IQ Was Not Improved by Post-Discharge Fortification of Breastmilk in Very Preterm Infants
Abstract
:1. Introduction
2. Materials and Methods
Statistics
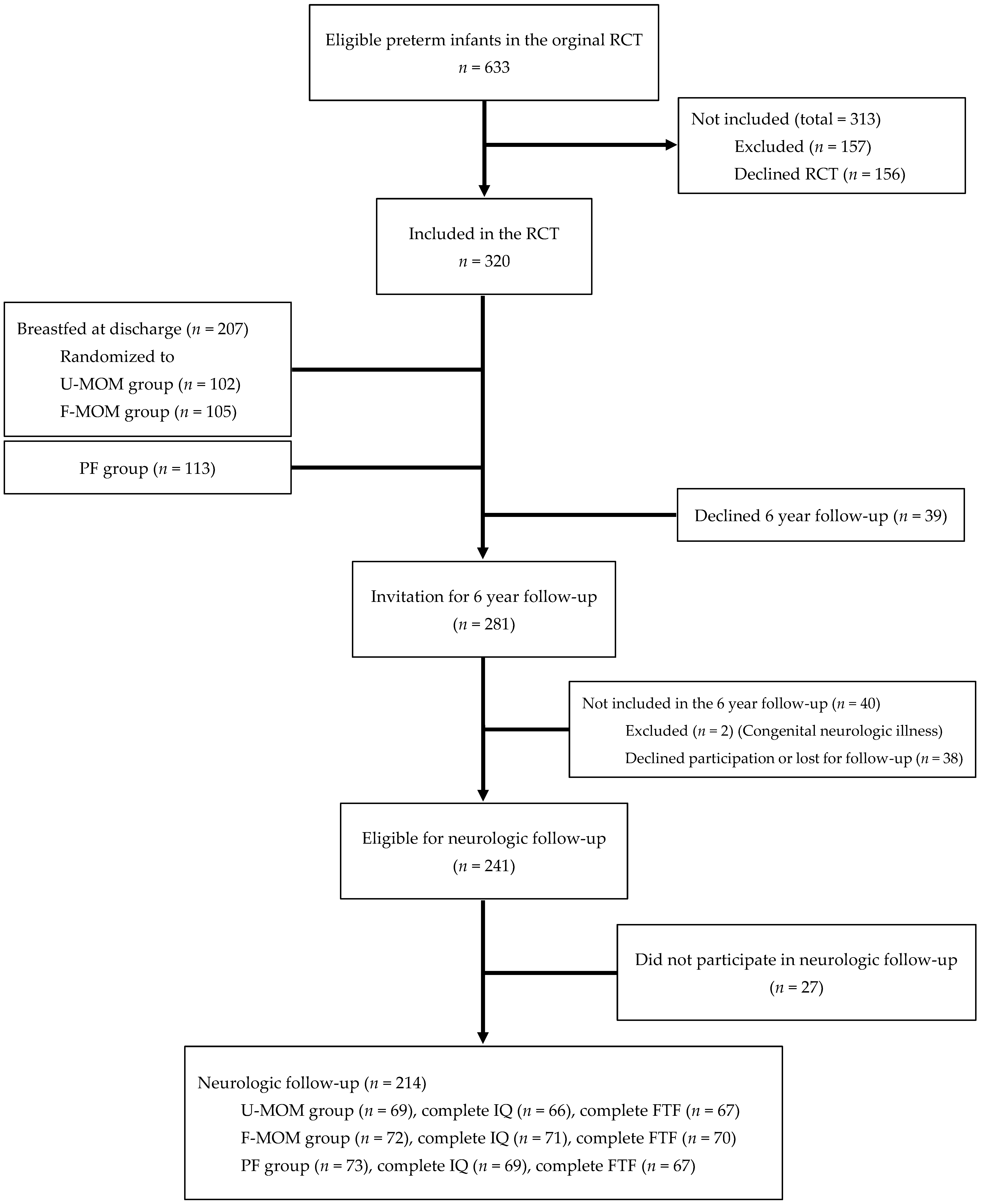
3. Results
3.1. Main Outcome on WISC-IV
3.1.1. WISC-IV in the U-MOM Group vs. the F-MOM Group
3.1.2. WISC-IV in the MOM Group vs. the PF Group
3.2. Five to Fifteen Questionnaire
3.2.1. FTF in the U-MOM Group vs. the F-MOM Group
3.2.2. FTF in the MOM Group vs. the PF Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MOM | mother’s own milk (both U-MOM and F-MOM) |
| U-MOM | unfortified MOM |
| F-MOM | fortified MOM |
| DHM | donor human milk |
| PF | preterm formula |
| FTF | The Five to Fifteen (FTF) parent questionnaire |
| VPI | Very preterm infants |
| WISC-IV | The Wechsler Intelligence Scale for Children IV |
| ADHD | Attention Deficit/Hyperactivity Disorder |
| IQ | Intelligence Quotient |
| VC | Verbal Comprehension |
| PR | Perceptual Reasoning |
| WM | Working Memory |
| PS | Processing Speed |
References
- Twilhaar, E.S.; Wade, R.M.; de Kieviet, J.F.; van Goudoever, J.B.; van Elburg, R.M.; Oosterlaan, J. Cognitive Outcomes of Children Born Extremely or Very Preterm Since the 1990s and Associated Risk Factors: A Meta-analysis and Meta-regression. JAMA Pediatr. 2018, 172, 361–367. [Google Scholar] [CrossRef]
- Johnson, S.; Marlow, N. Preterm birth and childhood psychiatric disorders. Pediatr. Res. 2011, 69, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.M.; Rehman Mian, M.O.; Nuyt, A.M. Long-Term Impact of Preterm Birth: Neurodevelopmental and Physical Health Outcomes. Clin. Perinatol. 2017, 44, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Coviello, C.; Keunen, K.; Kersbergen, K.J.; Groenendaal, F.; Leemans, A.; Peels, B.; Isgum, I.; Viergever, M.A.; de Vries, L.S.; Buonocore, G.; et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr. Res. 2018, 83, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslanoglu, S.; Boquien, C.Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of Human Milk for Preterm Infants: Update and Recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front. Pediatr. 2019, 7, 76. [Google Scholar] [CrossRef]
- Hay, W.W., Jr.; Hendrickson, K.C. Preterm formula use in the preterm very low birth weight infant. Semin. Fetal Neonatal Med. 2017, 22, 15–22. [Google Scholar] [CrossRef]
- Lucas, A.; Morley, R.; Cole, T.J. Randomised trial of early diet in preterm babies and later intelligence quotient. BMJ 1998, 317, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.J.; Patel, A.L.; Bigger, H.R.; Engstrom, J.L.; Meier, P.P. Economic benefits and costs of human milk feedings: A strategy to reduce the risk of prematurity-related morbidities in very-low-birth-weight infants. Adv. Nutr. 2014, 5, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.L.; Johnson, T.J.; Robin, B.; Bigger, H.R.; Buchanan, A.; Christian, E.; Nandhan, V.; Shroff, A.; Schoeny, M.; Engstrom, J.L.; et al. Influence of own mother’s milk on bronchopulmonary dysplasia and costs. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F256–F261. [Google Scholar] [CrossRef] [Green Version]
