Exercise: A Possibly Effective Way to Improve Vitamin D Nutritional Status
Abstract
1. Introduction
2. Endurance Exercise and Vitamin D
2.1. The Effect of Acute Endurance Exercise
2.1.1. Human Studies
2.1.2. Animal Studies
2.2. The Effect of Chronic Endurance Exercise Training
2.2.1. Human Studies
2.2.2. Animal Studies
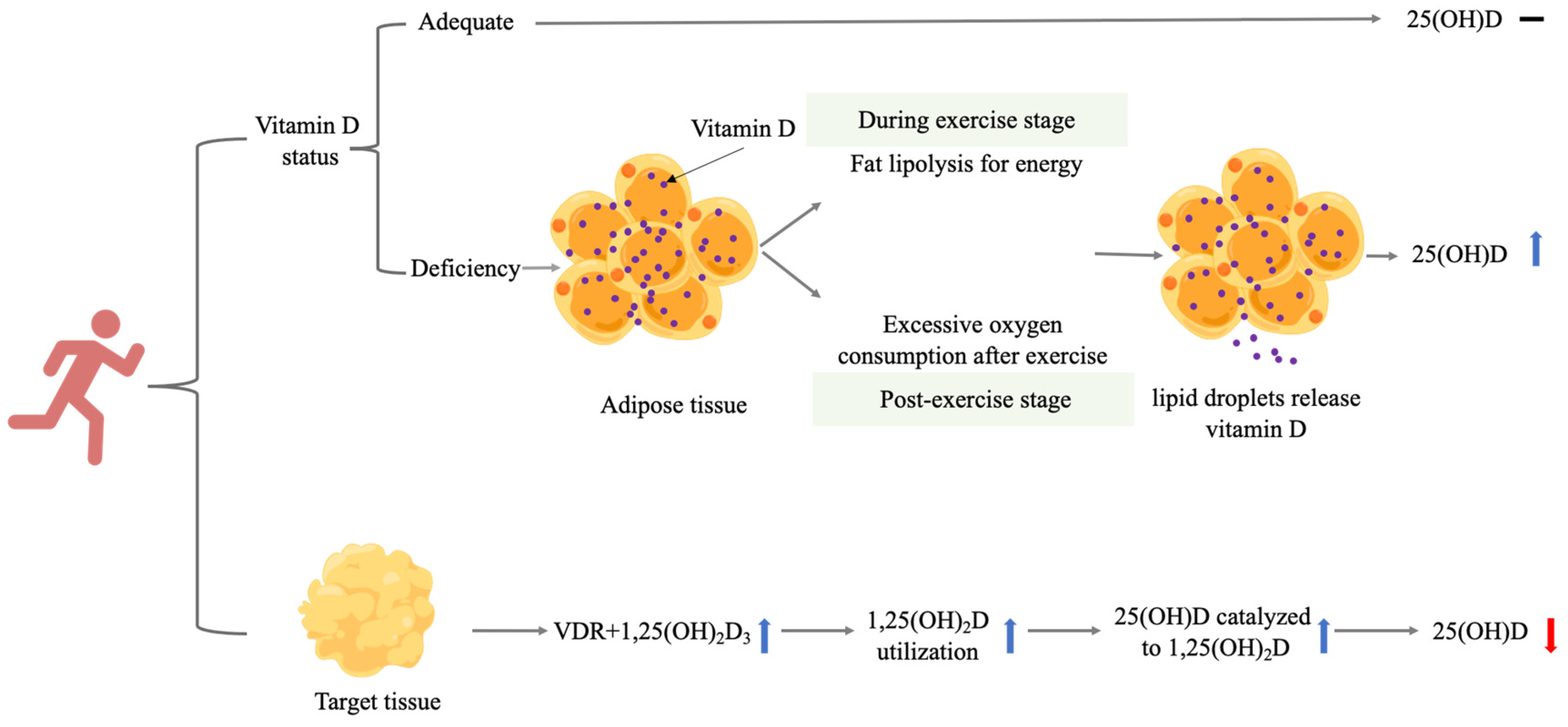
2.3. Mechanism
3. Resistance Exercise
3.1. The Effect of Acute Resistance Exercise
Human and Animal Studies
3.2. The Effect of Chronic Resistance Exercise Training
3.2.1. Human Studies
3.2.2. Animal Studies
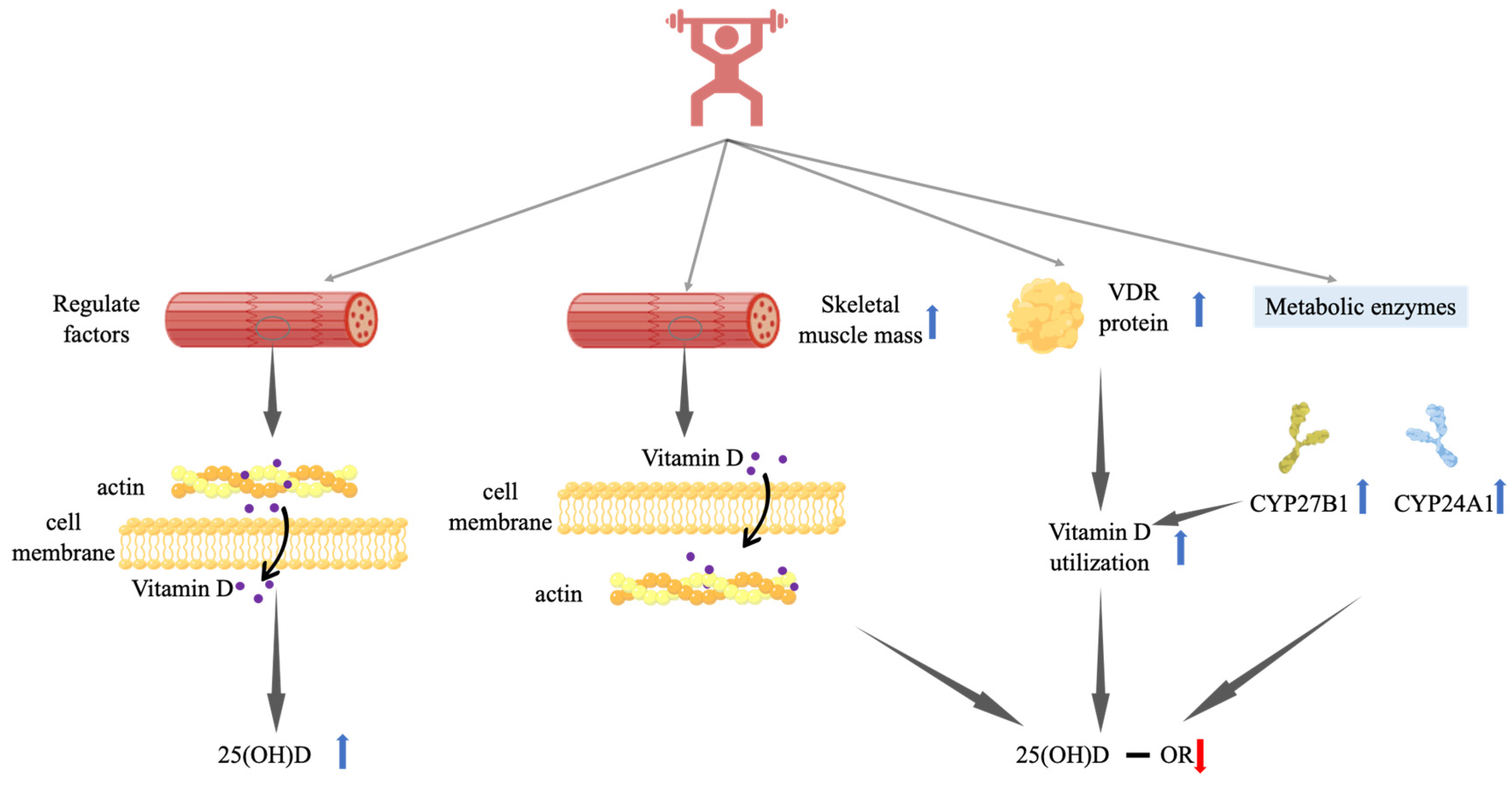
3.3. Mechanisms
4. Others
5. Limitations and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maddaloni, E.; Cavallari, I.; Napoli, N.; Conte, C. Vitamin D and diabetes mellitus. Front. Horm. Res. 2018, 50, 161–176. [Google Scholar]
- Sun, X.; Cao, Z.B.; Tanisawa, K.; Ito, T.; Oshima, S.; Higuchi, M. Vitamin D supplementation reduces insulin resistance in Japanese adults: A secondary analysis of a double-blind, randomized, placebo-controlled trial. Nutr. Res. 2016, 36, 1121–1129. [Google Scholar] [CrossRef]
- Yan, C.L. Molecular mechanism of vitamin D in the cardiovascular system. J. Investig. Med. 2015, 59, 868–871. [Google Scholar]
- Artaza-Artabe, I.; Sáez-López, P.; Sánchez-Hernández, N.; Fernández-Gutierrez, N.; Malafarina, V. The relationship between nutrition and frailty: Effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas 2016, 93, 89–99. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Spira, A.; Holick, M.F. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: A randomized double-blind clinical trial. PLoS ONE 2013, 8, e58725. [Google Scholar] [CrossRef]
- Rosas-Peralta, M.; Holick, M.F.; Borrayo-Sanchez, G.; Madrid-Miller, A.; Ramirez-Arias, E.; Arizmendi-Uribe, E. Dysfunctional immunometabolic effects of vitamin D deficiency, increased cardiometabolic risk. Potential epidemiological alert in America? Endocrinol. Diabetes Nutr. 2017, 64, 162–173. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid. Biochem. Mol. Biol. 2014, 144PA, 138–145. [Google Scholar] [CrossRef]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef]
- Han, B.; Wang, X.; Wang, N.; Li, Q.; Chen, Y.; Zhu, C.; Chen, Y.; Xia, F.; Pu, X.; Cang, Z.; et al. Investigation of vitamin D status and its correlation with insulin resistance in a Chinese population. Public Health Nutr. 2017, 20, 1602–1608. [Google Scholar] [CrossRef][Green Version]
- Prasad, P.; Kochhar, A. Interplay of vitamin D and metabolic syndrome: A review. Diabetes Metab. Syndr. 2016, 10, 105–112. [Google Scholar] [CrossRef]
- Poskitt, E.M.; Cole, T.J.; Lawson, D.E. Diet, sunlight, and 25-hydroxy vitamin D in healthy children and adults. Br. Med. J. 1979, 1, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.E.; Paul, A.A.; Black, A.E.; Cole, T.J.; Mandal, A.R.; Davie, M. Relative contributions of diet and sunlight to vitamin D state in the elderly. Br. Med. J. 1979, 2, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Philipsen, P.A.; Olsen, P.; Bogh, M.K.; Johansen, P.; Schmedes, A.V.; Morling, N.; Wulf, H.C. The half-life of 25(OH)D after UVB exposure depends on gender and vitamin D receptor polymorphism but mainly on the start level. Photochem. Photobiol. Sci. 2017, 16, 985–995. [Google Scholar] [CrossRef]
- Bikle, D.D.; Gee, E.; Halloran, B.; Kowalski, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Kiourtzidis, M.; Kühn, J.; Brandsch, C.; Baur, A.C.; Wensch-Dorendorf, M.; Stangl, G.I. Markers indicating body vitamin D stores and responses of liver and adipose tissues to changes in vitamin D intake in male mice. Nutrients 2020, 12, 1391. [Google Scholar] [CrossRef]
- Zhang, R.H.; He, D.H.; Zhou, B.; Zhu, Y.B.; Zhao, D.; Huang, L.C.; Ding, G.Q. Analysis of vitamin D status in men highly exposed to sunlight. Biomed. Environ. Sci. 2015, 28, 913–916. [Google Scholar]
- Leffell, D.J.; Brash, D.E. Sunlight and skin cancer. Sci. Am. 1996, 275, 52–53, 56–59. [Google Scholar] [CrossRef]
- Webb, A.R.; DeCosta, B.R.; Holick, M.F. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J. Clin. Endocrinol. Metab. 1989, 68, 882–887. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef]
- Webb, A.R. Who, what, where and when-influences on cutaneous vitamin D synthesis. Prog. Biophys Mol. Biol. 2006, 92, 17–25. [Google Scholar] [CrossRef]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Ritz, C.; Kiely, M.; Odin, C. Improved dietary guidelines for vitamin D: Application of individual participant aata (IPD)-level meta-regression analyses. Nutrients 2017, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Chinese Nutrition Society. Dietary Guidelines for Chinese Residents 2016; People’s Medical Publishing House: Beijing, China, 2016; pp. 201–202. (In Chinese) [Google Scholar]
