1. The Effect of GOS on the Body Weight and Food Intake of Mice
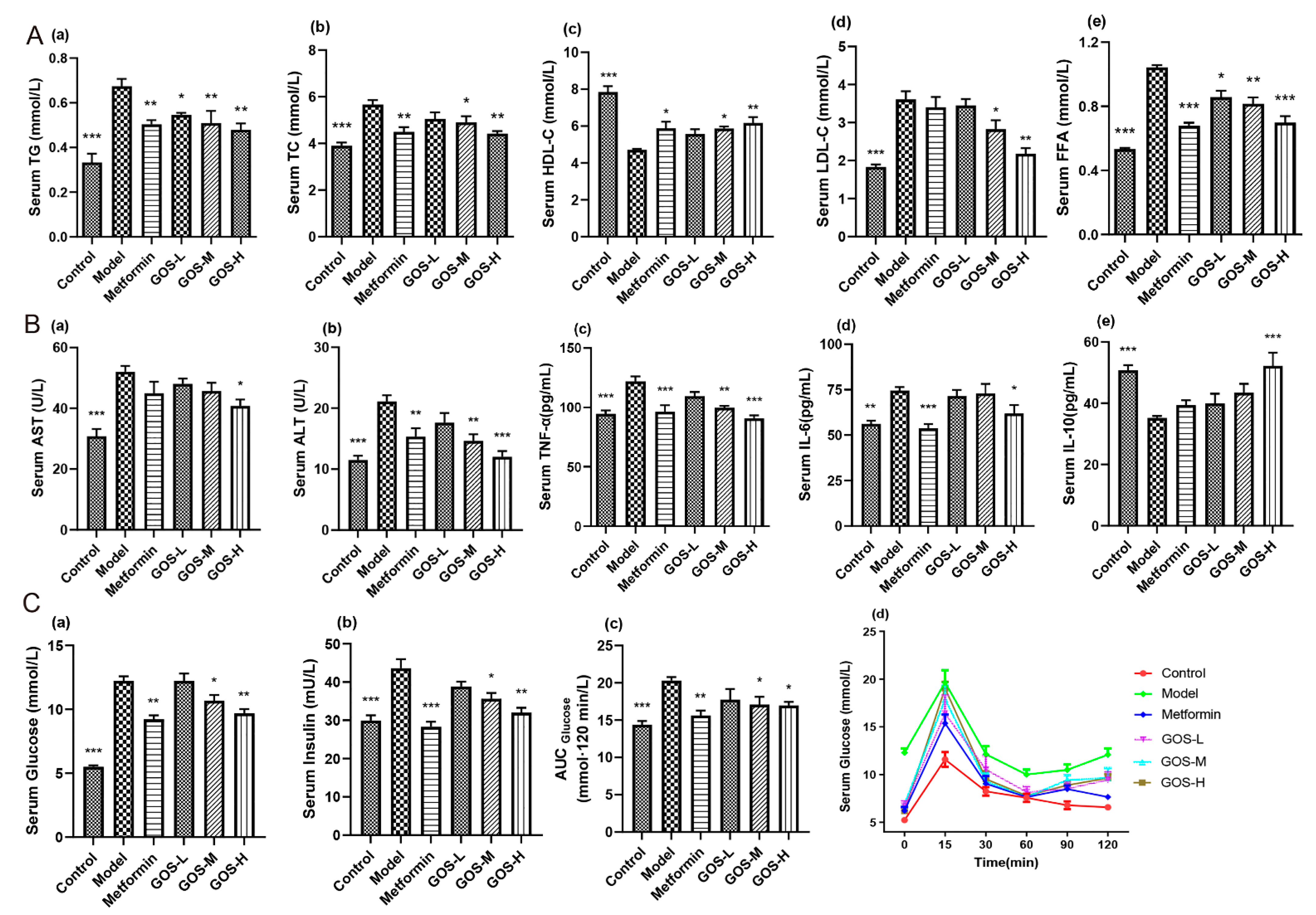
3.1.2. The Effect of GOS on Mouse Serum
3.1.3. The Effect of GOS on the Mouse Liver
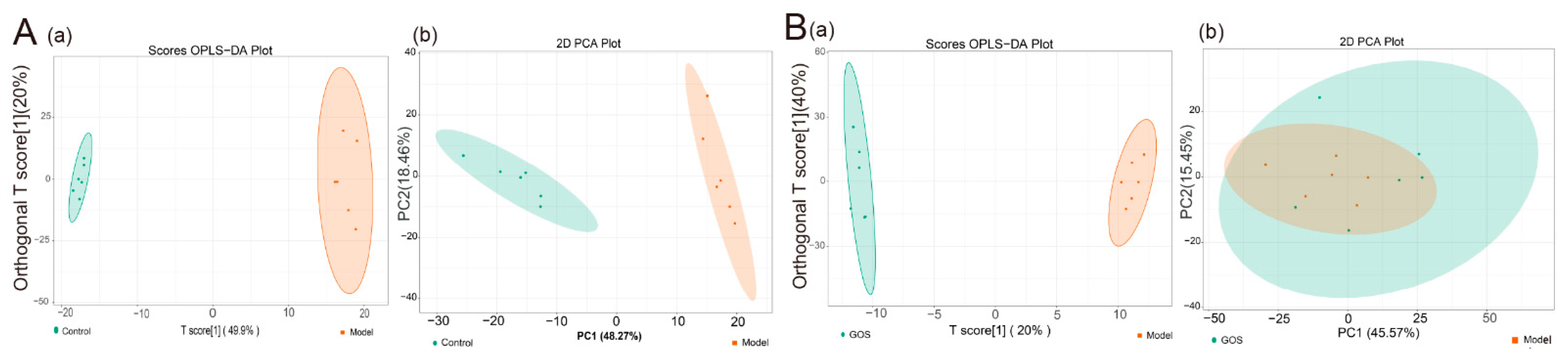
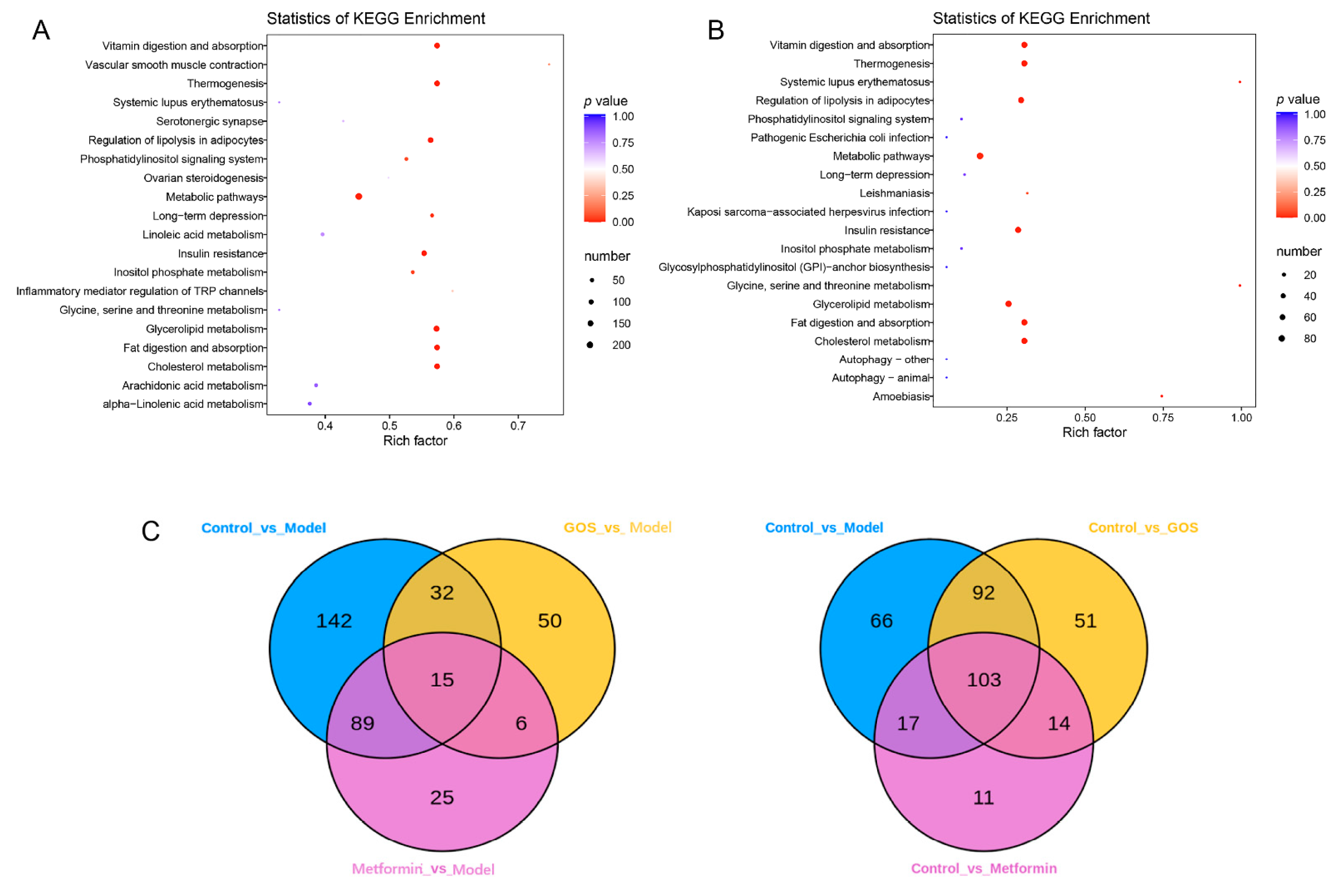
3.1.4. Mouse Liver Lipidomics
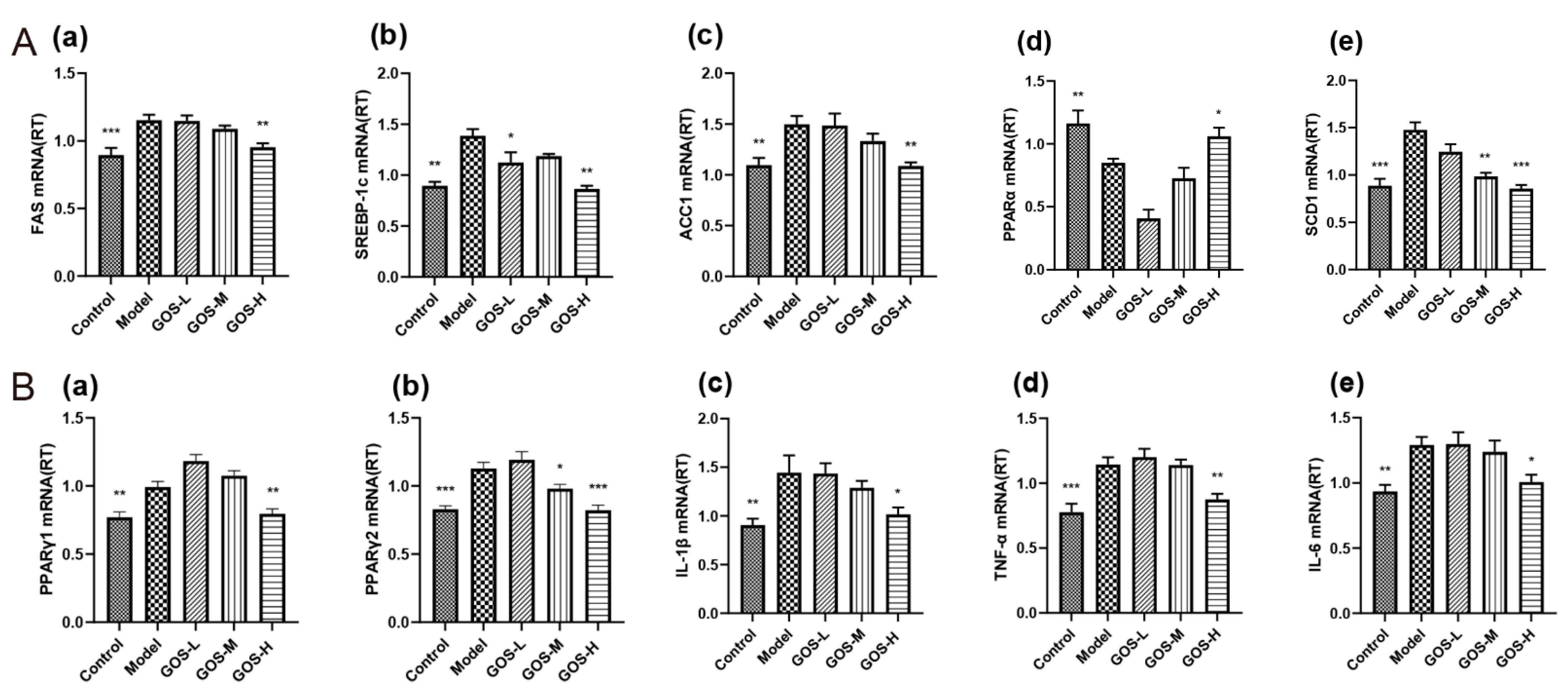
3.1.5. The Effect of GOS on the mRNA Expression Levels in Mouse Liver
3.1.6. The Effect of GOS on the mRNA Expression Levels in Mouse Liver
16S rRNA Sequencing Data Processing and Analysis
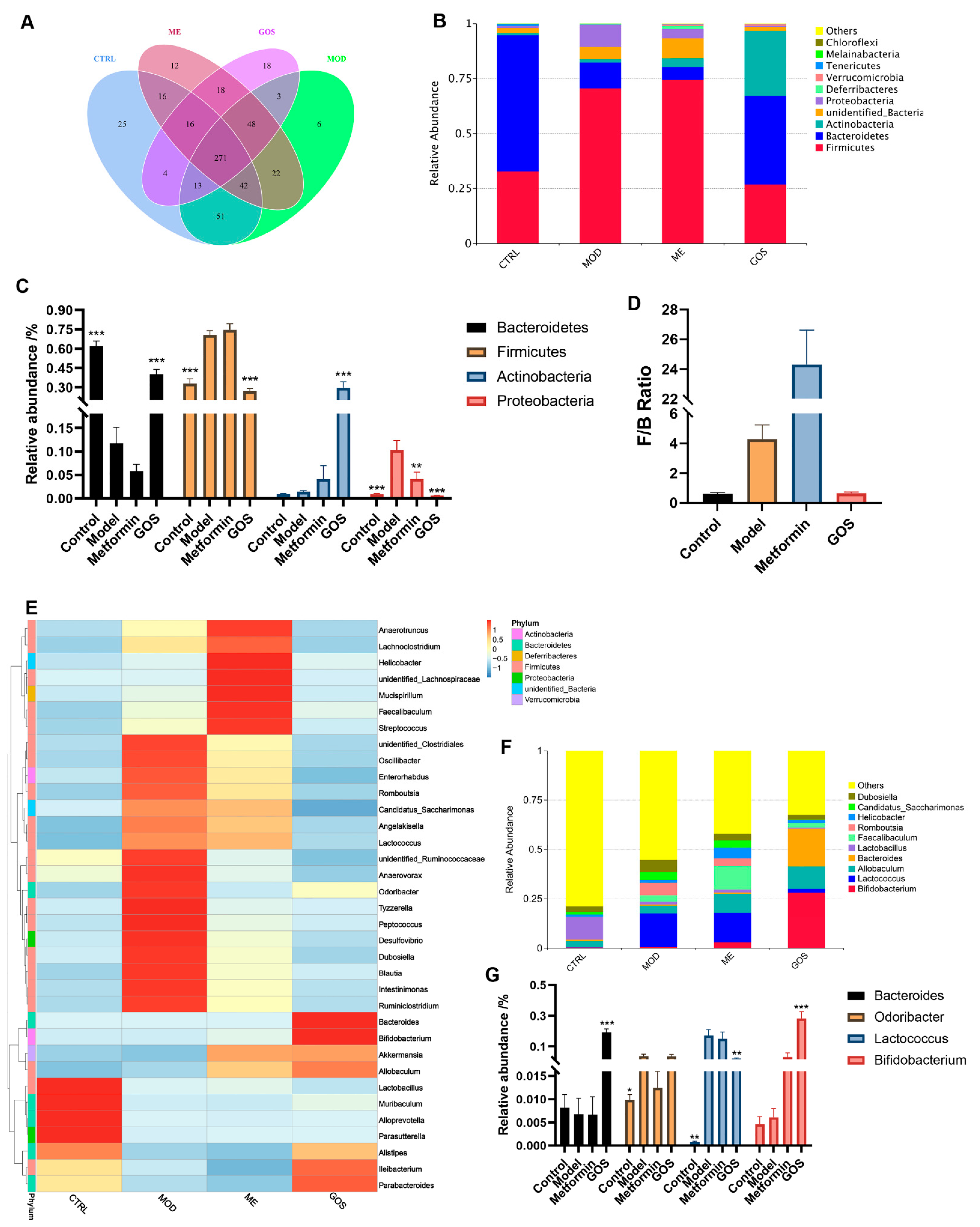
Analysis of GOS on the Relative Abundance of Intestinal Microbial Species in NAFLD Mice
Effect of GOS on the Diversity of Intestinal Microbial Communities in NAFLD Mice
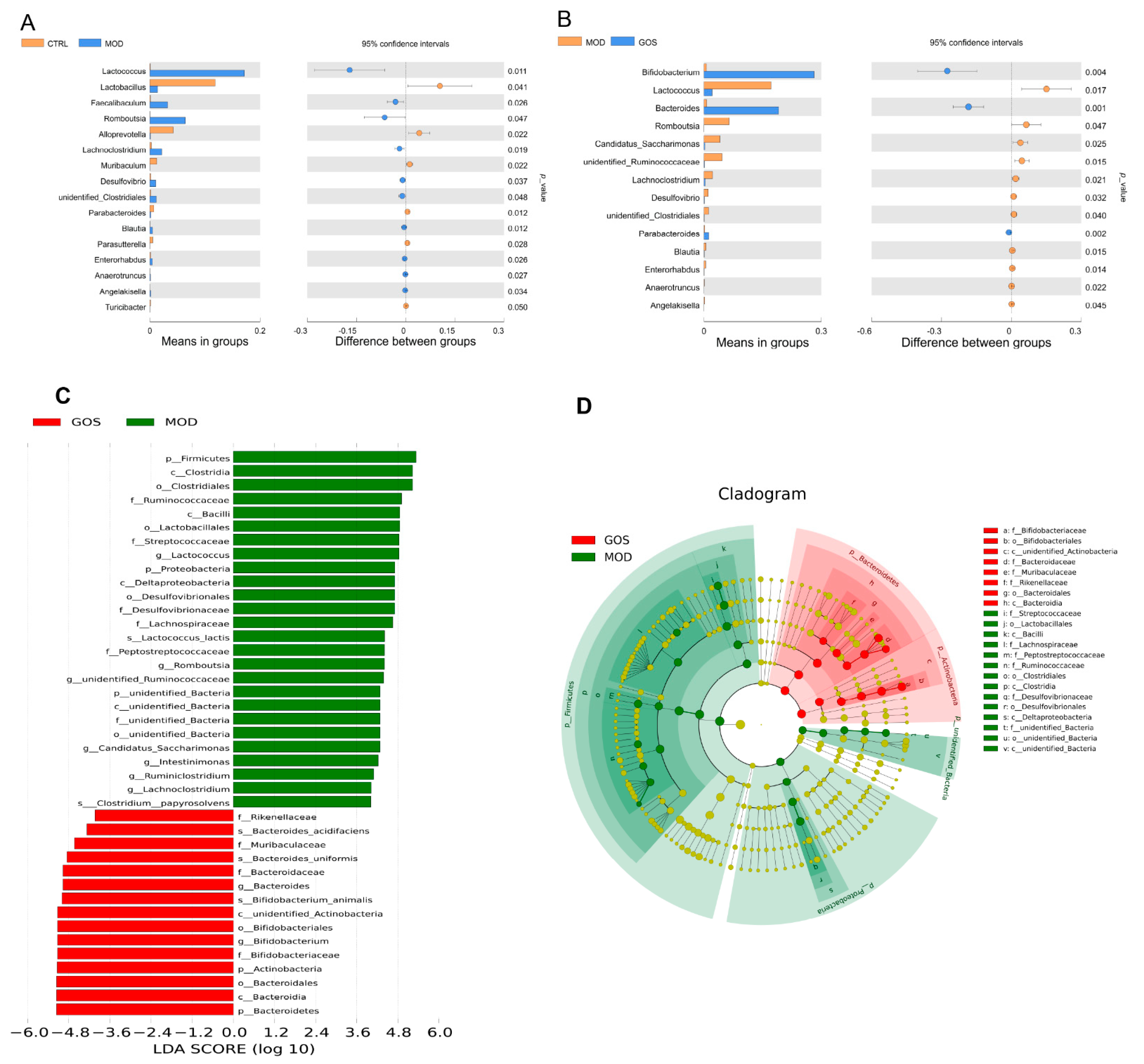
Analysis of the Effect of GOS on Different Species of Intestinal Microbial Colonies in NAFLD Mice
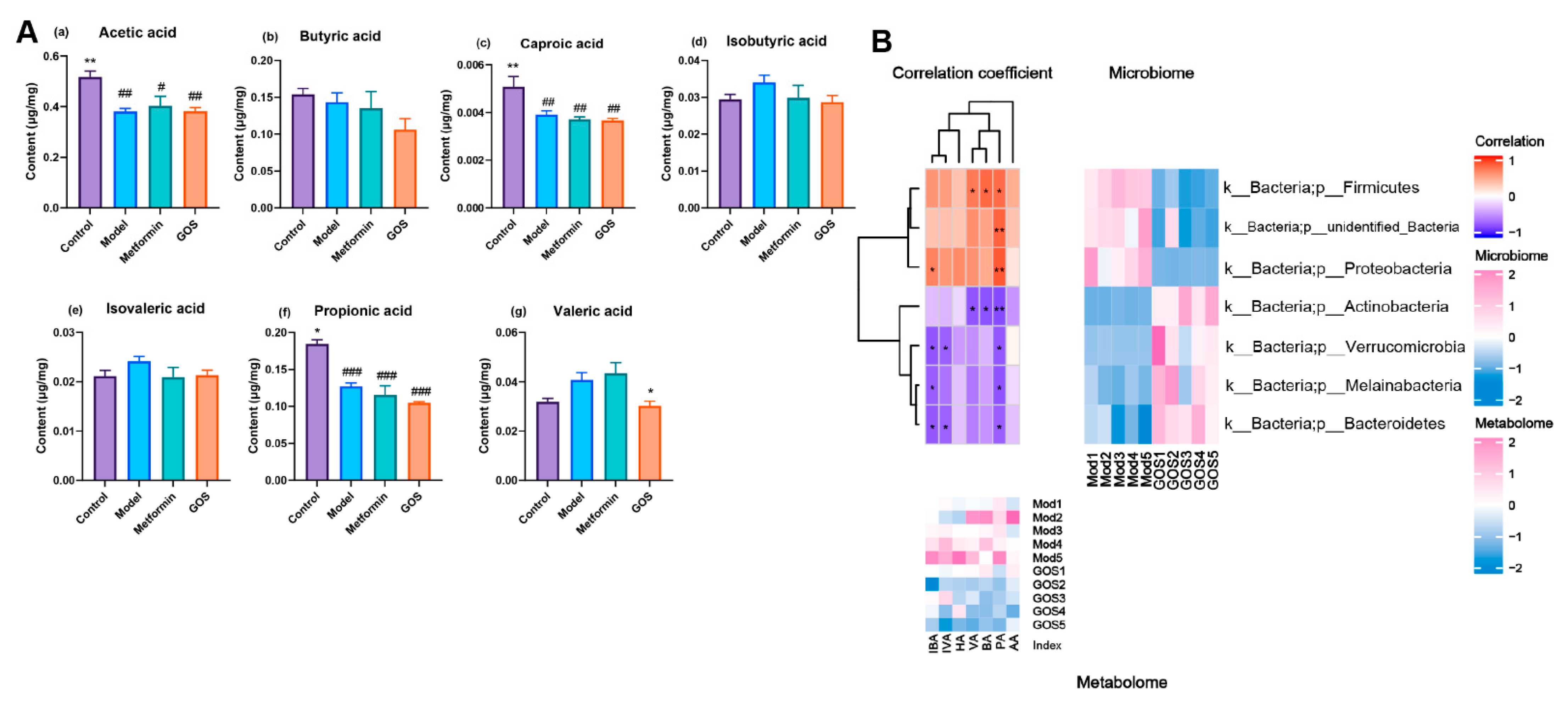
The Effect of GOS on Short-Chain Fatty Acids in the Cecal Contents of NAFLD Mice
Correlation between Intestinal Flora and Metabolites
3.2. The Effect of GOS on Fatty Degeneration of HepG2 Cells
3.2.1. GOS Reduces FFA-Induced HepG2 Hepatocyte Damage
3.2.2. GOS Reduces FFA-Induced Degeneration of HepG2 Hepatocytes
3.2.3. The Effect of GOS on the Lipid Metabolism Indexes of HepG2 Cells