- Lechner, B.E.; Vohr, B.R. Neurodevelopmental Outcomes of Preterm Infants Fed Human Milk: A Systematic Review. Clin. Perinatol. 2017, 44, 69–83. [Google Scholar] [CrossRef]
- Ong, K.K.; Kennedy, K.; Castaneda-Gutierrez, E.; Forsyth, S.; Godfrey, K.M.; Koletzko, B.; Latulippe, M.E.; Ozanne, S.E.; Rueda, R.; Schoemaker, M.H.; et al. Postnatal growth in preterm infants and later health outcomes: A systematic review. Acta Paediatr. 2015, 104, 974–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, A.; Fewtrell, M.S.; Morley, R.; Singhal, A.; Abbott, R.A.; Isaacs, E.; Stephenson, T.; MacFadyen, U.M.; Clements, H. Randomized trial of nutrient-enriched formula versus standard formula for postdischarge preterm infants. Pediatrics 2001, 108, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Zachariassen, G.; Faerk, J.; Grytter, C.; Esberg, B.H.; Hjelmborg, J.; Mortensen, S.; Thybo Christesen, H.; Halken, S. Nutrient enrichment of mother’s milk and growth of very preterm infants after hospital discharge. Pediatrics 2011, 127, e995–e1003. [Google Scholar] [CrossRef] [PubMed]
- Zachariassen, G.; Faerk, J.; Grytter, C.; Esberg, B.; Juvonen, P.; Halken, S. Factors associated with successful establishment of breastfeeding in very preterm infants. Acta Paediatr. 2010, 99, 1000–1004. [Google Scholar] [CrossRef]
- Niklasson, A.; Albertsson-Wikland, K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr. 2008, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Toftlund, L.H.; Agertoft, L.; Halken, S.; Zachariassen, G. Improved lung function at age 6 in children born very preterm and fed extra protein post-discharge. Pediatr. Allergy Immunol. 2019, 30, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Toftlund, L.H.; Halken, S.; Agertoft, L.; Zachariassen, G. Early nutrition and signs of metabolic syndrome at 6 y of age in children born very preterm. Am. J. Clin. Nutr. 2018, 107, 717–724. [Google Scholar] [CrossRef]
- Toftlund, L.H.; Halken, S.; Agertoft, L.; Zachariassen, G. Catch-Up Growth, Rapid Weight Growth, and Continuous Growth from Birth to 6 Years of Age in Very-Preterm-Born Children. Neonatology 2018, 114, 285–293. [Google Scholar] [CrossRef]
- Kadesjo, B.; Janols, L.O.; Korkman, M.; Mickelsson, K.; Strand, G.; Trillingsgaard, A.; Gillberg, C. The FTF (Five to Fifteen): The development of a parent questionnaire for the assessment of ADHD and comorbid conditions. Eur. Child Adolesc. Psychiatry 2004, 13 (Suppl. S3), 3–13. [Google Scholar] [CrossRef]
- Bohlin, G.; Janols, L.O. Behavioural problems and psychiatric symptoms in 5-13 year-old Swedish children-a comparison of parent ratings on the FTF (Five to Fifteen) with the ratings on CBCL (Child Behavior Checklist). Eur. Child Adolesc. Psychiatry 2004, 13 (Suppl. S3), 14–22. [Google Scholar] [CrossRef]
- Korkman, M.; Jaakkola, M.; Ahlroth, A.; Pesonen, A.E.; Turunen, M.M. Screening of developmental disorders in five-year-olds using the FTF (Five to Fifteen) questionnaire: A validation study. Eur. Child Adolesc. Psychiatry 2004, 13 (Suppl. S3), 31–38. [Google Scholar] [CrossRef] [PubMed]