- Razzaque, M.S. Can adverse effects of excessive vitamin D supplementation occur without developing hypervitaminosis D? J. Steroid. Biochem. Mol. Biol. 2018, 180, 81–86. [Google Scholar] [CrossRef]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Palaniswamy, S.; Hyppönen, E.; Williams, D.M.; Jokelainen, J.; Lowry, E.; Keinänen-Kiukaanniemi, S.; Herzig, K.H.; Järvelin, M.R.; Sebert, S. Potential determinants of vitamin D in Finnish adults: A cross-sectional study from the Northern Finland birth cohort 1966. BMJ Open 2017, 7, e013161. [Google Scholar] [CrossRef] [PubMed]
- Wanner, M.; Richard, A.; Martin, B.; Linseisen, J.; Rohrmann, S. Associations between objective and self-reported physical activity and vitamin D serum levels in the US population. Cancer Causes Control 2015, 26, 881–891. [Google Scholar] [CrossRef]
- Touvier, M.; Deschasaux, M.; Montourcy, M.; Sutton, A.; Charnaux, N.; Kesse-Guyot, E.; Assmann, K.E.; Fezeu, L.; Latino-Martel, P.; Druesne-Pecollo, N.; et al. Determinants of vitamin D status in Caucasian adults: Influence of sun exposure, dietary intake, sociodemographic, lifestyle, anthropometric, and genetic factors. J. Investig. Derm. 2015, 135, 378–388. [Google Scholar] [CrossRef]
- Scragg, R.; Holdaway, I.; Jackson, R.; Lim, T. Plasma 25-hydroxyvitamin D3 and its relation to physical activity and other heart disease risk factors in the general population. Ann. Epidemiol. 1992, 2, 697–703. [Google Scholar] [CrossRef]
- Scragg, R.; Holdaway, I.; Singh, V.; Metcalf, P.; Baker, J.; Dryson, E. Serum 25-hydroxyvitamin D3 is related to physical activity and ethnicity but not obesity in a multicultural workforce. Aust. N. Z. J. Med. 1995, 25, 218–223. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, K.; Cao, Z. Effects of physical activity on vitamin D: A systematic review and meta-analysis of observational and experimental studies. J. Shanghai Univ. Sport 2021, 45, 81–96. (In Chinese) [Google Scholar]
- Mieszkowski, J.; Stankiewicz, B.; Kochanowicz, A.; Niespodziński, B.; Kowalik, T.; Żmijewski, M.; Kowalski, K.; Rola, R.; Bieńkowski, T.; Antosiewicz, J. Ultra-marathon-induced increase in serum levels of vitamin D metabolites: A double-blind randomized controlled trial. Nutrients 2020, 12, 3629. [Google Scholar] [CrossRef]
- Dzik, K.P.; Grzywacz, T.; Łuszczyk, M.; Kujach, S.; Flis, D.J.; Kaczor, J.J. Single bout of exercise triggers the increase of vitamin D blood concentration in adolescent trained boys: A pilot study. Sci. Rep. 2022, 12, 1825. [Google Scholar] [CrossRef]
- Maimoun, L.; Manetta, J.; Couret, I.; Dupuy, A.M.; Mariano-Goulart, D.; Micallef, J.P.; Peruchon, E.; Rossi, M. The intensity level of physical exercise and the bone metabolism response. Int. J. Sports Med. 2006, 27, 105–111. [Google Scholar] [CrossRef]
- Maimoun, L.; Simar, D.; Caillaud, C.; Coste, O.; Barbotte, E.; Peruchon, E.; Rossi, M.; Mariano-Goulart, D. Response of calciotropic hormones and bone turnover to brisk walking according to age and fitness level. J. Sci. Med. Sport 2009, 12, 463–467. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.B.; Taniguchi, H.; Tanisawa, K.; Higuchi, M. Effect of an acute bout of endurance exercise on serum 25(OH)D concentrations in young adults. J. Clin. Endocrinol. Metab. 2017, 102, 3937–3944. [Google Scholar] [CrossRef]
- Makanae, Y.; Ogasawara, R.; Sato, K.; Takamura, Y.; Matsutani, K.; Kido, K.; Shiozawa, N.; Nakazato, K.; Fujita, S. Acute bout of resistance exercise increases vitamin D receptor protein expression in rat skeletal muscle. Exp. Physiol. 2015, 100, 1168–1176. [Google Scholar] [CrossRef]
- Puangthong, C.; Sukhong, P.; Saengnual, P.; Srikuea, R.; Chanda, M. A single bout of high-intensity exercise modulates the expression of vitamin D receptor and vitamin D-metabolising enzymes in horse skeletal muscle. Equine Vet. J. 2021, 53, 796–805. [Google Scholar] [CrossRef]
- Farag, H.A.M.; Hosseinzadeh-Attar, M.J.; Muhammad, B.A.; Esmaillzadeh, A.; el Bilbeisi, A.H. Effects of vitamin D supplementation along with endurance physical activity on lipid profile in metabolic syndrome patients: A randomized controlled trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1093–1098. [Google Scholar] [CrossRef]
- Mieszkowski, J.; Niespodzinski, B.; Kochanowicz, A.; Gmiat, A.; Prusik, K.; Prusik, K.; Kortas, J.; Ziemann, E.; Antosiewicz, J. The effect of nordic walking training combined with vitamin D supplementation on postural control and muscle strength in elderly people-a randomized controlled trial. Int. J. Env. Res. Public Health 2018, 15, 1951. [Google Scholar] [CrossRef]
- Prusik, K.; Kortas, J.; Prusik, K.; Mieszkowski, J.; Jaworska, J.; Skrobot, W.; Lipinski, M.; Ziemann, E.; Antosiewicz, J. Nordic walking training causes a decrease in blood cholesterol in elderly women supplemented with vitamin D. Front. Endocrinol. 2018, 9, 42. [Google Scholar] [CrossRef]
- Malandish, A.; Tartibian, B.; Sheikhlou, Z.; Afsargharehbagh, R.; Rahmati, M. The effects of short-term moderate intensity aerobic exercise and long-term detraining on electrocardiogram indices and cardiac biomarkers in postmenopausal women. J. Electrocardiol. 2020, 60, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, R.; Xu, l.; Chen, X. Effect of high intensity interval training on rehabilitation of elderly chronic obstructive pulmonary disease patients with osteoporosis. Chin. J. Front. Med. Sci. 2019, 11, 30–34. (In Chinese) [Google Scholar]
