Nutrition and Immunity in Perinatal Hypoxic-Ischemic Injury
Abstract
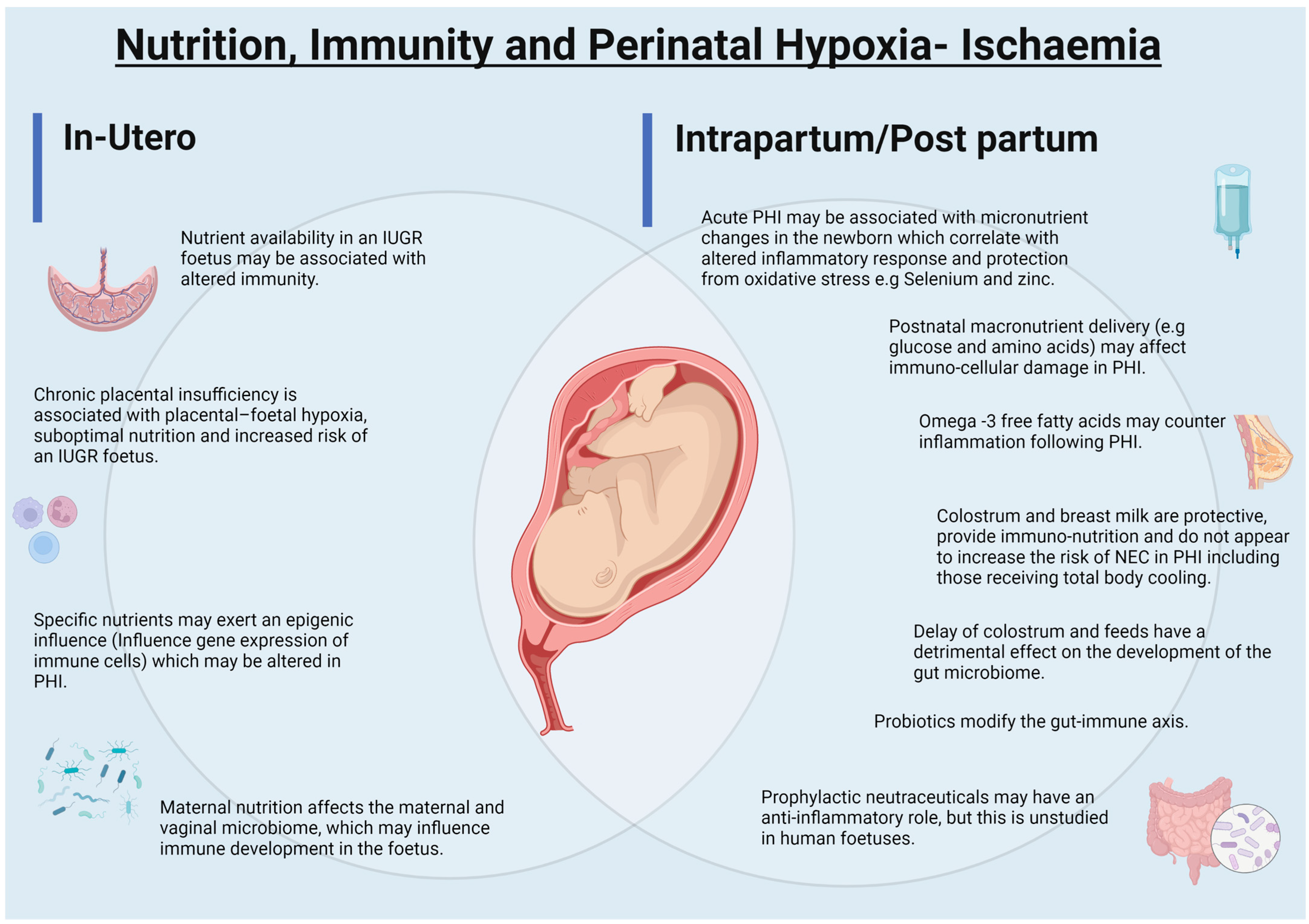
1. Introduction
2. Evolution of Immune Function in the Foetus and Perinatal Period
3. Nutrition–Immunity Interdependence
3.1. Immunometabolism
3.2. Nutritional Immunology: Macro and Micronutrients and Immune Function
3.3. The Nutrition–Microbiome–Immune Axis in the Foetus and Baby
4. Immunological Changes in Perinatal Hypoxic Ischaemic Injury
4.1. Intrauterine Growth Restriction, Chronic Placental Insufficiency and Development of Immune Functions
4.2. Intrauterine Growth Restriction, Chronic Placental Insufficiency, Nutrition and Nutritional-Epigenetics
4.3. Intrauterine Growth Restriction and Autophagy
5. Acute Hypoxia-Ischaemia, Immunity, Nutrients and Trace Elements
5.1. Glucose
5.2. Amino Acids and Fatty Acids
5.3. Micronutrients and Trace Elements
6. Early Enteral Nutritional Support and Immunity in Perinatal Hypoxia-Ischaemia
6.1. Breast Milk and Colostrum
6.2. Prebiotics and Probiotics
6.3. Neuroprotection
6.4. Prophylactic Nutraceuticals
6.5. Placental Nutrient Sensing Maternal—Foetal Resource Allocation
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novak, C.M.; Ozen, M.; Burd, I. Perinatal Brain Injury. Clin. Perinatol. 2018, 45, 357–375. [Google Scholar] [CrossRef]
- Kollmann, T.R.; Kampmann, B.; Mazmanian, S.K.; Marchant, A.; Levy, O. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity 2017, 46, 350–363. [Google Scholar] [CrossRef]
- Zhang, X.; Zhivaki, D.; Lo-Man, R. Unique Aspects of the Perinatal Immune System. Nat. Rev. Immunol. 2017, 17, 495–507. [Google Scholar] [CrossRef]
- Rizzuto, G.; Erlebacher, A. Trophoblast Antigens, Fetal Blood Cell Antigens, and the Paradox of Fetomaternal Tolerance. J. Exp. Med. 2022, 219, e20211515. [Google Scholar] [CrossRef]
- Petroff, M.G. Review: Fetal Antigens—Identity, Origins, and Influences on the Maternal Immune System. Placenta 2011, 32, S176–S181. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Khodadadi, H.; Malik, A.; Davidson, B.; da Silva Lopes Salles, É.; Bhatia, J.; Hale, V.L.; Baban, B. Innate Immunity of Neonates and Infants. Front. Immunol. 2018, 9, 1759. [Google Scholar] [CrossRef] [PubMed]
- Leeansyah, E.; Loh, L.; Nixon, D.F.; Sandberg, J.K. Acquisition of Innate-like Microbial Reactivity in Mucosal Tissues during Human Fetal MAIT-Cell Development. Nat. Commun. 2014, 5, 3143. [Google Scholar] [CrossRef] [PubMed]
- Viemann, D. S100-Alarmins Are Essential Pilots of Postnatal Innate Immune Adaptation. Front. Immunol. 2020, 11, 688. [Google Scholar] [CrossRef]
- Gervassi, A.; Lejarcegui, N.; Dross, S.; Jacobson, A.; Itaya, G.; Kidzeru, E.; Gantt, S.; Jaspan, H.; Horton, H. Myeloid Derived Suppressor Cells Are Present at High Frequency in Neonates and Suppress in Vitro T Cell Responses. PLoS ONE 2014, 9, e107816. [Google Scholar] [CrossRef]
- Dowling, D.J.; Levy, O. Ontogeny of Early Life Immunity. Trends Immunol. 2014, 35, 299–310. [Google Scholar] [CrossRef]
- Miller, D.; Motomura, K.; Garcia-Flores, V.; Romero, R.; Gomez-Lopez, N. Innate Lymphoid Cells in the Maternal and Fetal Compartments. Front. Immunol. 2018, 9, 2396. [Google Scholar] [CrossRef]
- Mendoza, G.R.; Minagawa, K.; Orner, F.B.; Stiehm, E.R. Basophil Releasability in the Newborn: Factors Limiting Immunoglobulin E-Mediated Histamine Release. Pediatrics 1982, 69, 188–192. [Google Scholar] [CrossRef]
- de Kleer, I.; Willems, F.; Lambrecht, B.; Goriely, S. Ontogeny of Myeloid Cells. Front. Immunol. 2014, 5, 423. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the Immune System in Humans from Infancy to Old Age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Sherrid, A.M.; Kollmann, T.R. Age-Dependent Differences in Systemic and Cell-Autonomous Immunity to L. Monocytogenes. Clin. Dev. Immunol. 2013, 2013, 917198. [Google Scholar] [CrossRef]
- Torow, N.; Marsland, B.J.; Hornef, M.W.; Gollwitzer, E.S. Neonatal Mucosal Immunology. Mucosal Immunol. 2017, 10, 5–17. [Google Scholar] [CrossRef]
- Marchini, G.; Lindow, S.; Brismar, H.; Stabi, B.; Berggren, V.; Ulfgren, A.-K.; Lonne-Rahm, S.; Agerberth, B.; Gudmundsson, G.H. The Newborn Infant Is Protected by an Innate Antimicrobial Barrier: Peptide Antibiotics Are Present in the Skin and Vernix Caseosa. Br. J. Dermatol. 2002, 147, 1127–1134. [Google Scholar] [CrossRef]
- Nyangahu, D.D.; Jaspan, H.B. Influence of Maternal Microbiota during Pregnancy on Infant Immunity. Clin. Exp. Immunol. 2019, 198, 47–56. [Google Scholar] [CrossRef]