- Trillingsgaard, A.; Damm, D.; Sommer, S.; Jepsen, J.R.; Ostergaard, O.; Frydenberg, M.; Thomsen, P.H. Developmental profiles on the basis of the FTF (Five to Fifteen) questionnaire-clinical validity and utility of the FTF in a child psychiatric sample. Eur. Child Adolesc. Psychiatry 2004, 13 (Suppl. S3), 39–63. [Google Scholar] [CrossRef] [PubMed]
- Trillingsgaard, A. 5-15 (FTF) Nordisk Skema Til Vurdering af Børns Udvikling og Adfærd; Psykologisk Institut Aarhus Universitet: Århus, Denmark, 2005. [Google Scholar]
- Aimone, A.; Rovet, J.; Ward, W.; Jefferies, A.; Campbell, D.M.; Asztalos, E.; Feldman, M.; Vaughan, J.; Westall, C.; Whyte, H.; et al. Growth and body composition of human milk-fed premature infants provided with extra energy and nutrients early after hospital discharge: 1-year follow-up. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 456–466. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, R.D.; Lamy Filho, F.; Rafael, E.V.; Lamy, Z.C.; de Queiroz, A.L. Breast milk supplementation and preterm infant development after hospital discharge: A randomized clinical trial. J. Pediatr. 2016, 92, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabydeen, L.; Thomas, J.E.; Aston, T.J.; Hartley, H.; Sinha, S.K.; Eyre, J.A. High-energy and -protein diet increases brain and corticospinal tract growth in term and preterm infants after perinatal brain injury. Pediatrics 2008, 121, 148–156. [Google Scholar] [CrossRef]
- Honore, K.D.; Johansen, M.N.; Rasmussen, L.; Zachariassen, G. Stoma Closure Improves Head Circumference Growth in Very Preterm Infants after Necrotizing Enterocolitis. Eur. J. Pediatr. Surg. 2021, 31, 504–508. [Google Scholar] [CrossRef]
- Lucas, A.; Morley, R.; Cole, T.J.; Lister, G.; Leeson-Payne, C. Breast milk and subsequent intelligence quotient in children born preterm. Lancet 1992, 339, 261–264. [Google Scholar] [CrossRef]
- Linsell, L.; Malouf, R.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Prognostic Factors for Poor Cognitive Development in Children Born Very Preterm or With Very Low Birth Weight: A Systematic Review. JAMA Pediatr. 2015, 169, 1162–1172. [Google Scholar] [CrossRef] [Green Version]
- Wood, N.S.; Costeloe, K.; Gibson, A.T.; Hennessy, E.M.; Marlow, N.; Wilkinson, A.R.; Group, E.P.S. The EPICure study: Associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F134–F140. [Google Scholar] [CrossRef] [Green Version]
- Kyhl, H.B.; Jensen, T.K.; Barington, T.; Buhl, S.; Norberg, L.A.; Jorgensen, J.S.; Jensen, D.F.; Christesen, H.T.; Lamont, R.F.; Husby, S. The Odense Child Cohort: Aims, design, and cohort profile. Paediatr. Perinat. Epidemiol. 2015, 29, 250–258. [Google Scholar] [CrossRef]