- Song, L.; Xuan, Y.; Yang, J.; Xuan, M.; Wang, Y.; Song, L.; Wang, W.; Zhang, X. The association among the management of diet and sport, glucose metabolism, bone metabolism, and bone mineral density in postmenopausal women with type II diabetes and osteoporosis: A clinical study. Chin. J. Front. Med. Sci. 2014, 20, 156–160. (In Chinese) [Google Scholar]
- Shi, D.; Shi, X.; Li, F.; Ren, J.; Gu, L. Clinical effect of exercise therapy on the patients with osteoporosis. Chin. J. Geriatr. 2013, 32, 872–874. (In Chinese) [Google Scholar]
- Klausen, T.; Breum, L.; Sørensen, H.A.; Schifter, S.; Sonne, B. Plasma levels of parathyroid hormone, vitamin D, calcitonin, and calcium in association with endurance exercise. Calcif. Tissue Int. 1993, 52, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Pilch, W.; Tyka, A.; Cebula, A.; Sliwicka, E.; Pilaczynska-Szczesniak, L.; Tyka, A. Effects of a 6-week Nordic walking training on changes in 25(OH)D blood concentration in women aged over 55. J. Sports Med. Phys. Fit. 2017, 57, 124–129. [Google Scholar] [CrossRef]
- Lithgow, H.M.; Florida-James, G.; Leggate, M. The combined effect of high-intensity intermittent training and vitamin D supplementation on glycemic control in overweight and obese adults. Physiol. Rep. 2018, 6, e13684. [Google Scholar] [CrossRef]
- Hossain, M.J.; Levinson, A.; George, D.; Canas, J.; Kumar, S.; Balagopal, P.B. Vitamin D status and cardiovascular risk in obesity: Effect of physical activity in nonvitamin D supplemented adolescents. Metab. Syndr. Relat. Disord. 2018, 16, 197–203. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.B.; Tanisawa, K.; Taniguchi, H.; Kubo, T.; Higuchi, M. Effects of chronic endurance exercise training on serum 25(OH)D concentrations in elderly Japanese men. Endocrine 2018, 59, 330–337. [Google Scholar] [CrossRef]
- Aly, Y.E.; Abdou, A.S.; Rashad, M.M.; Nassef, M.M. Effect of exercise on serum vitamin D and tissue vitamin D receptors in experimentally induced type 2 Diabetes Mellitus. J. Adv. Res. 2016, 7, 671–679. [Google Scholar] [CrossRef]
- Buskermolen, J.; van der Meijden, K.; Furrer, R.; Mons, D.J.; van Essen, H.W.; Heijboer, A.C.; Lips, P.; Jaspers, R.T.; Bravenboer, N. Effects of different training modalities on phosphate homeostasis and local vitamin D metabolism in rat bone. PeerJ 2019, 24, e6184. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.K.; Aloia, J.F.; Yasumura, S. Effect of physical activity on calcium and phosphorus metabolism in the rat. Am. J. Physiol. 1989, 256, E1–E6. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, J.; Shimamura, C.; Takeda, T.; Abe, H.; Ichimura, S.; Sato, Y.; Toyama, Y. Effects of treadmill exercise on bone mass, bone metabolism, and calciotropic hormones in young growing rats. J. Bone Min. Metab. 2004, 22, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hou, J. Effects of chronic aerobic exercise training on expression of vitamin D receptor in bone tissue of aged rats. J. Xi’An Technol. Univ. 2018, 38, 318–323. (In Chinese) [Google Scholar]
- Xu, S.; Li, S.; Chen, X. The effect of exercise on the expression of FGF23—klotho/FGFR1 axis and related factors of male mice. Chin. J. Sports Med. 2019, 38, 882–889. (In Chinese) [Google Scholar]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Fleet, J.C.; Gliniak, C.; Zhang, Z.; Xue, Y.; Smith, K.B.; McCreedy, R.; Adedokun, S.A. Serum metabolite profiles and target tissue gene expression define the effect of cholecalciferol intake on calcium metabolism in rats and mice. J. Nutr. 2008, 138, 1114–1120. [Google Scholar] [CrossRef]
- Lee, G.Y.; Park, C.Y.; Cha, K.S.; Lee, S.E.; Pae, M.; Han, S.N. Differential effect of dietary vitamin D supplementation on natural killer cell activity in lean and obese mice. J. Nutr. Biochem. 2018, 55, 178–184. [Google Scholar] [CrossRef]
- Maia-Ceciliano, T.C.; Dutra, R.R.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. The deficiency and the supplementation of vitamin D and liver: Lessons of chronic fructose-rich diet in mice. J. Steroid. Biochem. Mol. Biol. 2019, 192, 105399. [Google Scholar] [CrossRef]
- Evans, P.L.; McMillin, S.L.; Weyrauch, L.A.; Witczak, C.A. Regulation of skeletal muscle glucose transport and glucose metabolism by exercise training. Nutrients 2019, 11, 2432. [Google Scholar] [CrossRef]
- Scott, K.; Edward, T. Exercise Physiology: Theory and Application to Fitness and Performance Tenth Edition; McGraw-Hill Education: New York, NY, USA, 2019; pp. 302–320. [Google Scholar]
- Heaney, R.P.; Horst, R.L.; Cullen, D.M.; Armas, L.A. Vitamin D3 distribution and status in the body. J. Am. Coll. Nutr. 2009, 28, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Hengist, A.; Perkin, O.; Gonzalez, J.T.; Betts, J.A.; Hewison, M.; Manolopoulos, K.N.; Jones, K.S.; Koulman, A.; Thompson, D. Mobilising vitamin D from adipose tissue: The potential impact of exercise. Nutr. Bull. 2019, 44, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Lafontan, M.; Moro, C.; Berlan, M.; Crampes, F.; Sengenes, C.; Galitzky, J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol. Metab. 2008, 19, 130–137. [Google Scholar] [CrossRef] [PubMed]