- Pillay, T.; Zhang, H.-T.; Drijfhout, J.W.; Robinson, N.; Brown, H.; Khan, M.; Moodley, J.; Adhikari, M.; Pfafferott, K.; Feeney, M.E.; et al. Unique Acquisition of Cytotoxic T-Lymphocyte Escape Mutants in Infant Human Immunodeficiency Virus Type 1 Infection. J. Virol. 2005, 79, 12100–12105. [Google Scholar] [CrossRef]
- Mishra, A.; Lai, G.C.; Yao, L.J.; Aung, T.T.; Shental, N.; Rotter-Maskowitz, A.; Shepherdson, E.; Singh, G.S.N.; Pai, R.; Shanti, A.; et al. Microbial Exposure during Early Human Development Primes Fetal Immune Cells. Cell 2021, 184, 3394–3409.e20. [Google Scholar] [CrossRef]
- Yeo, K.T.; Embury, P.; Anderson, T.; Mungai, P.; Malhotra, I.; King, C.; Kazura, J.; Dent, A. HIV, Cytomegalovirus, and Malaria Infections during Pregnancy Lead to Inflammation and Shifts in Memory B Cell Subsets in Kenyan Neonates. J. Immunol. 2019, 202, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T.; Colgan, S.P. Regulation of Immunity and Inflammation by Hypoxia in Immunological Niches. Nat. Rev. Immunol. 2017, 17, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Rundles, S.; Lin, H.; Ho-Lin, D.; Dnistrian, A.; Cassileth, B.R.; Perlman, J.M. Role of Nutrients in the Development of Neonatal Immune Response. Nutr. Rev. 2009, 67, S152–S163. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Gallagher, K.; Beck, C.; Kumar, R.; Gernand, A.D. Maternal-Fetal Inflammation in the Placenta and the Developmental Origins of Health and Disease. Front. Immunol. 2020, 11, 531543. [Google Scholar] [CrossRef]
- Williams, J.J.; Mayurasakorn, K.; Vannucci, S.J.; Mastropietro, C.; Bazan, N.G.; Ten, V.S.; Deckelbaum, R.J. N-3 Fatty Acid Rich Triglyceride Emulsions Are Neuroprotective after Cerebral Hypoxic-Ischemic Injury in Neonatal Mice. PLoS ONE 2013, 8, e56233. [Google Scholar] [CrossRef]
- Gale, C.; Longford, N.T.; Jeyakumaran, D.; Ougham, K.; Battersby, C.; Ojha, S.; Dorling, J. Feeding during Neonatal Therapeutic Hypothermia, Assessed Using Routinely Collected National Neonatal Research Database Data: A Retrospective, UK Population-Based Cohort Study. Lancet Child Adolesc. Health 2021, 5, 408–416. [Google Scholar] [CrossRef]
- Reyes-Corral, M.; Sola-Idígora, N.; de la Puerta, R.; Montaner, J.; Ybot-González, P. Nutraceuticals in the Prevention of Neonatal Hypoxia–Ischemia: A Comprehensive Review of Their Neuroprotective Properties, Mechanisms of Action and Future Directions. Int. J. Mol. Sci. 2021, 22, 2524. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Kishton, R.J.; Rathmell, J. A Guide to Immunometabolism for Immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Zhang, X.; Zink, F.; Hezel, F.; Vogt, J.; Wachter, U.; Wepler, M.; Loconte, M.; Kranz, C.; Hellmann, A.; Mizaikoff, B.; et al. Metabolic Substrate Utilization in Stress-Induced Immune Cells. Intensive Care Med. Exp. 2020, 8, 28. [Google Scholar] [CrossRef]
- Wood, T.R.; Stubbs, B.J.; Juul, S.E. Exogenous Ketone Bodies as Promising Neuroprotective Agents for Developmental Brain Injury. Dev. Neurosci. 2018, 40, 451–462. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, X.; Cheng, X.; Lin, X.; Zhao, T.; Wu, L.; Yu, X.; Wu, K.; Fan, M.; Zhu, L. Ketogenic Diet Improves the Spatial Memory Impairment Caused by Exposure to Hypobaric Hypoxia through Increased Acetylation of Histones in Rats. PLoS ONE 2017, 12, e0174477. [Google Scholar] [CrossRef]
- Pinchefsky, E.F.; Schneider, J.; Basu, S.; Tam, E.W.Y.; Gale, C. Nutrition and Management of Glycemia in Neonates with Neonatal Encephalopathy Treated with Hypothermia. Semin. Fetal Neonatal Med. 2021, 26, 101268. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef]
- Zhang, P.; Cui, T.-T.; Zhang, Z.-H.; Wang, Y.-Q. Low-Dose Vitamin A Therapy on T Lymphocyte Function in Neonatal Pneumonia. Eur. Rev. Med. Pharm. Sci. 2018, 22, 4371–4374. [Google Scholar] [CrossRef]
- Prentice, S. They Are What You Eat: Can Nutritional Factors during Gestation and Early Infancy Modulate the Neonatal Immune Response? Front. Immunol. 2017, 8, 1641. [Google Scholar] [CrossRef]
- Siddiqua, T.J.; Ahmad, S.M.; Ahsan, K.B.; Rashid, M.; Roy, A.; Rahman, S.M.; Shahab-Ferdows, S.; Hampel, D.; Ahmed, T.; Allen, L.H.; et al. Vitamin B12 Supplementation during Pregnancy and Postpartum Improves B12 Status of Both Mothers and Infants but Vaccine Response in Mothers Only: A Randomized Clinical Trial in Bangladesh. Eur. J. Nutr. 2016, 55, 281–293. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Li, Z.; Jian, L.; Gao, Y.; Qu, Y.; Liu, C.; Xu, C.; Li, Y.; Diao, Z.; et al. Maternal Folic Acid Supplementation Modulates the Growth Performance, Muscle Development and Immunity of Hu Sheep Offspring of Different Litter Size. J. Nutr. Biochem. 2019, 70, 194–201. [Google Scholar] [CrossRef]