- Beck, I.H.; Bilenberg, N.; Davidsen, K.A.; Rasmussen, A.A.; Boye, H.; Jensen, T.K. Prenatal and early childhood predictors of intelligence quotient (IQ) in 7-year-old Danish children from the Odense Child Cohort. Scand. J. Public Health 2022. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.; Hagglof, B.; Serenius, F. Behaviours related to executive functions and learning skills at 11 years of age after extremely preterm birth: A Swedish national prospective follow-up study. Acta Paediatr. 2013, 102, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Rao, S.C.; Bulsara, M.K.; Patole, S.K. Prevalence of Autism Spectrum Disorder in Preterm Infants: A Meta-analysis. Pediatrics 2018, 142, e20180134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linsell, L.; Malouf, R.; Johnson, S.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Prognostic Factors for Behavioral Problems and Psychiatric Disorders in Children Born Very Preterm or Very Low Birth Weight: A Systematic Review. J. Dev. Behav. Pediatr. 2016, 37, 88–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Amissah, E.; Gamble, G.D.; Crowther, C.A.; Harding, J.E. Impact of macronutrient supplements for children born preterm or small for gestational age on developmental and metabolic outcomes: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002952. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Lee, K.J.; Anderson, P.J. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2010, 52, 232–237. [Google Scholar] [CrossRef]
- Lambek, R.; Trillingsgaard, A.; Kadesjo, B.; Damm, D.; Thomsen, P.H. Gender differences on the Five to Fifteen questionnaire in a non-referred sample with inattention and hyperactivity-impulsivity and a clinic-referred sample with hyperkinetic disorder. Scand. J. Psychol. 2010, 51, 540–547. [Google Scholar] [CrossRef]
- Duncan, A.F.; Matthews, M.A. Neurodevelopmental Outcomes in Early Childhood. Clin. Perinatol. 2018, 45, 377–392. [Google Scholar] [CrossRef]
- Linsell, L.; Johnson, S.; Wolke, D.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Trajectories of behavior, attention, social and emotional problems from childhood to early adulthood following extremely preterm birth: A prospective cohort study. Eur. Child Adolesc. Psychiatry 2019, 28, 531–542. [Google Scholar] [CrossRef] [Green Version]
| Unfortified Mother’s Milk (U-MOM) | Fortified Mother’s Milk (F-MOM) | Preterm Formula (PF) | |
|---|---|---|---|
| Preterm infants | 69 | 72 | 73 |
| Boys % (n) | 49.3% (34) | 43.1% (31) | 63% (46) |
| Singletons % (n) | 82.6% (57) | 61.1% (44) | 54.7% (40) |
| GA at birth (Weeks + days) (Median; min–max) | 29 + 4 (24 + 1–32 + 0) | 30 + 2 (24 + 3–32 + 0) | 29 + 4 (26 + 2–31 + 5) |
| Birth weight (grams) (Mean ± SD) | 1284 ± 376 | 1318 ± 335 | 1280 ± 339 |
| Weight z-score (SDS) at birth (Mean ± SD) | −0.96 ± 1.12 | −1.04 ± 1.04 | −1.25 ± 1.14 |
| SGA % (n) | 18.8% (13) | 19.4% (14) | 26% (19) |
| PMA at discharge (Weeks and days) (Median; min–max) | 37 + 3 (34 + 5–45 +4) | 37 + 2 (34 + 5–42 + 3) | 36 + 6 (35 + 0–40 + 6) |
| Weight at discharge (g) (Mean ± SD) | 2679 ± 469 | 2612 ± 343 | 2696 ± 427 |
| Weight z-score (SDS) at discharge (Mean ± SD) | −1.19 ± 0.87 | −1.20 ± 0.83 | −0.96 ± 0.95 |
| Head circumference z-score (SDS) at 4-month CA (Mean ± SD) | 0.24 ± 0.94 | 0.26 ± 1.08 | 0.44 ± 1.29 |
| Weight z-score (SDS) at 4-month CA (Mean ± SD) | −0.65 ± 1.19 | −0.54 ± 1.20 | −0.17 ± 1.17 |
| Length z-score (SDS) at 4-month CA (Mean ± SD) | −0.36 ± 1.42 | −0.14 ± 1.34 | 0.16 ± 1.41 |