- de Glisezinski, I.; Larrouy, D.; Bajzova, M.; Koppo, K.; Polak, J.; Berlan, M.; Bulow, J.; Langin, D.; Marques, M.A.; Crampes, F.; et al. Adrenaline but not noradrenaline is a determinant of exercise-induced lipid mobilization in human subcutaneous adipose tissue. J. Physiol. 2009, 587, 3393–3404. [Google Scholar] [CrossRef]
- Panissa, V.L.G.; Fukuda, D.H.; Staibano, V.; Marques, M.; Franchini, E. Magnitude and duration of excess of post-exercise oxygen consumption between high-intensity interval and moderate-intensity continuous exercise: A systematic review. Obes. Rev. 2021, 22, e13099. [Google Scholar] [CrossRef]
- Newsom, S.A.; Schenk, S.; Thomas, K.M.; Harber, M.P.; Knuth, N.D.; Goldenberg, N.; Horowitz, J.F. Energy deficit after exercise augments lipid mobilization but does not contribute to the exercise-induced increase in insulin sensitivity. J. Appl. Physiol. 1985 2010, 108, 554–560. [Google Scholar] [CrossRef]
- Abboud, M.; Rybchyn, M.S.; Liu, J.; Ning, Y.; Gordon-Thomson, C.; Brennan-Speranza, T.C.; Cole, L.; Greenfield, H.; Fraser, D.R.; Mason, R.S. The effect of parathyroid hormone on the uptake and retention of 25-hydroxyvitamin D in skeletal muscle cells. J. Steroid. Biochem. Mol. Biol. 2017, 173, 173–179. [Google Scholar] [CrossRef]
- Blau, J.E.; Collins, M.T. The PTH-vitamin D-FGF23 axis. Rev. Endocr. Metab. Disord. 2015, 16, 165–174. [Google Scholar] [CrossRef]
- Kägi, L.; Bettoni, C.; Pastor-Arroyo, E.M.; Schnitzbauer, U.; Hernando, N.; Wagner, C.A. Regulation of vitamin D metabolizing enzymes in murine renal and extrarenal tissues by dietary phosphate, FGF23, and 1,25(OH)2D3. PLoS ONE 2018, 13, e0195427. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Liu, S.; Tang, W.; Zhou, J.; Stubbs, J.R.; Luo, Q.; Pi, M.; Quarles, L.D. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 2006, 17, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.; Henriksen, V.T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Schneider, E.D.; Dixon, B.M.; Weaver, L.K. Higher serum 25-hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Nutrients 2013, 5, 1253–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Mao, H.; Song, H.; Chen, Y. Effects of lower extremity intensive weight-bearing exercise training on osteoporosis in patients with post-stroke hemiplegia. Chin. J. Gerontol. 2017, 37, 5382–5383. (In Chinese) [Google Scholar]
- Bass, J.J.; Nakhuda, A.; Deane, C.S.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; Kadi, F.; Andersen, D.; et al. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Mol. Metab. 2020, 42, 101059. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, X.-K.; Zhang, L.; Cao, Z.-B. Effects of resistance training on serum 25(OH)D concentrations in young men: A randomized controlled trial. Nutr. Metab. 2020, 17, 1–7. [Google Scholar] [CrossRef]
- Aschauer, R.; Unterberger, S.; Zöhrer, P.A.; Draxler, A.; Franzke, B.; Strasser, E.M.; Wagner, K.H.; Wessner, B. Effects of vitamin D3 supplementation and resistance training on 25-hydroxyvitamin D status and functional performance of older adults: A randomized placebo-controlled trial. Nutrients 2021, 14, 86. [Google Scholar] [CrossRef]
- Agergaard, J.; Trostrup, J.; Uth, J.; Iversen, J.V.; Boesen, A.; Andersen, J.L.; Schjerling, P.; Langberg, H. Does vitamin-D intake during resistance training improve the skeletal muscle hypertrophic and strength response in young and elderly men?—A randomized controlled trial. Nutr. Metab. 2015, 12, 32. [Google Scholar] [CrossRef]
- Mason, R.S.; Rybchyn, M.S.; Abboud, M.; Brennan-Speranza, T.C.; Fraser, D.R. The role of skeletal muscle in maintaining vitamin D status in winter. Curr. Dev. Nutr. 2019, 3, nzz087. [Google Scholar] [CrossRef]
- Abboud, M.; Puglisi, D.A.; Davies, B.N.; Rybchyn, M.; Whitehead, N.P.; Brock, K.E.; Cole, L.; Gordon-Thomson, C.; Fraser, D.R.; Mason, R.S. Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology 2013, 154, 3022–3030. [Google Scholar] [CrossRef]
- Abboud, M.; Gordon-Thomson, C.; Hoy, A.J.; Balaban, S.; Rybchyn, M.S.; Cole, L.; Su, Y.; Brennan-Speranza, T.C.; Fraser, D.R.; Mason, R.S. Uptake of 25-hydroxyvitamin D by muscle and fat cells. J. Steroid. Biochem. Mol. Biol. 2013, 144 Pt A, 232–236. [Google Scholar] [CrossRef]
- Abboud, M.; Rybchyn, M.S.; Ning, Y.J.; Brennan-Speranza, T.C.; Girgis, C.M.; Gunton, J.E.; Fraser, D.R.; Mason, R.S. 1,25-Dihydroxycholecalciferol (calcitriol) modifies uptake and release of 25-hydroxycholecalciferol in skeletal muscle cells in culture. J. Steroid. Biochem. Mol. Biol. 2018, 177, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Mokbel, N.; Cha, K.M.; Houweling, P.J.; Abboud, M.; Fraser, D.R.; Mason, R.S.; Clifton-Bligh, R.J.; Gunton, J.E. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 2014, 155, 3227–3237. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.K.; Romundstad, P.R.; Stafne, S.N.; Helvik, A.S.; Stunes, A.K.; Morkved, S.; Salvesen, K.A.; Thorsby, P.M.; Mosti, M.P.; Syversen, U. The effect of an exercise program in pregnancy on vitamin D status among healthy, pregnant Norwegian women: A randomized controlled trial. BMC Pregnancy Childbirth 2019, 19, 76. [Google Scholar] [CrossRef]