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byrne, C.D.; Targher, G. NAFLD as a driver of chronic kidney disease. J. Hepatol. 2020, 72, 785–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratakis, N.; Golden-Mason, L.; Margetaki, K.; Zhao, Y.; Valvi, D.; Garcia, E.; Maitre, L.; Andrusaityte, S.; Basagana, X.; Borràs, E.; et al. In Utero Exposure to Mercury Is Associated with Increased Susceptibility to Liver Injury and Inflammation in Childhood. Hepatology 2021, 74, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, F.; Wang, W.; Zhang, X.-J.; Ji, Y.-X.; Zhang, P.; She, Z.-G.; Zhu, L.; Cai, J.; Li, H. Epidemiological Features of NAFLD from 1999 to 2018 in China. Hepatology 2020, 71, 1851–1864. [Google Scholar] [CrossRef]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, V.W.-S.; Wong, G.L.-H.; Chan, R.S.-M.; Shu, S.S.-T.; Cheung, B.H.-K.; Li, L.S.; Chim, A.M.-L.; Chan, C.K.-M.; Leung, J.K.-Y.; Chu, W.C.-W.; et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 1349–1356. [Google Scholar] [CrossRef]
- Guarino, M.P.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef] [Green Version]
- Vulevic, J.; Rastall, R.A.; Gibson, G.R. Developing a quantitative approach for determining the in vitro prebiotic potential of dietary oligosaccharides. FEMS Microbiol. Lett. 2004, 236, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; do Rosario, V.A.; Mocellin, M.C.; Kuntz, M.G.F.; Trindade, E.B.S.M. Effects