- Zhao, X.; Pang, X.; Wang, F.; Cui, F.; Wang, L.; Zhang, W. Maternal Folic Acid Supplementation and Antibody Persistence 5 Years after Hepatitis B Vaccination among Infants. Hum. Vaccines Immunother. 2018, 14, 2478–2484. [Google Scholar] [CrossRef]
- Carr, A.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Wassall, H.; Devereux, G.; Seaton, A.; Barker, R. Complex Effects of Vitamin E and Vitamin C Supplementation on in Vitro Neonatal Mononuclear Cell Responses to Allergens. Nutrients 2013, 5, 3337–3351. [Google Scholar] [CrossRef]
- Hicks, S.D.; Confair, A.; Warren, K.; Chandran, D. Levels of Breast Milk MicroRNAs and Other Non-Coding RNAs Are Impacted by Milk Maturity and Maternal Diet. Front. Immunol. 2022, 12, 785217. [Google Scholar] [CrossRef]
- Hornsby, E.; Pfeffer, P.E.; Laranjo, N.; Cruikshank, W.; Tuzova, M.; Litonjua, A.A.; Weiss, S.T.; Carey, V.J.; O’Connor, G.; Hawrylowicz, C. Vitamin D Supplementation during Pregnancy: Effect on the Neonatal Immune System in a Randomized Controlled Trial. J. Allergy Clin. Immunol. 2018, 141, 269–278.e1. [Google Scholar] [CrossRef]
- Alhussien, M.N.; Tiwari, S.; Panda, B.S.K.; Pandey, Y.; Lathwal, S.S.; Dang, A.K. Supplementation of Antioxidant Micronutrients Reduces Stress and Improves Immune Function/Response in Periparturient Dairy Cows and Their Calves. J. Trace Elem. Med. Biol. 2021, 65, 126718. [Google Scholar] [CrossRef]
- Wieringa, F.T.; Dijkhuizen, M.A.; Muhilal; van der Meer, J.W.M. Maternal Micronutrient Supplementation with Zinc and β-Carotene Affects Morbidity and Immune Function of Infants during the First 6 Months of Life. Eur. J. Clin. Nutr. 2010, 64, 1072–1079. [Google Scholar] [CrossRef]
- Brabin, L.; Brabin, B.J.; Gies, S. Influence of Iron Status on Risk of Maternal or Neonatal Infection and on Neonatal Mortality with an Emphasis on Developing Countries. Nutr. Rev. 2013, 71, 528–540. [Google Scholar] [CrossRef]
- Rodríguez, A.M.; López Valiente, S.; Mattioli, G.; Maresca, S. Effects of Inorganic Copper Injection in Beef Cows at Late Gestation on Fetal and Postnatal Growth, Hematology and Immune Function of Their Progeny. Res. Vet. Sci. 2021, 139, 11–17. [Google Scholar] [CrossRef]
- Garg, B.D.; Bansal, A.; Kabra, N.S. Role of Selenium Supplementation in Prevention of Late Onset Sepsis among Very Low Birth Weight Neonates: A Systematic Review of Randomized Controlled Trials. J. Matern. Fetal Neonatal Med. 2019, 32, 4159–4165. [Google Scholar] [CrossRef]
- Holm, S.R.; Jenkins, B.J.; Cronin, J.G.; Jones, N.; Thornton, C.A. A Role for Metabolism in Determining Neonatal Immune Function. Pediatric Allergy Immunol. 2021, 32, 1616–1628. [Google Scholar] [CrossRef]
- Haase, B.; Faust, K.; Heidemann, M.; Scholz, T.; Demmert, M.; Tröger, B.; Herz, A.; Härtel, C. The Modulatory Effect of Lipids and Glucose on the Neonatal Immune Response Induced by Staphylococcus Epidermidis. Inflamm. Res. 2011, 60, 227–232. [Google Scholar] [CrossRef]
- van Sadelhoff, J.H.J.; Wiertsema, S.P.; Garssen, J.; Hogenkamp, A. Free Amino Acids in Human Milk: A Potential Role for Glutamine and Glutamate in the Protection Against Neonatal Allergies and Infections. Front. Immunol. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.-L.; Li, D.; Woo Kim, S.; Wu, G. Amino Acids and Immune Function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Hu, L.; Liu, Y.; Yan, C.; Peng, X.; Xu, Q.; Wang, R.; Cheng, Y.; Chen, H.; Fang, Z.; et al. Dietary Nucleotides Supplementation Improves the Intestinal Development and Immune Function of Neonates with Intra-Uterine Growth Restriction in a Pig Model. PLoS ONE 2016, 11, e0157314. [Google Scholar] [CrossRef] [PubMed]
- Carver, J. Dietary Nucleotides: Effects on the Immune and Gastrointestinal Systems. Acta Paediatr. 2007, 88, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shao, X.M.; Neu, J. Immunonutrients and Neonates. Eur. J. Pediatrics 2003, 162, 122–128. [Google Scholar] [CrossRef]
- Phillips-Farfán, B.; Gómez-Chávez, F.; Medina-Torres, E.A.; Vargas-Villavicencio, J.A.; Carvajal-Aguilera, K.; Camacho, L. Microbiota Signals during the Neonatal Period Forge Life-Long Immune Responses. Int. J. Mol. Sci. 2021, 22, 8162. [Google Scholar] [CrossRef]