- Li, R.; Yang, Z.; Han, W.; Tian, X.; Li, Q.; Shuai, S.; Liu, Y. Effects of combined exercise therapy on bone metabolism in patients with postmenopausal osteoporosis. Int. J. Orthop. 2019, 40, 52–62. (In Chinese) [Google Scholar]
- Evans, R.K.; Antczak, A.J.; Lester, M.; Yanovich, R.; Israeli, E.; Moran, D.S. Effects of a 4-month recruit training program on markers of bone metabolism. Med. Sci. Sports Exerc. 2008, 40, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Drincic, A.T.; Armas, L.A.; Van Diest, E.E.; Heaney, R.P. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012, 20, 1444–1448. [Google Scholar] [CrossRef]
- Jungert, A.; Neuhäuser-Berthold, M. Sex-specific determinants of serum 25-hydroxyvitamin D3 concentrations in an elderly German cohort: A cross-sectional study. Nutr. Metab. 2015, 12, 1–9. [Google Scholar] [CrossRef]
- Fiamenghi, V.I.; Mello, E.D. Vitamin D deficiency in children and adolescents with obesity: A meta-analysis. J. Pediatr. 2021, 97, 273–279. [Google Scholar] [CrossRef]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef]
- Di Nisio, A.; De Toni, L.; Sabovic, I.; Rocca, M.S.; De Filippis, V.; Opocher, G.; Azzena, B.; Vettor, R.; Plebani, M.; Foresta, C. Impaired release of vitamin D in dysfunctional adipose tissue: New cues on vitamin D supplementation in obesity. J. Clin. Endocrinol. Metab. 2017, 102, 2564–2574. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, L.; Hachemi, M.A.; Karkeni, E.; Couturier, C.; Astier, J.; Defoort, C.; Svilar, L.; Martin, J.C.; Tourniaire, F.; Landrier, J.F. Diet induced obesity modifies vitamin D metabolism and adipose tissue storage in mice. J. Steroid. Biochem. Mol. Biol. 2019, 185, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.F.; Costa, R.R.; Macedo, R.C.; Coconcelli, L.; Kruel, L.F. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: A systematic review and meta-analysis. Br. J. Nutr. 2016, 116, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Aird, T.P.; Davies, R.W.; Carson, B.P. Effects of fasted vs fed-state exercise on performance and post-exercise metabolism: A systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2018, 28, 1476–1493. [Google Scholar] [CrossRef] [PubMed]

| Study | Participants/Animal, n | Endurance Exercise Intervention | Sunlight Exposure | Main Findings |
|---|---|---|---|---|
| Acute endurance exercise intervention-human studies | ||||
| Mieszkowski (2020) [32] | Experimental, n = 13, 42.00 ± 8.44 years old, Ultra-Marathon Race, 150,000 vitamin D3; Control, n = 14, 40.00 ± 8.11 years old, Ultra-Marathon Race, placebo solution | Ultra-Marathon Race | 18:00 h, 19 July; most of the time, the sky was overcast | 25(OH)D3: significantly increased immediately and 24 h after the ultra-marathon vs. 24 h before the ultra-marathon in both groups |
| Dzik (2022) [33] | Male soccer players, n = 12 (pre-pubertal, n = 5; pubertal, n = 7) | VO2max test | - | 25(OH)D3: significantly increased at 15 min and 1 h after exercise vs. before; increased 15 min after the VO2max test and dropped one hour after exercise, but not significantly different. |
| Maimoun (2006) [34] | Male competitive road cyclists, n = 7, 20–30 years old | 47% Wmax; 64% Wmax | - | 25(OH)D: no change 1,25(OH)2D: no change |
| Maimoun (2009) [35] | Elderly moderately active (ModEl, n = 18), 71.9 ± 7.3 years old; Elderly active (HAcEl; n = 18), 71.7 ± 8.6 years old; Young active (AcYo; n = 9), 25.8 ± 2.3 years old | maximal incremental exercise | - | 25(OH)D: significantly increased in HAcEl, but not in ModEl and AcYo 1,25(OH)2D: no change |
| Sun (2017) [36] | Healthy young men, n = 10, 18–22 years old; Healthy young women, n = 10, 19–22 years old | cycling exercise for 30 min at 70% VO2max | at the laboratory | 25(OH)D: significantly greater at 0 h, 1 h, 3 h and 24 h after exercise vs. before exercise; subgroup analysis: significantly increased at 24 h after exercise in women only 1,25(OH)2D: no change |
| Acute endurance exercise intervention: animal studies | ||||
| Makanae (2015) [37] | Adult male Sprague–Dawley rats, 10 weeks age | 60 min, 25 m/min | at the laboratory | 25(OH)D3: no change |
| Puangthong (2021) [38] | Healthy ponies, n = 6 (5 geldings, 1 mare), 6.3 ± 2.2 years age | 77–93% of HRmax, 16.5 ± 1 min, 5.2 ± 0.3 km | at the laboratory | 25(OH)D2: significantly reduced at 30 min, 1 week, and 3 weeks after high-intensity exercise |
| Chronic endurance exercise intervention-human studies | ||||