| Corrected age (years) at WISC-IV (Median; min-max) | 6.4 (5.4–8.3) | 6.4 (5.2–7.5) | 6.3 (5.9–7.7) |
| Corrected age (years) at FTF questionnaire (Median; min–max) | 6.5 (6.1–8.3) | 6.4 (6.0–7.5) | 6.3 (5.9–7.7) |
| Maternal age (years) (Mean ± SD) | 30.5 ± 4.6 | 31.3 ± 4.5 | 30.9 ± 5.4 |
| Young mother % (n) * | 8.7% (6) | 5.6% (4) | 12.3% (9) |
| Maternal low social group (group 3–5) % (n) | 53.7% (36) | 51.4% (37) | 71.2% (52) |
| U-MOM Group | F-MOM Group | MOM Group | PF Group | |
|---|---|---|---|---|
| Full Scale IQ (Median; min–max) | 105.5 (63–122) | 104 (64–132) | 105 (63–132) | 104 (71–127) * |
| Verbal Comprehension index (Median; min–max) | 112 (83–130) | 110 (63–130) | 110 (63–130) | 106 (73–128) * |
| Perceptual Reasoning index (Median; min–max) | 109 (62–128) | 105 (77–141) | 107 (62–141) | 101 (69–126) * |
| Working Memory index (Median; min–max) | 98 (60–122) | 98 (63–116) | 98 (60–122) | 95 (57–116) |
| Processing Speed index (Median; min–max) | 104 (69–133) | 104 (75–141) | 104 (69–141) | 104 (69–136) |
| Skill | Percentile | Post Discharge Nutrition Group | |||
|---|---|---|---|---|---|
| U-MOM Group | F-MOM Group | MOM Group | PF Group | ||
| Motor skills (n) | ≥90th <90th | 22.1% (15) 77.9% (53) | 9.7% (7) 90.3% ^ (65) | 15.7% (22) 84.3% * (118) | 33.8% (24) 66.2% (47) |
| Executive functions (n) | ≥90th <90th | 14.5% (10) 85.5% (59) | 8.5% (6) 91.5% (65) | 11.4% (16) 88.6% (124) | 12.7% (9) 87.3% (62) |
| Perception (n) | ≥90th <90th | 42.4% (22) 67.6% (46) | 22.2% (16) 77.8% (56) | 27.1% (38) 72.9% * (102) | 43.7% (31) 56.3% (40) |
| Memory (n) | ≥90th <90th | 28.4% (19) 71.6% (48) | 22.2% (16) 77.8% (56) | 25.2% (35) 74.8% (104) | 35.7% (25) 64.3% (45) |
| Language (n) | ≥90th <90th | 16.4% (11) 83.6% (56) | 12.5% (9) 87.5% (63) | 14.4% (20) 85.6% * (119) | 29.4% (20) 70.6% (48) |
| Social skills (n) | ≥90th <90th | 29% (20) 71% (49) | 16.7% (12) 83.3% (60) | 22.7% (32) 77.3% * (109) | 39.1% (27) 60.9% (42) |
| Emotional/behavioural problems (n) | ≥90th <90th | 23.9% (16) 76.1% (51) | 18.1% (13) 81.9% (59) | 20.9% (29) 79.1% (110) | 31.9% (22) 68.1% (47) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klamer, A.; Toftlund, L.H.; Grimsson, K.; Halken, S.; Zachariassen, G. IQ Was Not Improved by Post-Discharge Fortification of Breastmilk in Very Preterm Infants. Nutrients 2022, 14, 2709. https://doi.org/10.3390/nu14132709
Klamer A, Toftlund LH, Grimsson K, Halken S, Zachariassen G. IQ Was Not Improved by Post-Discharge Fortification of Breastmilk in Very Preterm Infants. Nutrients. 2022; 14(13):2709. https://doi.org/10.3390/nu14132709
Chicago/Turabian StyleKlamer, Anja, Line H. Toftlund, Kristjan Grimsson, Susanne Halken, and Gitte Zachariassen. 2022. "IQ Was Not Improved by Post-Discharge Fortification of Breastmilk in Very Preterm Infants" Nutrients 14, no. 13: 2709. https://doi.org/10.3390/nu14132709
APA StyleKlamer, A., Toftlund, L. H., Grimsson, K., Halken, S., & Zachariassen, G. (2022). IQ Was Not Improved by Post-Discharge Fortification of Breastmilk in Very Preterm Infants. Nutrients, 14(13), 2709. https://doi.org/10.3390/nu14132709