- Miles, E.A.; Childs, C.E.; Calder, P.C. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients 2021, 13, 247. [Google Scholar] [CrossRef]
- Field, C.J. The Immunological Components of Human Milk and Their Effect on Immune Development in Infants. J. Nutr. 2005, 135, 1–4. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Fuhler, G.M. The Immune System and Microbiome in Pregnancy. Best Pract. Res. Clin. Gastroenterol. 2020, 44–45, 101671. [Google Scholar] [CrossRef]
- Lambring, C.B.; Siraj, S.; Patel, K.; Sankpal, U.T.; Mathew, S.; Basha, R. Impact of the Microbiome on the Immune System. Crit. Rev. Immunol. 2019, 39, 313–328. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the Microbiome in Human Development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Singhal, R.; Shah, Y.M. Oxygen Battle in the Gut: Hypoxia and Hypoxia-Inducible Factors in Metabolic and Inflammatory Responses in the Intestine. J. Biol. Chem. 2020, 295, 10493–10505. [Google Scholar] [CrossRef]
- Paparo, L.; di Costanzo, M.; di Scala, C.; Cosenza, L.; Leone, L.; Nocerino, R.; Canani, R. The Influence of Early Life Nutrition on Epigenetic Regulatory Mechanisms of the Immune System. Nutrients 2014, 6, 4706–4719. [Google Scholar] [CrossRef]
- Rainaldi, M.A.; Perlman, J.M. Pathophysiology of Birth Asphyxia. Clin. Perinatol. 2016, 43, 409–422. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, K.T.E.; Lian, D.W.Q.; Lu, H.; Roy, S.; Laksmi, N.K.; Low, Y.; Krishnaswamy, G.; Pierro, A.; Ong, C.C.P. The Role of Ischemia in Necrotizing Enterocolitis. J. Pediatric Surg. 2016, 51, 1255–1261. [Google Scholar] [CrossRef]
- Povroznik, J.M.; Engler-Chiurazzi, E.B.; Nanavati, T.; Pergami, P. Absolute Lymphocyte and Neutrophil Counts in Neonatal Ischemic Brain Injury. SAGE Open Med. 2018, 6, 205031211775261. [Google Scholar] [CrossRef]
- Li, B.; Concepcion, K.; Meng, X.; Zhang, L. Brain-Immune Interactions in Perinatal Hypoxic-Ischemic Brain Injury. Prog. Neurobiol. 2017, 159, 50–68. [Google Scholar] [CrossRef]
- Qin, X.; Cheng, J.; Zhong, Y.; Mahgoub, O.K.; Akter, F.; Fan, Y.; Aldughaim, M.; Xie, Q.; Qin, L.; Gu, L.; et al. Mechanism and Treatment Related to Oxidative Stress in Neonatal Hypoxic-Ischemic Encephalopathy. Front. Mol. Neurosci. 2019, 12, 88. [Google Scholar] [CrossRef]
- Li, K.; Zheng, Y.; Wang, X. The Potential Relationship Between HIF-1α and Amino Acid Metabolism After Hypoxic Ischemia and Dual Effects on Neurons. Front. Neurosci. 2021, 15, 676553. [Google Scholar] [CrossRef]
- Hu, L.; Peng, X.; Chen, H.; Yan, C.; Liu, Y.; Xu, Q.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; et al. Effects of Intrauterine Growth Retardation and Bacillus Subtilis PB6 Supplementation on Growth Performance, Intestinal Development and Immune Function of Piglets during the Suckling Period. Eur. J. Nutr. 2017, 56, 1753–1765. [Google Scholar] [CrossRef]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus Donor Breast Milk for Feeding Preterm or Low Birth Weight Infants. Cochrane Database Syst. Rev. 2018, 6, CD002971. [Google Scholar] [CrossRef]
- Neumann, C.G.; Stiehm, E.R.; Zahradnick, J.; Newton, C.; Weber, H.; Swendseid, M.E.; Cherry, J.D.; Carney, J.M.; Bwibo, N.; Chotskey, N.; et al. Immune Function in Intrauterine Growth Retardation. Nutr. Res. 1998, 18, 201–224. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; Zhou, L.; He, J.; Huang, Q.; Siyal, F.A.; Zhang, L.; Zhong, X.; Wang, T. Intrauterine Growth Retardation Promotes Fetal Intestinal Autophagy in Rats via the Mechanistic Target of Rapamycin Pathway. J. Reprod. Dev. 2017, 63, 547–554. [Google Scholar] [CrossRef][Green Version]
- Dong, L.; Zhong, X.; Ahmad, H.; Li, W.; Wang, Y.; Zhang, L.; Wang, T. Intrauterine Growth Restriction Impairs Small Intestinal Mucosal Immunity in Neonatal Piglets. J. Histochem. Cytochem. 2014, 62, 510–518. [Google Scholar] [CrossRef]
- Herz, J.; Bendix, I.; Felderhoff-Müser, U. Peripheral Immune Cells and Perinatal Brain Injury: A Double-Edged Sword? Pediatric Res. 2022, 91, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Boardman, J.P.; Hawdon, J.M. Hypoglycaemia and Hypoxic-Ischaemic Encephalopathy. Dev. Med. Child Neurol. 2015, 57, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-C.; Yang, J.-J.; Liou, Y.-M. Early Blood Glucose Level Post-Admission Correlates with the Outcomes and Oxidative Stress in Neonatal Hypoxic-Ischemic Encephalopathy. Antioxidants 2021, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Pinchefsky, E.F.; Hahn, C.D.; Kamino, D.; Chau, V.; Brant, R.; Moore, A.M.; Tam, E.W.Y. Hyperglycemia and Glucose Variability Are Associated with Worse Brain Function and Seizures in Neonatal Encephalopathy: A Prospective Cohort Study. J. Pediatrics 2019, 209, 23–32. [Google Scholar] [CrossRef]