| Farag (2019) [39] | Vitamin D plus PA group: n = 21, 40.42 ± 5.89 years old, 2000IU/day, endurance PA | Endurance PA: 12 weeks, daily endurance PA, 30 min/day | Either at morning, 7:30 a.m. or afternoon after 3:00 p.m. | 25(OH)D: significantly increased |
| Mieszkowski (2018) [40] | High-intensity interval training group (HI-NW): LD (n = 8, 67.37 ± 6.30 years old, 800 IU/day vitamin D3), and HD (n = 8, 67.63 ± 7.29 years old, 4000 IU/day vitamin D3); Moderate-inteensity continuous training group (MI-NW): LD (n = 13, 69.08 ± 4.87 years old, 800 IU/day vitamin D3) and HD (n = 13, 70.85 ± 4.61 years old, 4000 IU/day vitamin D3) | Nordic walking training: 12 weeks, two hours, three times a week. HI-NW: 30 s acceleration going uphill,60 s release going downhill for eight time; 70% HRmax for 28 min. MI-NW: 60–70 HRmax for 40 min | morning hours | 25(OH)D3: significantly increased in HI-NW with LD and HD group and MI-NW with HD group; no change in MI-NW with LD group. |
| Prusik (2018) [41] | Experimental group (EG), n = 35, 68.4 ± 5.0 years old | EG: Nordic walking training, 12 weeks, three times a week, 60–70% HRmax for 45–55 min; 4000 IU/day vitamin D supplement | 1 h after breakfast | 25(OH)D3: significantly increased after 12 weeks of Nordic walking training with vitamin D supplementation; no change after 6 months without training and vitamin D supplementation |
| Malandish (2020) [42] | Postmenopausal women Exercise group (EX), n = 13, 53.36 ± 3.98 years old; Control group (C), n = 13, 53.00 ± 3.26 years old | EX: 12 weeks training, 3 sessions per week, 55–60 min per session, 40 min of walking or jogging aerobic exercise on treadmill C: no intervention | - | 25(OH)D: significantly increased after exercise vs. before exercise in EX group and compared to C group; no change in C group |
| Li (2019) [43] | elderly chronic obstructive pulmonary disease patients with osteoporosis, 65–82 years old Experimental group, n = 31; Control group, n = 31 | Experimental group: 12 weeks, 4 times/week, 5 set/session, 5 min/set, 5 min between sets, 75% CPET, 25 min/session. Control group: 12 weeks, 4 times/week, 5 set/session, 5 min/set, 5 min between sets, 50% CPET, 25 min/session. | - | 25(OH)D: significantly increased after exercise in experimental group and control group; significantly increased after exercise in experimental group vs. control group after exercise intervention |
| Song (2014) [44] | postmenopausal women with type II diabetes and osteoporosis Experimental group: n = 278, 52.82 ± 5.12 years old; Control group: n = 284, 53.26 ± 5.12 years old | Experimental group: 48 weeks, moderate intensity, 20–30 min/time, two times/day, 0.25 ug/day Calcitriol and 600 mg vitamin D supplementation Control group: 0.25 ug/day Calcitriol and 600 mg vitamin D supplementation | - | 25(OH)D: significantly increased 24 weeks and 48 weeks after exercise vs. before exercise in experimental group and higher than control group at same time points |
| Shi (2013) [45] | Patients with osteoporosis, 50–89 years old, n = 82 exercise group (n = 40); control group (n = 42) | exercise group: Wu xing Bone gymnastics, 90 days, 30–45 min/time, two times/day control group: calcium and Calcitriol supplementation | - | 25(OH)D: significantly increased after exercise intervention vs. before exercise intervention in exercise group; no change in control group |
| Klausen (1993) [46] | Male marathon runners, n = 9, 41–50 years old | Endurance training: median running distance was 61 km per week, 4 weeks | the months of December and January | 25(OH)D3: no change at 2 week and 4 week retraining. 1,25(OH)2D3: significantly reduced at 4 week retraining vs. before retraining |
| Pilch (2017) [47] | Women, n = 17, 57 ± 4.20 years old | Nordic walking training, 6 weeks, three times a week, 90 min/time, 60–70% HRmax. | morning hours | 25(OH)D: significantly reduced after exercise intervention |
| Lithgow (2018) [48] | Overweight and obese adults Placebo group: n = 10, 34 ± 10 years old; Vitamin D group: n = 10, 34 ± 9 years old | Placebo group: HIIT intervention, 6 weeks, 3 sessions/week, 10 repetitions of 1 min intervals interspersed with 1 min active recovery at a power output of 50 W. placebo tablets Vitamin D group: HIIT with 4000 IU/day vitamin D3 | - | 25(OH)D3: significantly increased in vitamin D group than placebo group; no change between before and after exercise in placebo group |
| Hossain (2018) [49] | Intervention group: n = 7, 14–18 years old; Control group: n = 7, 14–18 years old | Intervention group: brisk walking, 12 weeks, 45 min/time, three times a week Control group: no change routine lifestyle | - | 25(OH)D: no change in both groups |
| Sun (2018) [50] | The 5-week endurance exercise training group (ET group), n = 10, 66.5–75.3 years old; Sedentary control group (SC group), n = 10, 63.8–73.0 years old | ET group: aerobic exercise, 5 weeks, three times per week, 60% VO2max during week 1, 70% during weeks 2 and 3, and 75% during weeks 4 and 5, 30 min for weeks 1 and 2, and 45 min for weeks 3–5 SC group: no intervention | From October to November | 25(OH)D: significantly reduced after exercise in SC group; no change in ET group |
