Hydroxycitric Acid Inhibits Chronic Myelogenous Leukemia Growth through Activation of AMPK and mTOR Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Proliferation Assay
2.3. Quantification of Apoptotic Cells
2.4. DNA Fragmentation
2.5. Cell Cycle Analysis
2.6. Protein Extraction and Western Blotting
2.7. Immunoprecipitation
2.8. Size-Exclusion Chromatography
2.9. Animal Studies
2.10. Statistical Analysis
3. Results
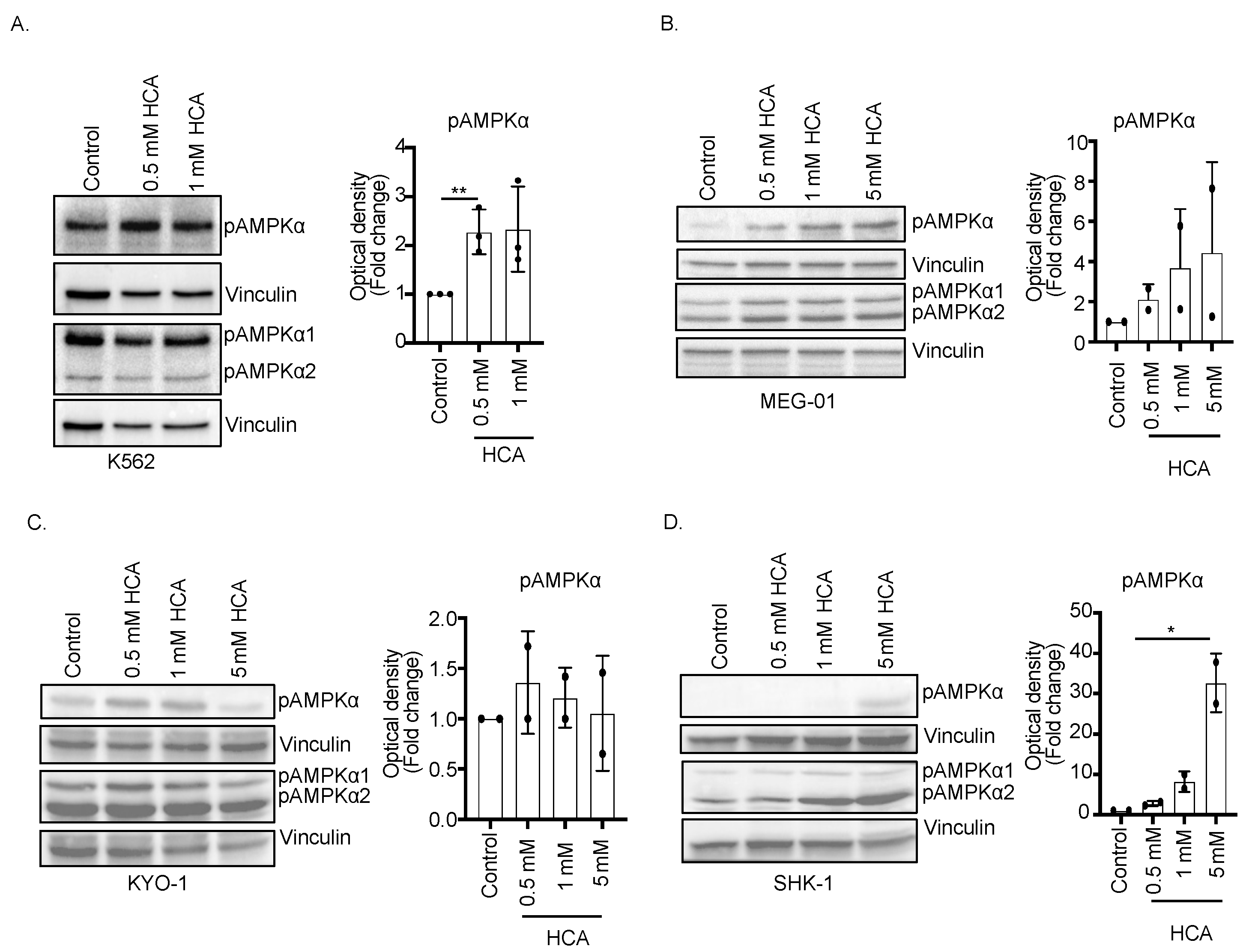
3.1. Hydroxycitric Acid Promotes AMPK Phosphorylation in CML Cells
3.2. ACLY, a Direct Target of Hydroxycitric Acid, Interacts with AMPK
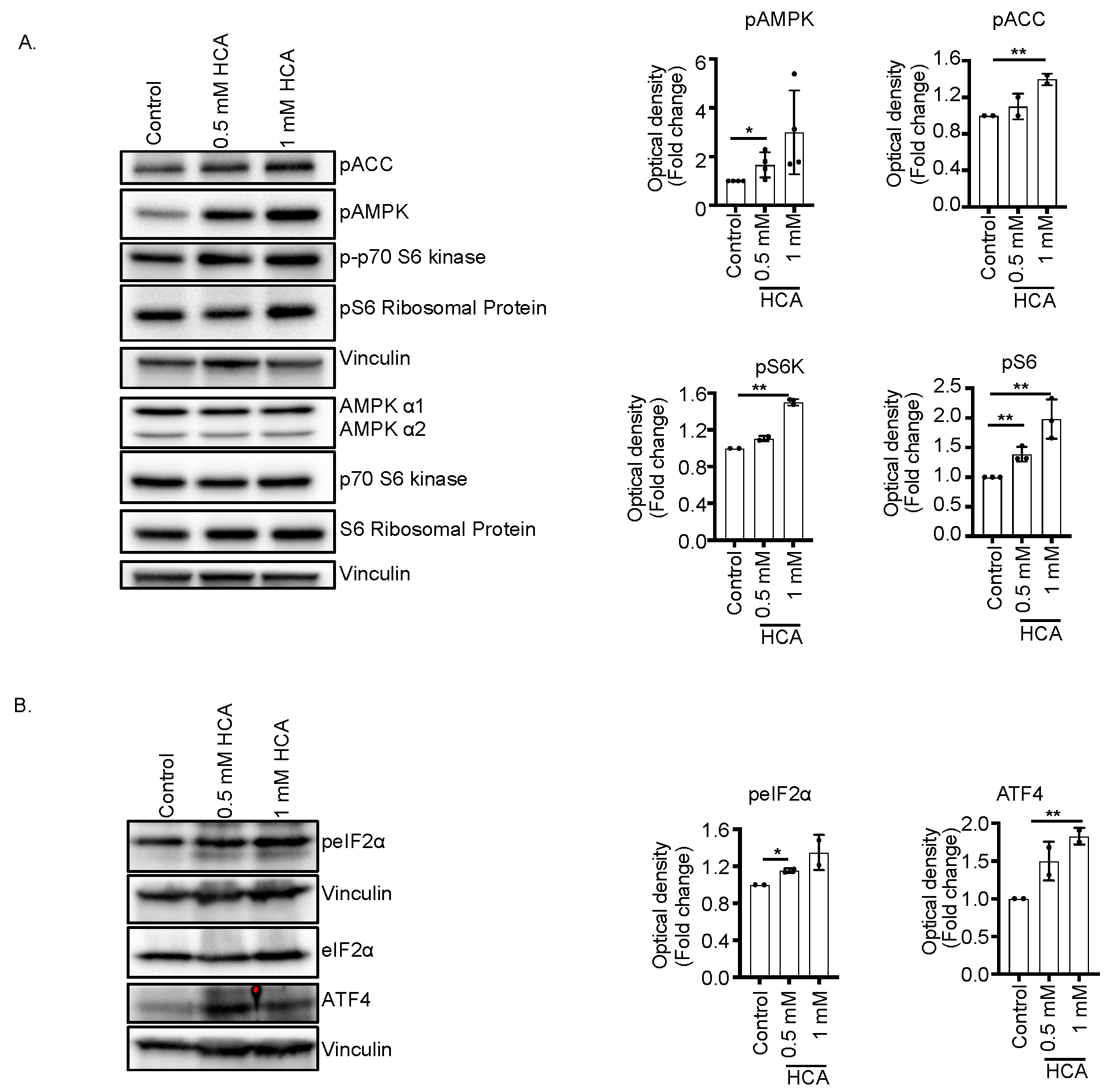
3.3. Concurrent Activation of AMPK and mTOR Pathway Increases Metabolic Stress Pathway
3.4. HCA Induces Cell Cycle Arrest at the G2/M Phase in K562 Cells
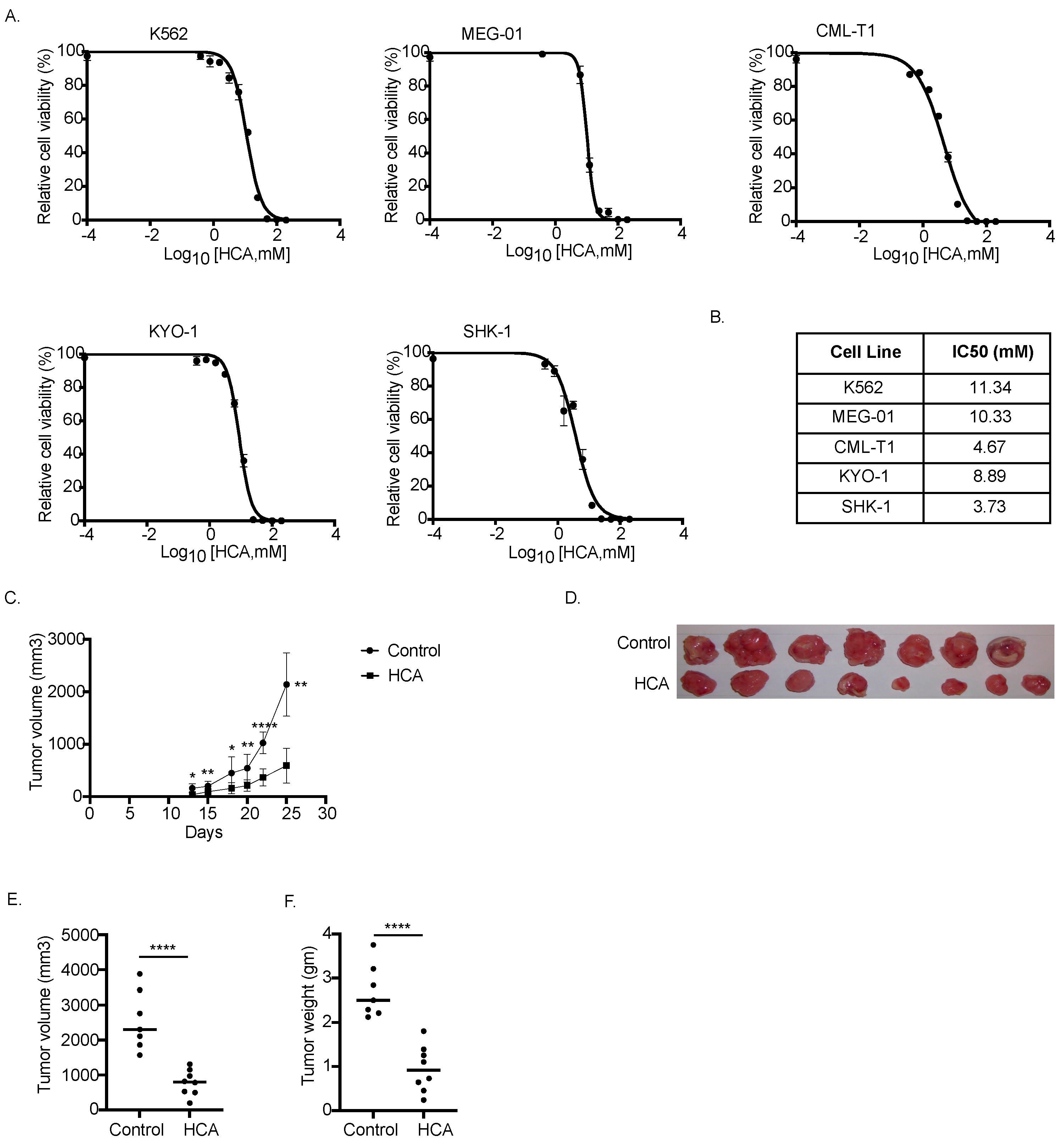
3.5. HCA Inhibited Tumor Cell Growth In Vitro and In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardie, D.G. AMPK—Sensing Energy While Talking to Other Signaling Pathways. Cell Metab. 2014, 20, 939–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A Nutrient and Energy Sensor That Maintains Energy Homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haurie, V.; Boucherie, H.; Sagliocco, F. The Snf1 Protein Kinase Controls the Induction of Genes of the Iron Uptake Pathway at the Diauxic Shift in Saccharomyces Cerevisiae. J. Biol. Chem. 2003, 278, 45391–45396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting AMPK for Cancer Prevention and Treatment. Oncotarget 2015, 6, 7365. [Google Scholar] [CrossRef] [Green Version]
- Shaw, R.J.; Kosmatka, M.; Bardeesy, N.; Hurley, R.L.; Witters, L.A.; DePinho, R.A.; Cantley, L.C. The Tumor Suppressor LKB1 Kinase Directly Activates AMP-Activated Kinase and Regulates Apoptosis in Response to Energy Stress. Proc. Natl. Acad. Sci. USA 2004, 101, 3329–3335. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G. Molecular Pathways: Is AMPK a Friend or a Foe in Cancer? Clin. Cancer Res. 2015, 21, 3836–3840. [Google Scholar] [CrossRef] [Green Version]