- Iqbal, A.; Prince, L.R.; Novodvorsky, P.; Bernjak, A.; Thomas, M.R.; Birch, L.; Lambert, D.; Kay, L.J.; Wright, F.J.; Macdonald, I.A.; et al. Effect of Hypoglycemia on Inflammatory Responses and the Response to Low-Dose Endotoxemia in Humans. J. Clin. Endocrinol. Metab. 2019, 104, 1187–1199. [Google Scholar] [CrossRef]
- Boscarino, G.; Conti, M.G.; Gasparini, C.; Onestà, E.; Faccioli, F.; Dito, L.; Regoli, D.; Spalice, A.; Parisi, P.; Terrin, G. Neonatal Hyperglycemia Related to Parenteral Nutrition Affects Long-Term Neurodevelopment in Preterm Newborn: A Prospective Cohort Study. Nutrients 2021, 13, 1930. [Google Scholar] [CrossRef]
- Sangild, P.T.; Strunk, T.; Currie, A.J.; Nguyen, D.N. Editorial: Immunity in Compromised Newborns. Front. Immunol. 2021, 12, 732332. [Google Scholar] [CrossRef]
- Joffre, C.; Rey, C.; Layé, S. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front. Pharmacol. 2019, 10, 1022. [Google Scholar] [CrossRef]
- El-Mazary, A.-A.M.; Abdel- Aziz, R.A.; Mahmoud, R.A.; El-Said, M.A.; Mohammed, N.R. Correlations between Maternal and Neonatal Serum Selenium Levels in Full Term Neonates with Hypoxic Ischemic Encephalopathy. Ital. J. Pediatrics 2015, 41, 83. [Google Scholar] [CrossRef]
- Avery, J.; Hoffmann, P. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Yousuf, S.; Atif, F.; Ahmad, M.; Hoda, N.; Badruzzaman Khan, M.; Ishrat, T.; Islam, F. Selenium Plays a Modulatory Role against Cerebral Ischemia-Induced Neuronal Damage in Rat Hippocampus. Brain Res. 2007, 1147, 218–225. [Google Scholar] [CrossRef]
- Sarada, S.K.S.; Himadri, P.; Ruma, D.; Sharma, S.K.; Pauline, T. Mrinalini Selenium Protects the Hypoxia Induced Apoptosis in Neuroblastoma Cells through Upregulation of Bcl-2. Brain Res. 2008, 1209, 29–39. [Google Scholar] [CrossRef]
- Ward, R.J.; Crichton, R.R.; Taylor, D.L.; della Corte, L.; Srai, S.K.; Dexter, D.T. Iron and the Immune System. J. Neural Transm. 2011, 118, 315–328. [Google Scholar] [CrossRef]
- Terrin, G.; Berni Canani, R.; di Chiara, M.; Pietravalle, A.; Aleandri, V.; Conte, F.; de Curtis, M. Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients 2015, 7, 10427–10446. [Google Scholar] [CrossRef]
- Prasad, A.S. Clinical, Endocrinological and Biochemical Effects of Zinc Deficiency. Clin. Endocrinol. Metab. 1985, 14, 567–589. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and Its Role in Immunity and Inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human Milk Composition. Pediatric Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Conti, M.G.; Terreri, S.; Piano Mortari, E.; Albano, C.; Natale, F.; Boscarino, G.; Zacco, G.; Palomba, P.; Cascioli, S.; Corrente, F.; et al. Immune Response of Neonates Born to Mothers Infected With SARS-CoV-2. JAMA Netw. Open 2021, 4, e2132563. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.; Kalanetra, K.M.; Mills, D.A.; Underwood, M.A. Buccal Administration of Human Colostrum: Impact on the Oral Microbiota of Premature Infants. J. Perinatol. 2016, 36, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Yao, X.; Shi, L. The Effects of Colostrum on Gastrointestinal Function and Related Diseases in Premature Infants: A Comprehensive Meta-Analysis of Randomized Controlled Trials. Yangtze Med. 2018, 2, 271–280. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.-S.; Jung, Y.H.; Choi, K.Y.; Shin, S.H.; Kim, E.-K.; Choi, J.-H. Oropharyngeal Colostrum Administration in Extremely Premature Infants: An RCT. Pediatrics 2015, 135, e357–e366. [Google Scholar] [CrossRef]
- Pan, X.; Gong, D.; Gao, F.; Sangild, P.T. Diet-Dependent Changes in the Intestinal DNA Methylome after Introduction of Enteral Feeding in Preterm Pigs. Epigenomics 2018, 10, 395–408. [Google Scholar] [CrossRef]
- Garnica, A.D.; Chan, W.Y. The Role of the Placenta in Fetal Nutrition and Growth. J. Am. Coll. Nutr. 1996, 15, 206–222. [Google Scholar] [CrossRef]
- Manogura, A.C.; Turan, O.; Kush, M.L.; Berg, C.; Bhide, A.; Turan, S.; Moyano, D.; Bower, S.; Nicolaides, K.H.; Galan, H.L.; et al. Predictors of Necrotizing Enterocolitis in Preterm Growth-Restricted Neonates. Am. J. Obs. Gynecol. 2008, 198, 638.e1–638.e5. [Google Scholar] [CrossRef]