| Chronic endurance exercise intervention: animal studies | ||||
| Aly (2016) [51] | Adult male albino, Group I(a): control sedentary, n = 15; Group I(b): control exercised, n = 15; Group II(a): diabetic sedentary, n = 15; Group II(b): diabetic exercised, n = 15 | Group I(b) and Group II(b): swimming moderate exercise, 4 weeks, 60 min/time, 5 time per week Group I(a) and Group II(a): no intervention | at the laboratory | 25(OH)D: significantly increased in Group II(b) vs. Group II(a); no change between Group I(a) and Group I(b) |
| Buskermolen (2019) [52] | Female wistar rat, 13 weeks old Control group, n = 8; Endurance training group (ET), n = 10 | ET: treadmill running, 6 weeks, 10 min at a speed of 16 m/min without a slope, increased up to 45 min with a speed of 26 m/min on a 10% slope Control group: no intervention | at the laboratory | 25(OH)D: no change between ET and control group |
| Yeh (1989) [53] | Female Sprague-Dawley rats, 75 ± 5 g Exercise group; Pair-fed exercise group; control group; | Exercise group and Pair-fed exercise group: flat-bed treadmill running, 13 weeks, 60 min/time, 5 times per week, 18–25 m/min Control group: no intervention | at the laboratory | 25(OH)D: no change in the three groups 1,25(OH)2D3: significantly increased in Exercise group and Pair-fed exercise group vs. control |
| Iwamoto (2004) [54] | Female Wistar rats, 6 weeks old, n = 20 7 weeks of exercise (7EX), n = 5; 7 weeks of sedentary control (7CON), n = 5; 11 weeks of exercise (11EX), n = 5; 11 weeks of sedentary control (11CON), n = 5 | 7EX and 11EX: running on flat-bed treadmill, 7 weeks or 11 weeks, 60 min/time, 5 time a week 7CON and11CON: no intervention | at the laboratory | 1,25(OH)2D3: significantly increased in 7EX vs. 7CON; significantly increased in 11EX than 11CON |
| Wang (2018) [55] | Male F344 rats Sedentary young rats (Young), n = 9; Sedentary aged rats (Aged), n = 9; Aged rats with aerobic exercise training (Aged + EX), n = 9 | Aged + EX: running treadmill, 12 weeks, 7 times per week, 1 h/time, 10% slope, 8–20 m/min Young and Aged group: no intervention | at the laboratory | 1,25(OH)2D3: slightly increased, not significant |
| Xu (2019) [56] | C57BL/6 male mice, 5 weeks old Swimming group (group S), n = 7; Downhill running group (group R), n = 7; Control (group C), n = 7 | group S: swimming training, 8 weeks, 6 times per week, 50 min/time, 65–70%VO2max group R: downhill running, 8 weeks, 6 times per week, 50 min/time, −9% slope, 0.8 km/h group C: no intervention | at the laboratory | 1,25(OH)2D3: significantly reduced in group S and group R vs. group C |
| Study | Participants/Animal, n | Resistance Exercise Intervention | Sunlight Exposure | Main Findings |
|---|---|---|---|---|
| Acute resistance exercise intervention: human study | ||||
| Barker (2013) [74] | Recreationally active subjects Intense-stretch shortening contraction leg (SSC); Control leg (CON) | SSC: 10 sets of 10 jumps with a 20-s rest between each set at 75% of body mass on one leg only CON: no intervention | December to March; at the laboratory | 25(OH)D: significantly increased immediately after acute resistance exercise; decreased after 24, 48, 72, and 168 h |
| Acute resistance exercise intervention: animal study | ||||
| Makanae (2015) [37] | Male Sprague-Dawley, 10 weeks old | Isometrically exercise, five sets of ten 3 s contractions, with a 7 s interval between contractions and 3 min rest intervals between sets | at the laboratory | 25(OH)D3: no change |
| Chronic resistance exercise intervention: human study | ||||
| Zhang (2017) [75] | patients with post-stroke hemiplegia, 59.58 ± 4.39 years old Experimental group, n = 25; Control group, n = 25 | Experimental group: weight-bearing exercise training, one year, 40 min/time, two times/day. Routine rehabilitation. Calcium and calciferol supplement Control group: Routine rehabilitation. Calcium and calciferol supplement | - | 25(OH)D: significantly increased at 3 months and 1 year of intervention in Experimental group vs. before intervention and vs. control group at same time points. |
| Bass (2020) [76] | Male and female healthy participants, n = 37, 48.4 ± 2.6 years old | 20 weeks, three times a week, 70% 1 repetition max, single sets of 12 repetitions with 2-min rests between sets of seated chest press, lat pull down, seated lever row, leg extension, seated leg curl, seated leg press, back extension and abdominal curls | - | 25(OH)D: significantly increased after exercise intervention |
| Sun (2020) [77] | healthy men, n = 18, 19–39 years old resistance training group (RT), n = 9, 24.2 ± 3.1 years old; non-exercise control group (CON), n = 9, 26.7 ± 6.2 years old | RT: progressive resistance training, 12 weeks, 2–3 times per week, resistance workload gradually changed from light to heavy CON: no intervention | From March to July, Between 16:30 h and 20:00 h in a gymnasium | 25(OH)D: significantly increased after 12 weeks of exercise intervention vs. baseline in both groups; significantly higher at 6 weeks compared with the values at baseline in the CON group, whereas no notable differences were found in the RT group |