- Chuang, H.-C.; Chou, C.-C.; Kulp, S.; Chen, C.-S. AMPK as a Potential Anticancer Target—Friend or Foe? Curr. Pharm. Des. 2015, 20, 2607–2618. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G. AMP-Activated Protein Kinase: Maintaining Energy Homeostasis at the Cellular and Whole-Body Levels. Annu. Rev. Nutr. 2014, 34, 31. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK Activators: Mechanisms of Action and Physiological Activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef] [Green Version]
- Grahame Hardie, D. Regulation of AMP-Activated Protein Kinase by Natural and Synthetic Activators. Acta Pharm. Sin. B 2016, 6, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.; Kumar, S. Natural AMPK Activators: An Alternative Approach for the Treatment and Management of Metabolic Syndrome. Curr. Med. Chem. 2016, 24, 1007–1047. [Google Scholar] [CrossRef]
- Arkwright, R.; Deshmukh, R.; Adapa, N.; Stevens, R.; Zonder, E.; Zhang, Z.; Farshi, P.; Ahmed, R.; El-Banna, H.; Chan, T.-H.; et al. Lessons from Nature: Sources and Strategies for Developing AMPK Activators for Cancer Chemotherapeutics. Anti Cancer Agents Med. Chem. 2015, 15, 657–671. [Google Scholar] [CrossRef] [Green Version]
- Marín-Aguilar, F.; Pavillard, L.E.; Giampieri, F.; Bullón, P.; Cordero, M.D. Adenosine Monophosphate (AMP)-Activated Protein Kinase: A New Target for Nutraceutical Compounds. Int. J. Mol. Sci. 2017, 18, 288. [Google Scholar] [CrossRef] [Green Version]
- Jabbour, E.; Kantarjian, H. Chronic Myeloid Leukemia: 2020 Update on Diagnosis, Therapy and Monitoring. Am. J. Hematol. 2020, 95, 691–709. [Google Scholar] [CrossRef]
- Alves, R.; Gonçalves, A.C.; Rutella, S.; Almeida, A.M.; De Las Rivas, J.; Trougakos, I.P.; Ribeiro, A.B.S. Resistance to Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia—From Molecular Mechanisms to Clinical Relevance. Cancers 2021, 13, 4820. [Google Scholar] [CrossRef]
- Chan, O.; Talati, C.; Isenalumhe, L.; Shams, S.; Nodzon, L.; Fradley, M.; Sweet, K.; Pinilla-Ibarz, J. Side-Effects Profile and Outcomes of Ponatinib in the Treatment of Chronic Myeloid Leukemia. Blood Adv. 2020, 4, 530–538. [Google Scholar] [CrossRef]