- Che, L.; Thymann, T.; Bering, S.B.; le Huërou-Luron, I.; D’Inca, R.; Zhang, K.; Sangild, P.T. IUGR Does Not Predispose to Necrotizing Enterocolitis or Compromise Postnatal Intestinal Adaptation in Preterm Pigs. Pediatric Res. 2010, 67, 54–59. [Google Scholar] [CrossRef]
- Morgan, W.; Yardley, J.; Luk, G.; Niemiec, P.; Dudgeon, D. Total Parenteral Nutrition and Intestinal Development: A Neonatal Model. J. Pediatric Surg. 1987, 22, 541–545. [Google Scholar] [CrossRef]
- le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front. Immunol. 2018, 9, 361. [Google Scholar] [CrossRef]
- Patel, R.M.; Underwood, M.A. Probiotics and Necrotizing Enterocolitis. Semin. Pediatric Surg. 2018, 27, 39–46. [Google Scholar] [CrossRef]
- Hoang, T.K.; He, B.; Wang, T.; Tran, D.Q.; Rhoads, J.M.; Liu, Y. Protective Effect of Lactobacillus Reuteri DSM 17938 against Experimental Necrotizing Enterocolitis Is Mediated by Toll-like Receptor 2. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G231–G240. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.; Li, F.; Shi, Y. White Matter Injury in Preterm Infants: Pathogenesis and Potential Therapy From the Aspect of the Gut–Brain Axis. Front. Neurosci. 2022, 16, 849372. [Google Scholar] [CrossRef]
- Sokolov, A.v.; Dubrovskaya, N.M.; Kostevich, V.A.; Vasilev, D.S.; Voynova, I.v.; Zakharova, E.T.; Runova, O.L.; Semak, I.v.; Budevich, A.I.; Nalivaeva, N.N.; et al. Lactoferrin Induces Erythropoietin Synthesis and Rescues Cognitive Functions in the Offspring of Rats Subjected to Prenatal Hypoxia. Nutrients 2022, 14, 1399. [Google Scholar] [CrossRef]
- Ayuso, M.I.; Montaner, J. Advanced Neuroprotection for Brain Ischemia: An Alternative Approach to Minimize Stroke Damage. Expert. Opin. Investig. Drugs 2015, 24, 1137–1142. [Google Scholar] [CrossRef]
- Cho, K.H.; Davidson, J.O.; Dean, J.M.; Bennet, L.; Gunn, A.J. Cooling and Immunomodulation for Treating Hypoxic-Ischemic Brain Injury. Pediatrics Int. 2020, 62, 770–778. [Google Scholar] [CrossRef]
- Koehn, L.M.; Chen, X.; Logsdon, A.F.; Lim, Y.-P.; Stonestreet, B.S. Novel Neuroprotective Agents to Treat Neonatal Hypoxic-Ischemic Encephalopathy: Inter-Alpha Inhibitor Proteins. Int. J. Mol. Sci. 2020, 21, 9193. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Murtaza, G.; Metwally, E.; Yang, H.; Kalhoro, M.S.; Kalhoro, D.H.; Chughtai, M.I.; Yin, Y. Role of Dietary Amino Acids and Nutrient Sensing System in Pregnancy Associated Disorders. Front. Pharmacol. 2020, 11, 586979. [Google Scholar] [CrossRef]

| Nutritional Component | Contribution to Immune Function [23,33] | Potential Impact of Supplementation on Perinatal Immunity | |
|---|---|---|---|
| MICRONUTRIENTS | Innate | Adaptive | |
| Vitamin A [34] | Structural and functional integrity of mucosal cells in innate barriers (e.g., skin, respiratory tract). Function of innate immune cells (e.g., natural killer (NK) cells, macrophages, neutrophils) | Important in T and B lymphocytes function. Involved in development and differentiation of Th1 and Th2 cells. Supports Th2 anti-inflammatory response | Adjuvant vitamin A in neonatal pneumonia increases IgM and IgG levels and shortens duration of infection Al-trans-retinoic acid supplementation in rat pups resulted in significantly higher levels of intestinal superoxide dismutase and glutathione peroxidase with reduced tissue tumour necrosis factor-α levels. These suggest a protective effect |
| Vitamin B [35,36,37,38] | Various B Vitamins impact function and activity of innate immune cells including dendritic cells (B6), NK cells (B6, B9/Folate, B12) and phagocytes (B2). B6 has a role in production of cytokine. | Important in synthesis and modulation of lymphocytes and activation of antibody production (B6, B9/Folate, B12) Role in supporting antibody response to antigens and Th1 response (B6, B9/Folate). | Maternal vitamin B12 supplementation may cause a slower decline in H1N1-IgG levels in neonates Antenatal supplementation with B9 in sheep was associated with increased levels of IgM and IgA in the offspring Maternal folic acid supplementation is associated with persistence of protective anti hepatitis B surface antigen five years after primary vaccination in the infant. Few other B vitamins have been studied in terms of their impact on immunity. Those that have shown little to no effect. |