| Aschauer (2021) [78] | Older adults, n = 85, 65–85 years old Control group (CON), Placebo, 400 mg calcium/day; Vitamin D3 daily group (VDD), 800 IU vitamin D3/day, 400 mg calcium/day; Vitamin D3 monthly group (VDM), 50,000 IU vitamin D3/month, 400 mg calcium/day | Three groups have conducted Resistance training: 10 weeks, twice a week, 60–90 min/session | From mid-February to mid-July | 25(OH)D: no change in CON; significantly increased in both VDD and VDM |
| Agergaard (2015) [79] | Healthy sedentary young and elderly men Young vitamin D group, n = 7, 23.3 ± 2.0 years old; Young placebo group, n = 10, 22.4 ± 1.8 years old; elderly vitamin D group, n = 7, 67.1 ± 2.9 years old ; elderly placebo group, n = 10, 66.6 ± 4.2 years old | Four groups have conducted resistance training exercise: 12 weeks, 3 sessions/week, Progressive loading levels | From November to April | 25(OH)D: significantly reduced at 0, 2, 6, and 12 weeks in young placebo group vs. at −4 weeks; significantly reduced at 0, 6, and 12 weeks in young placebo group vs. at −4 weeks; significantly increased at 0, 2, 6, and 12 weeks in young vitamin D group and elderly vitamin D group vs. at −4 weeks |
| Acute resistance exercise intervention: animal studies | ||||
| Buskermolen (2019) [52] | Female wistar rat, 13 weeks old peak power training (PT), n = 10; Control group, n = 8 | PT: peak power training, 10 sprints of 15 s in gallop at a maximal attainable velocity on a progressively increasing slope starting at 10% reaching up to 40% by the end Control group: no intervention | at the laboratory | 25(OH)D: no change |
| Xu (2019) [56] | C57BL/6 male mice, 5 weeks old Jumping group (group J), n = 7; Control group (group C), n = 7 | Group J: jumping training, 8 weeks, 6 times per week, 6–7 sets/min, 50 min/time Group C: no intervention | at the laboratory | 1,25(OH)2D3: significantly reduced in group J vs. group C |
| Study | Participants/Animal, n | Endurance Exercise Intervention | Sunlight Exposure | Main Findings |
|---|---|---|---|---|
| Endurance combined with resistance exercise intervention: human studies | ||||
| Gustafsson (2019) [85] | healthy, pregnant Norwegian women Intervention group: n = 429, 30.5 ± 4.4 years old; Control group: n = 426, 30.4 ± 4.3 years old | Intervention group: aerobic and strength training, 12 weeks, 3 times per week, 60 min/time | - | 25(OH)D: no significant effect of the exercise program on levels of total, free, or bioavailable 25(OH)D in only baseline level adjust model; additionally adjusted for study site and sampling month, revealed a significant between-group difference in levels of total, free, and bioavailable 25(OH)D. |
| Li (2019) [86] | Patients with postmenopausal osteoporosis Training group: n = 26, 55.46 ± 4.12 years old; Control group: n = 26, 56.25 ± 3.75 years old | Training group: 12 weeks, (a) endurance exercise training, brisk walk outdoors, 4 times per week, 30 min/ time, 50%VO2max; (b) progressive resistance training. calcium and Calcitriol supplementation Control group: calcium and Calcitriol supplementation | brisk walk outdoors | 25(OH)D: significantly increased after intervention in both groups; significantly increased in Training group vs. control group |
| Evans [87] | Healthy men, n = 41, 19.3 ± 1.2 years old; Healthy women, n = 153, 19.0 ± 1,0 years old | Marching under load, running and jumping, battle drills, and walking and standing for prolonged periods of time | - | 25(OH)D: significantly reduced at 4 months in male participants; no change in female participants |
| Endurance combined with resistance exercise intervention-animal study | ||||
| Buskermolen [52] | Female wistar rat, 13 weeks old peak power training and endurance training group, n = 10; Control group, n = 8 | Peak power training: 10 sprints of 15 s in gallop at a maximal attainable velocity on a progressively increasing slope starting at 10% reaching up to 40% by the end endurance training: treadmill running, 6 weeks, 10 min at a speed of 16 m/min without a slope, increased up to 45 min with a speed of 26 m/min on a 10% slope Control group: no intervention | - | 25(OH)D: no change |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Cao, Z.-B. Exercise: A Possibly Effective Way to Improve Vitamin D Nutritional Status. Nutrients 2022, 14, 2652. https://doi.org/10.3390/nu14132652
Zhang J, Cao Z-B. Exercise: A Possibly Effective Way to Improve Vitamin D Nutritional Status. Nutrients. 2022; 14(13):2652. https://doi.org/10.3390/nu14132652
Chicago/Turabian StyleZhang, Jinghua, and Zhen-Bo Cao. 2022. "Exercise: A Possibly Effective Way to Improve Vitamin D Nutritional Status" Nutrients 14, no. 13: 2652. https://doi.org/10.3390/nu14132652
APA StyleZhang, J., & Cao, Z.-B. (2022). Exercise: A Possibly Effective Way to Improve Vitamin D Nutritional Status. Nutrients, 14(13), 2652. https://doi.org/10.3390/nu14132652