of inulin-type fructans, galacto-oligosaccharides and related synbiotics on inflammatory markers in adult patients with overweight or obesity: A systematic review. Clin. Nutr. 2017, 36, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Morel, F.B.; Dai, Q.; Ni, J.; Thomas, D.; Parnet, P.; Fança-Berthon, P. α-Galacto-oligosaccharides Dose-Dependently Reduce Appetite and Decrease Inflammation in Overweight Adults. J. Nutr. 2015, 145, 2052–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of the extension of use of galacto-oligosaccharides as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06844. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Hu, Z.; Cui, A.; Liu, Z.; Ma, F.; Xue, Y.; Liu, Y.; Zhang, F.; Zhao, Z.; Yu, Y.; et al. Post-translational regulation of lipogenesis via AMPK-dependent phosphorylation of insulin-induced gene. Nat. Commun. 2019, 10, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Wang, L.; Yang, D.; Li, L.; Togo, J.; Wu, Y.; Liu, Q.; Li, B.; Li, M.; Wang, G.; et al. Dietary Fat, but Not Protein or Carbohydrate, Regulates Energy Intake and Causes Adiposity in Mice. Cell Metab. 2018, 28, 415–431.e414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexopoulos, A.-S.; Crowley, M.J.; Wang, Y.; Moylan, C.A.; Guy, C.D.; Henao, R.; Piercy, D.L.; Seymour, K.A.; Sudan, R.; Portenier, D.D.; et al. Glycemic Control Predicts Severity of Hepatocyte Ballooning and Hepatic Fibrosis in Nonalcoholic Fatty Liver Disease. Hepatology 2021, 74, 1220–1233. [Google Scholar] [CrossRef]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Knights, A.J.; Wu, J.; Tseng, Y.-H. The Heating Microenvironment: Intercellular Cross Talk Within Thermogenic Adipose Tissue. Diabetes 2020, 69, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Carr, R.M. A new perspective on NAFLD: Focusing on lipid droplets. J. Hepatol. 2022, 76, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Heine, M.; Fischer, A.W.; Schlein, C.; Jung, C.; Straub, L.G.; Gottschling, K.; Mangels, N.; Yuan, Y.; Nilsson, S.K.; Liebscher, G.; et al. Lipolysis Triggers a Systemic Insulin Response Essential for Efficient Energy Replenishment of Activated Brown Adipose Tissue in Mice. Cell Metab. 2018, 28, 644–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rom, O.; Liu, Y.; Liu, Z.; Zhao, Y.; Wu, J.; Ghrayeb, A.; Villacorta, L.; Fan, Y.; Chang, L.; Wang, L.; et al. Glycine-based treatment ameliorates NAFLD by modulating fatty acid oxidation, glutathione synthesis, and the gut microbiome. Sci. Transl. Med. 2020, 12, eaaz2841. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.-H.; Ou, X.; Ouyang, X.-P.; Tang, C.-K. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Prog. Lipid Res. 2021, 83, 101109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Coker, O.O.; Chu, E.S.H.; Fu, K.; Lau, H.C.H.; Wang, Y.-X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761. [Google Scholar] [CrossRef] [PubMed]
- Noth, R.; Lange-Grumfeld, J.; Stüber, E.; Kruse, M.-L.; Ellrichmann, M.; Häsler, R.; Hampe, J.; Bewig, B.; Rosenstiel, P.; Schreiber, S.; et al. Increased intestinal permeability and tight junction disruption by altered expression and localization of occludin in a murine graft versus host disease model. BMC Gastroenterol. 2011, 11, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.Y.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.S.; Lim, H.S.; Kim, M.S.; et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zocco, M.A.; Ainora, M.E.; Gasbarrini, G.; Gasbarrini, A. Bacteroides thetaiotaomicron in the gut: Molecular aspects of their interaction. Dig. Liver Dis. 2007, 39, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.L.; Yang, D.H.; Zhang, Y.T.; Zhang, Y.; Wang, Z.Q.; Wang, Y.S.; Cai, S.Q.; Liu, S.L. Cloning, expression, and characterization of the β-glucosidase hydrolyzing secoisolariciresinol diglucoside to secoisolariciresinol from Bacteroides uniformis ZL1. Appl. Microbiol. Biotechnol. 2014, 98, 2519–2531. [Google Scholar] [CrossRef]