- Vakana, E.; Platanias, L.C. AMPK in BCR-ABL Expressing Leukemias. Regulatory Effects and Therapeutic Implications. Oncotarget 2011, 2, 1322. [Google Scholar] [CrossRef] [Green Version]
- Vakana, E.; Altman, J.K.; Glaser, H.; Donato, N.J.; Platanias, L.C. Antileukemic Effects of AMPK Activators on BCR-ABL-Expressing Cells. Blood 2011, 118, 6399–6402. [Google Scholar] [CrossRef] [Green Version]
- Puissant, A.; Robert, G.; Fenouille, N.; Luciano, F.; Cassuto, J.P.; Raynaud, S.; Auberger, P. Resveratrol Promotes Autophagic Cell Death in Chronic Myelogenous Leukemia Cells via JNK-Mediated P62/SQSTM1 Expression and AMPK Activation. Cancer Res. 2010, 70, 1042–1052. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, T.K.; Leclerc, G.M.; Hsieh-Kinser, T.T.; Leclerc, G.J.; Singh, I.; Barredo, J.C. Cytotoxic Effect of 5-Aminoimidazole-4-Carboxamide-1-β-4-Ribofuranoside (AICAR) on Childhood Acute Lymphoblastic Leukemia (ALL) Cells: Implication for Targeted Therapy. Mol. Cancer 2007, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Na, Y.J.; Yu, E.S.; Kim, D.S.; Lee, D.H.; Oh, S.C.; Choi, C.W. Metformin Enhances the Cytotoxic Effect of Nilotinib and Overcomes Nilotinib Resistance in Chronic Myeloid Leukemia Cells. Korean J. Intern. Med. 2021, 36, S196. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Rink, C.; Khanna, S.; Phillips, C.; Bagchi, D.; Bagchi, M.; Sen, C.K. Body Weight and Abdominal Fat Gene Expression Profile in Response to a Novel Hydroxycitric Acid-Based Dietary Supplement. Gene Expr. 2003, 11, 251–262. [Google Scholar] [CrossRef]
- Watson, J.A.; Fang, M.; Lowenstein, J.M. Tricarballylate and Hydroxycitrate: Substrate and Inhibitor of ATP: Citrate Oxaloacetate Lyase. Arch. Biochem. Biophys. 1969, 135, 209–217. [Google Scholar] [CrossRef]