| Vitamin C [39,40] | Antioxidant properties Promotes epithelial integrity Increases complement Promotes structure, function and movement of neutrophils, phagocytes and lymphocytes Important in NK cell activity and chemotaxis Role in apoptosis and clearance of neutrophils from infection site by macrophages | Increased antibody levels Increased lymphocyte differentiation and proliferation | Improved neutrophil chemotaxis in neonates with suspected sepsis Maternal vitamin C supplementation influenced cord blood mononuclear cell function by increasing cytokine (IFN-γ and IL-4) production, and decreasing IL-10 production |
| Vitamin D [41,42] | Promotes macrophage differentiation from monocytes Immune cell proliferation and cytokine production 1,25 dihydroxyvitamin D3 regulates defensins and cathelicidins (antimicrobial proteins that can directly kill pathogens) | Suppresses antibody production, inhibits T cell proliferation | Maternal Vit D correlates with leucocyte antigenic responses in breast feeding infants Newton 2022 High dose maternal vitamin D supplementation enhances proinflammatory cytokines response in cord blood. IL-17 A production increased (important in defence against respiratory pathogens) |
| Vitamin E [43] | Protects against free radical damage Enhances IL-2 and NK cell cytotoxic activity | Enhances T cell mediated function, promotes Th1 and suppresses Th2 | Maternal peripartum supplement in calves improved phagocytic activity of neutrophils |
| Zinc [44] | Protects against free radical damage Modulates cytokines enhancing CD8+ proliferation Maintains physical immune barriers | Important in immune cell growth and differentiation Essential for T cell development and activation Supports Th1 response | Maternal supplementation improved IL-6 production and reduced number of episodes of diarrhoea in infants at 6 months of age |
| Iron [45] | Regulates cytokine production and function Supports killing of bacteria by neutrophilsImportant in the generation of free radicals | Supports differentiation and proliferation of T cells Component of enzymes essential for function of immune cells | Supplementation in neonates linked to increased Gram-negative infection. In vitro studies have shown overgrowth of pathogens that are implicated in neonatal sepsis in neonatal blood |
| Copper [46] | Free-radical scavenger Antimicrobial properties Important for IL-2 production and inflammatory response | Role in T cell proliferation, antibody production and cellular immunity | Perinatal supplementation in maternal cows increased antibody response and a reduction in respiratory infection in their calves |
| Selenium [47] | Essential for enzyme function (selenoproteins) counteracting free radicals Affects function of NK cells and leukocytes | Supports T cell proliferation Role in antibody mediated immunity | Systematic review identified a 12% reduction in incidence of late onset sepsis in very low birthweight neonates when supplemented postnatally with selenium |
| MACRONUTRIENTS | Innate | Adaptive | |
| Glucose/Oligosaccharide [48,49] | Metabolites are used as immune cell substrates. Type 2 innate lymphoid cells require glucose to proliferate Required for the effector functions of human NK cells, such as GLUT1, CD98 and CD71 Required for the activation of dendritic cells to express HLA-DR, CD80, CD86 and IFN-α | Role in class switch recombination in B cells Role in IFN-γ production from GAPDH.Helps express of Th2 cytokines (IL-4, IL-13) Supports proliferation of CD8+ T cells Role in activation of T reg cells | Innate: Increased cord blood cytokines (IL-6, IL-8 and TNF- α) when exposed to high glucose concentration post staphylococcal infection Inhibition of mTORC1 in murine NK cells prevents glycolysis required for granzyme b and IFN-γ production.Adaptive: required to drive proliferation and differentiation of CD4+ T cells in adult studies. Oligosaccharide diet may contribute to regulating Th1 cells in mice. |
| Amino acids [50,51] | Reduces TNF-α production by macrophages reducing the signalling to T-Lymphocytes High levels of adenosine increase cAMP, affecting neutrophil response and reduced expression of TNF-α. May also affect NK regulation. Glutamine: can affect eosinophil metabolic plasticity, required for T-cell function related to myeloid derived suppressor cells Arginine: required for T-cell function related to myeloid-derived suppressor cellsAlanine: a significant energy substrate for leucocytes Glycine: required in proliferation and antioxidative defence of leucocytesHistidine: required for the production of histamine required for macrophages and dendritic cell function | Glutamine: required for