- Yao, G.; Cao, C.; Zhang, M.; Kwok, L.-Y.; Zhang, H.; Zhang, W. Lactobacillus casei Zhang exerts probiotic effects to antibiotic-treated rats. Comput. Struct. Biotechnol. J. 2021, 19, 5888–5897. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D.; Vialettes, B.; Raoult, D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. 2012, 36, 817–825. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-S.; Kim, T.-Y.; Kim, Y.; Lee, S.-H.; Kim, S.; Kang, S.W.; Yang, J.-Y.; Baek, I.-J.; Sung, Y.H.; Park, Y.-Y.; et al. Microbiota-Derived Lactate Accelerates Intestinal Stem-Cell-Mediated Epithelial Development. Cell Host Microbe 2018, 24, 833–846.e836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yan, J.; Zhang, P.; Zhou, X. Berberine Maintains Gut Microbiota Homeostasis and Ameliorates Liver Inflammation in Experimental Non-alcoholic Fatty Liver Disease. Chin. J. Gastroenterol. 2018, 23, 209–215. [Google Scholar]
- Zhang-Sun, W.; Augusto, L.A.; Zhao, L.; Caroff, M. Desulfovibrio desulfuricans isolates from the gut of a single individual: Structural and biological lipid A characterization. FEBS Lett. 2015, 589, 165–171. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Liping, Z.; Feng, Z.; Xiaoying, D.; Guojun, W.; Yan, Y.L.; Xuejiao, W.; Huaqing, F.; Xinhe, X.; Chunhua, L.; Jilin, M.J.S. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Kuang, Z.; Yu, X.; Ruhn, K.A.; Kubo, M.; Hooper, L.V. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science 2017, 357, 912–916. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; He, G.; Peng, Y.; Zhong, W.; Wang, Y.; Zhang, B. Sodium butyrate alleviates adipocyte inflammation by inhibiting NLRP3 pathway. Sci. Rep. 2015, 5, 12676. [Google Scholar] [CrossRef] [Green Version]
- Pearson, J.R.; Gill, C.I.R.; Rowland, I.R. Diet, fecal water, and colon cancer—Development of a biomarker. Nutr. Rev. 2009, 67, 509–526. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Khoruts, A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J. Hepatol. 2020, 72, 1003–1027. [Google Scholar] [CrossRef] [Green Version]
- Quigley, E.M.M.; Stanton, C.; Murphy, E.F. The gut microbiota and the liver. Pathophysiological and clinical implications. J. Hepatol. 2013, 58, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.-C.; Beilin, L.J.; Ayonrinde, O.; Mori, T.A.; Olynyk, J.K.; Burrows, S.; Hands, B.; Adams, L.A. Importance of cardiometabolic risk factors in the association between nonalcoholic fatty liver disease and arterial stiffness in adolescents. Hepatology 2013, 58, 1306–1314. [Google Scholar] [CrossRef] [Green Version]
- Labayen, I.; Margareto, J.; Maldonado-Martin, S.; Gorostegi, I.; Illera, M.; Medrano, M.; Barrenechea, L.; Larrarte, E. Independent and combined influence of the FTO rs9939609 and MC4Rrs17782313 polymorphisms on hypocaloric