- Sullivan, A.C.; Singh, M.; Srere, P.A.; Glusker, J.P. Reactivity and Inhibitor Potential of Hydroxycitrate Isomers with Citrate Synthase, Citrate Lyase, and ATP Citrate Lyase. J. Biol. Chem. 1977, 252, 7583–7590. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, H.; Lee, S.M.; Lee, J.E.; Choi, J.; Kim, T.W.; Cho, E.J.; Youn, H.D. ATP-Citrate Lyase Regulates Cellular Senescence via an AMPK- and P53-Dependent Pathway. FEBS J. 2015, 282, 361–371. [Google Scholar] [CrossRef]
- Pezze, P.D.; Ruf, S.; Sonntag, A.G.; Langelaar-Makkinje, M.; Hall, P.; Heberle, A.M.; Navas, P.R.; van Eunen, K.; Tölle, R.C.; Schwarz, J.J.; et al. A Systems Study Reveals Concurrent Activation of AMPK and MTOR by Amino Acids. Nat. Commun. 2016, 7, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Sujobert, P.; Poulain, L.; Paubelle, E.; Zylbersztejn, F.; Grenier, A.; Lambert, M.; Townsend, E.C.; Brusq, J.M.; Nicodeme, E.; Decrooqc, J.; et al. Co-Activation of AMPK and MTORC1 Induces Cytotoxicity in Acute Myeloid Leukemia. Cell Rep. 2015, 11, 1446–1457. [Google Scholar] [CrossRef]
- Lee, W.; Yoo, W.; Chae, H. ER Stress and Autophagy. Curr. Mol. Med. 2015, 15, 735–745. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, J.; Altafaj, A.; Cardona, M.; Bahi, N.; Llovera, M.; Cañas, X.; Cook, S.A.; Comella, J.X.; Sanchis, D. Endog Links Bnip3-Induced Mitochondrial Damage and Caspase-Independent DNA Fragmentation in Ischemic Cardiomyocytes. PLoS ONE 2011, 6, e17998. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.; Nakamura, H.; Tsuchiya, S.; Horie, S.; Kashiwayanagi, M.; Saito, T.; Murayama, T. Quercetin-Induced PC12 Cell Death Accompanied by Caspase-Mediated DNA Fragmentation. Biol. Pharm. Bull. 2007, 30, 682–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, S.; Tandon, S. DNA Fragmentation and Cell Cycle Arrest: A Hallmark of Apoptosis Induced by Ruta Graveolens in Human Colon Cancer Cells. Homeopathy 2015, 104, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Salami, A.; Seydi, E.; Pourahmad, J. Use of Nutraceuticals for Prevention and Treatment of Cancer. Iran. J. Pharm. Res. 2013, 12, 219. [Google Scholar] [PubMed]