earliest stages of T-cell activation Asparagine: may modulate lymphocyte blastogenesis Aspartate is required lymphocyte proliferation Histidine: required for production of histamine required for T lymphocyte differentiation and function Lysine: required for proliferation of lymphocytes Tyrosine: the immediate precursor for catecholamine hormones, therefore important in the activation of T and B cells Serine: utilised for structural components and signalling in T and B cells. | Oral supplementation of glutamine enhances mucin synthesis in the small intestine of piglets. In rat pups and young piglets, dietary deprivation of glutamine has been associated with diminished intestinal integrity; supplementation improved growth, barrier function and protected against pathogen damage Arginine, glycine and histidine supplementation can improve immunological response. Lysine, tryptophan and tyrosine deficiency limits proliferation of lymphocytes and impairs response in chickens. Threonine improved outcome for immune responses in piglets challenged with E-Coli |
| Dietary Nucleotides [52,53] | Role in innate immunity Required for initial leukocytes stimulation | Required for initial lymphocyte activation | IUGR piglets have lower serum cytokine (IgA, IL-1β and IL-10), peripheral leucocyte levels and down regulation of innate immunity-related genes TOLLIP, TLR-9 and TLR-2. Dietary nucleotide supplementation improved peripheral leucocyte count, IgA and IL-1B and gene expression of TOLLIP, TLR-4 and TLR-9 in ileum A nucleotide free diet was associated with an increase in delayed cutaneous hypersensitivity, reduced NK cell and macrophage activity and spleen cell production of IL-2 in rodent studies |
| Glycoproteins [48,54] | Improved bactericidal properties of cells such as MDSC, e.g., with lactoferrin | Glutamine supplementation in low-birth-weight infants was associated with less translocated bacteria across the intestinal mucosa. This corresponded to a dampened immune response | |
| Short chain Fatty acids (SCFA) (for example, Beta hydroxybutyrate) [35,55] | Ketone bodies: neutrophil effector function Fatty acid oxidation: expansion and cytokine production by Type 2 innate lymphoid cells | Differentiation of CD8+ t cells into T cytotoxic cells. Promotes CD4+ T cells IL-4 production in response to allergens. Promotes nitric oxide, IL-6 and TNF-α release Beta-hydroxybutyrate reduces B cell function | SCFA boosts the inflammatory process in murine studies. Maternal supplementation can reverse viral-induced islet inflammatory processes and therefore type 1 diabetes via modification of the microbiota in rat pups |
| Long chain Polyunsaturated Fatty Acids (LCPUFAs) [35,56,57] | LCPUFAs are used in cP450 pathway and produce Prostaglandins (PGs). PGs have multiple effects on dendritic cells, basophils, eosinophils, mast cells and macrophages LCPUFAs such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are utilised in pathways to produce specialised pro-resolving mediators DHA and arachidonic acid modulate Th1 and Th2 cell generation Can support tolerance and priming of the immune system (through suppressive IL-10 and transforming growth factor B) | Utilised in cP450 pathway and important in production of PGs. PGs have effects on Th1, Th2 cells, B cells, cytotoxic T cells, NK cells and on cytokine production such as interleukins and IFN gamma | Mice pups showed better responses to infections and vaccination in mothers supplemented with PUFAs during pregnancy LCPUFA formula fed infants were more likely to produce cytokines and lymphocyte populations similar to breast fed infants |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandecha, H.; Kaur, A.; Sanghera, R.; Preece, J.; Pillay, T. Nutrition and Immunity in Perinatal Hypoxic-Ischemic Injury. Nutrients 2022, 14, 2747. https://doi.org/10.3390/nu14132747
Gandecha H, Kaur A, Sanghera R, Preece J, Pillay T. Nutrition and Immunity in Perinatal Hypoxic-Ischemic Injury. Nutrients. 2022; 14(13):2747. https://doi.org/10.3390/nu14132747
Chicago/Turabian StyleGandecha, Hema, Avineet Kaur, Ranveer Sanghera, Joanna Preece, and Thillagavathie Pillay. 2022. "Nutrition and Immunity in Perinatal Hypoxic-Ischemic Injury" Nutrients 14, no. 13: 2747. https://doi.org/10.3390/nu14132747
APA StyleGandecha, H., Kaur, A., Sanghera, R., Preece, J., & Pillay, T. (2022). Nutrition and Immunity in Perinatal Hypoxic-Ischemic Injury. Nutrients, 14(13), 2747. https://doi.org/10.3390/nu14132747