diet induced changes in body mass and composition and energy metabolism in non-morbid obese premenopausal women. Nutr. Hosp. 2015, 31, 2025–2032. [Google Scholar] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, S.; Chen, J.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. GOS Ameliorates Nonalcoholic Fatty Liver Disease Induced by High Fat and High Sugar Diet through Lipid Metabolism and Intestinal Microbes. Nutrients 2022, 14, 2749. https://doi.org/10.3390/nu14132749
Qiu S, Chen J, Bai Y, He J, Cao H, Che Q, Guo J, Su Z. GOS Ameliorates Nonalcoholic Fatty Liver Disease Induced by High Fat and High Sugar Diet through Lipid Metabolism and Intestinal Microbes. Nutrients. 2022; 14(13):2749. https://doi.org/10.3390/nu14132749
Chicago/Turabian StyleQiu, Shuting, Jiajia Chen, Yan Bai, Jincan He, Hua Cao, Qishi Che, Jiao Guo, and Zhengquan Su. 2022. "GOS Ameliorates Nonalcoholic Fatty Liver Disease Induced by High Fat and High Sugar Diet through Lipid Metabolism and Intestinal Microbes" Nutrients 14, no. 13: 2749. https://doi.org/10.3390/nu14132749
APA StyleQiu, S., Chen, J., Bai, Y., He, J., Cao, H., Che, Q., Guo, J., & Su, Z. (2022). GOS Ameliorates Nonalcoholic Fatty Liver Disease Induced by High Fat and High Sugar Diet through Lipid Metabolism and Intestinal Microbes. Nutrients, 14(13), 2749. https://doi.org/10.3390/nu14132749