- Han, J.; Li, L.; Wang, D.; Ma, H. (-)-Hydroxycitric Acid Reduced Fat Deposition via Regulating Lipid Metabolism-Related Gene Expression in Broiler Chickens. Lipids Health Dis. 2016, 15, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, H.; Yao, Y.; Yang, Z.; Ma, H. (-)-Hydroxycitric Acid Suppresses Lipid Droplet Accumulation and Accelerates Energy Metabolism via Activation of the Adiponectin-AMPK Signaling Pathway in Broiler Chickens. J. Agric. Food Chem. 2019, 67, 3188–3197. [Google Scholar] [CrossRef]
- Pinkosky, S.L.; Filippov, S.; Srivastava, R.A.K.; Hanselman, J.C.; Bradshaw, C.D.; Hurley, T.R.; Cramer, C.T.; Spahr, M.A.; Brant, A.F.; Houghton, J.L.; et al. AMP-Activated Protein Kinase and ATP-Citrate Lyase Are Two Distinct Molecular Targets for ETC-1002, a Novel Small Molecule Regulator of Lipid and Carbohydrate Metabolism. J. Lipid Res. 2013, 54, 134–151. [Google Scholar] [CrossRef] [Green Version]
- Migita, T.; Okabe, S.; Ikeda, K.; Igarashi, S.; Sugawara, S.; Tomida, A.; Taguchi, R.; Soga, T.; Seimiya, H. Inhibition of ATP Citrate Lyase Induces an Anticancer Effect via Reactive Oxygen Species: AMPK as a Predictive Biomarker for Therapeutic Impact. Am. J. Pathol. 2013, 182, 1800–1810. [Google Scholar] [CrossRef]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin Inhibits Hepatic MTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef] [Green Version]
- Nagalingam, A.; Arbiser, J.L.; Bonner, M.Y.; Saxena, N.K.; Sharma, D. Honokiol Activates AMP-Activated Protein Kinase in Breast Cancer Cells via an LKB1-Dependent Pathway and Inhibits Breast Carcinogenesis. Breast Cancer Res. 2012, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.M.; Jung, J.H.; Jeong, S.J.; Sohn, E.J.; Kim, B.; Kim, S.H. Tanshinone IIA Induces Autophagic Cell Death via Activation of Ampk and Erk and Inhibition of MTOR and P70 S6K in KBM-5 Leukemia Cells. Phytother. Res. 2014, 28, 458–464. [Google Scholar] [CrossRef]
- Chiang, P.C.; Lin, S.C.; Pan, S.L.; Kuo, C.H.; Tsai, I.L.; Kuo, M.T.; Wen, W.C.; Chen, P.; Guh, J.H. Antroquinonol Displays Anticancer Potential against Human Hepatocellular Carcinoma Cells: A Crucial Role of AMPK and MTOR Pathways. Biochem. Pharmacol. 2010, 79, 162–171. [Google Scholar] [CrossRef]
- Shieh, J.M.; Chen, Y.C.; Lin, Y.C.; Lin, J.N.; Chen, W.C.; Chen, Y.Y.; Ho, C.T.; Way, T.-D. Demethoxycurcumin Inhibits Energy Metabolic and Oncogenic Signaling Pathways through AMPK Activation in Triple-Negative Breast Cancer Cells. J. Agric. Food Chem. 2013, 61, 6366–6375. [Google Scholar] [CrossRef]
- Ozcan, U.; Ozcan, L.; Yilmaz, E.; Düvel, K.; Sahin, M.; Manning, B.D.; Hotamisligil, G.S. Loss of the Tuberous Sclerosis Complex Tumor Suppressors Triggers the Unfolded Protein Response to Regulate Insulin Signaling and Apoptosis. Mol. Cell 2008, 29, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Law, M.E.; Castellano, R.K.; Law, B.K. The Unfolded Protein Response as a Target for Anticancer Therapeutics. Crit. Rev. Oncol. Hematol. 2018, 127, 66–79. [Google Scholar] [CrossRef]
- Dolai, S.; Pal, S.; Yadav, R.K.; Adak, S. Endoplasmic Reticulum Stress-Induced Apoptosis in Leishmania through Ca2+-Dependent and Caspase-Independent Mechanism. J. Biol. Chem. 2011, 286, 13638–13646. [Google Scholar] [CrossRef] [Green Version]
- Tait, S.W.G.; Green, D.R. Caspase-Independent Cell Death: Leaving the Set without the Final Cut. Oncogene 2008, 27, 6452–6461. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, Y. Chromosomal DNA Fragmentation in Apoptosis and Necrosis Induced by Oxidative Stress. Biochem. Pharmacol. 2003, 66, 1527–1535. [Google Scholar] [CrossRef]
- Kitazumi, I.; Tsukahara, M. Regulation of DNA Fragmentation: The Role of Caspases and Phosphorylation. FEBS J. 2011, 278, 427–441. [Google Scholar] [CrossRef]
- Limonta, P.; Moretti, R.M.; Marzagalli, M.; Fontana, F.; Raimondi, M.; Marelli, M.M. Role of Endoplasmic Reticulum Stress in the Anticancer Activity of Natural Compounds. Int. J. Mol. Sci. 2019, 20, 961. [Google Scholar] [CrossRef] [Green Version]


| Nutraceuticals | Category | Food Source | Health Benefits |
|---|---|---|---|
| Cyanidine-3-O-glucoside chloride (Kuromanin chloride) | Flavonoids (Anthocyanin) | Berries such as blackberry, gooseberry, red raspberry, etc. Vegetables such as black olive, red lettuce, black beans | Cancer, inflammation, oxidative stress, and cardiovascular diseases |
| Hydroxycitric acid (HCA) | Organic acids | Fruit rinds of Garcinia; calyxes of hibiscus (used as a herbal tea | Weight loss, cancer |
| Protocatechuic acid (PCA) | Polyphenols | Olives, white wine grapes, calamondin citrus fruit | Cancer, inflammation, oxidative stress, and cardiovascular diseases |
| Quercetin | Flavonols | Red onions, kale, broccoli, berries, cherries, grapes, and citrus fruit | Diabetes, cancer, inflammation, aging, etc. |
| Naringenin | Citrus flavanones | Citrus fruits such as blood oranges, sour oranges, grapefruits, limes, mandarins, etc. | Cancer and cardiovascular diseases |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verrelli, D.; Dallera, L.; Stendardo, M.; Monzani, S.; Pasqualato, S.; Giorgio, M.; Pallavi, R. Hydroxycitric Acid Inhibits Chronic Myelogenous Leukemia Growth through Activation of AMPK and mTOR Pathway. Nutrients 2022, 14, 2669. https://doi.org/10.3390/nu14132669
Verrelli D, Dallera L, Stendardo M, Monzani S, Pasqualato S, Giorgio M, Pallavi R. Hydroxycitric Acid Inhibits Chronic Myelogenous Leukemia Growth through Activation of AMPK and mTOR Pathway. Nutrients. 2022; 14(13):2669. https://doi.org/10.3390/nu14132669
Chicago/Turabian StyleVerrelli, Doriana, Luca Dallera, Massimo Stendardo, Silvia Monzani, Sebastiano Pasqualato, Marco Giorgio, and Rani Pallavi. 2022. "Hydroxycitric Acid Inhibits Chronic Myelogenous Leukemia Growth through Activation of AMPK and mTOR Pathway" Nutrients 14, no. 13: 2669. https://doi.org/10.3390/nu14132669
APA StyleVerrelli, D., Dallera, L., Stendardo, M., Monzani, S., Pasqualato, S., Giorgio, M., & Pallavi, R. (2022). Hydroxycitric Acid Inhibits Chronic Myelogenous Leukemia Growth through Activation of AMPK and mTOR Pathway. Nutrients, 14(13), 2669. https://doi.org/10.3390/nu14132669







