Artificial Sweeteners in Breast Milk: A Clinical Investigation with a Kinetic Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Biochemical Analyses
2.4. Statistics
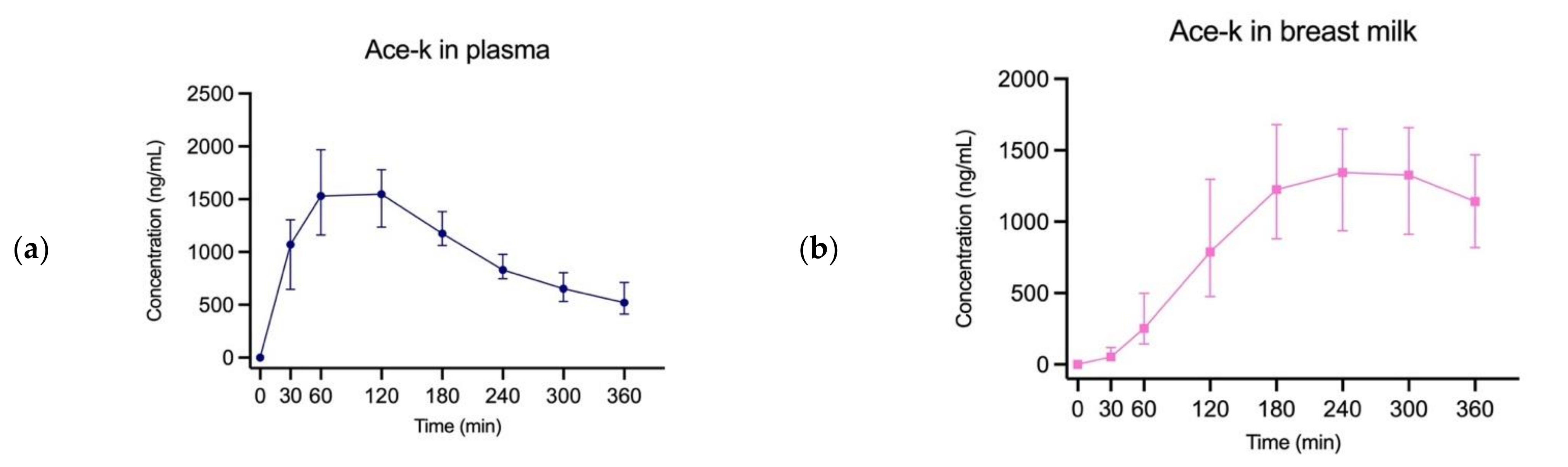
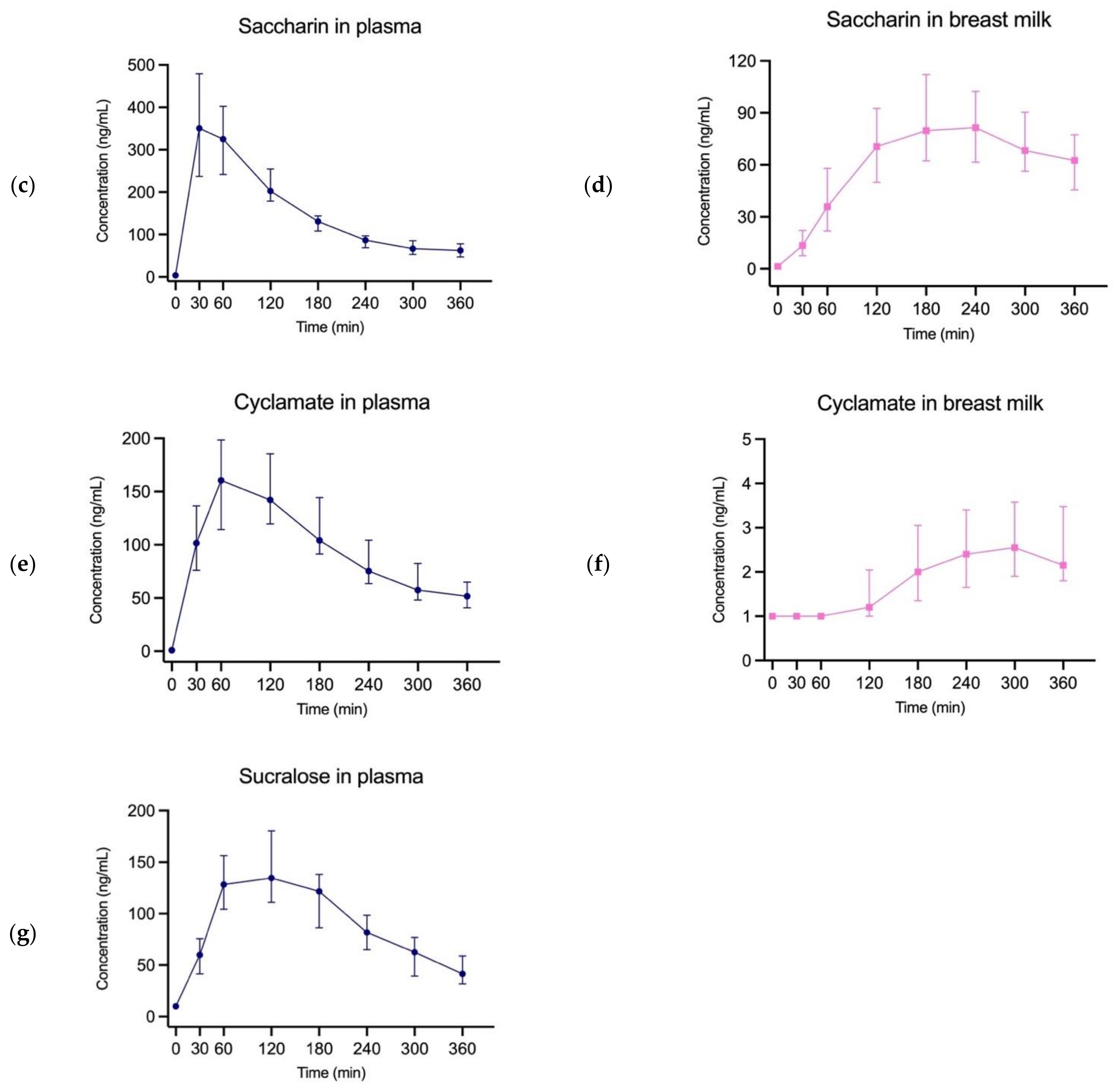
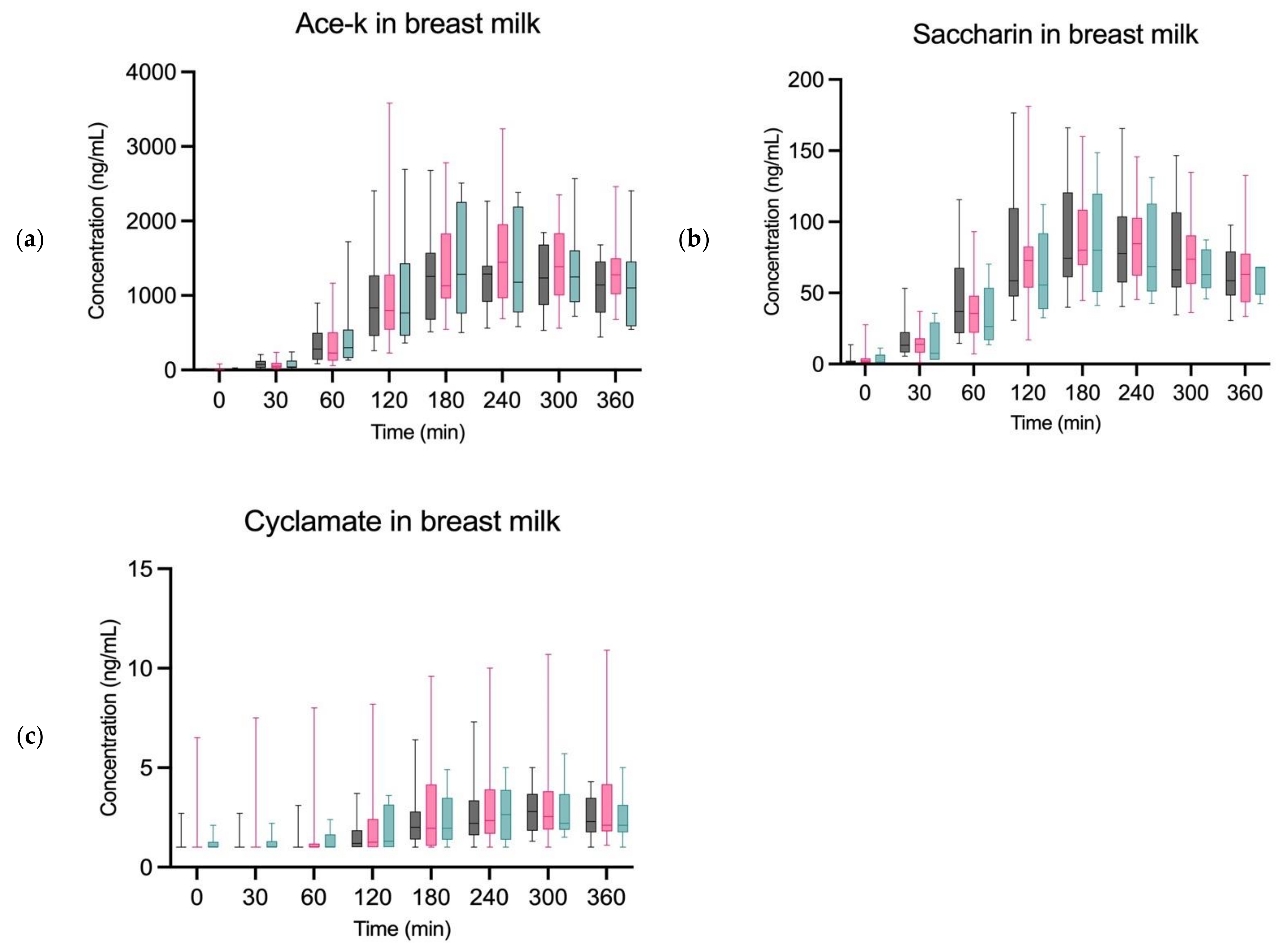
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, M.; Obert, J.; Casey, L. The Association Between Artificial Sweeteners and Obesity. Curr. Gastroenterol. Rep. 2017, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Sylvetsky, A.C.; Jin, Y.; Clark, E.J.; Welsh, J.A.; Rother, K.I.; Talegawkar, S.A. Consumption of Low-Calorie Sweeteners among Children and Adults in the United States. J. Acad. Nutr. Diet. 2017, 117, 441–448.e2. [Google Scholar] [CrossRef]
- Davidson, T.L.; Martin, A.; Clark, K.; Swithers, S.E. Intake of High-Intensity Sweeteners Alters the Ability of Sweet Taste to Signal Caloric Consequences: Implications for the Learned Control of Energy and Body Weight Regulation. Q. J. Exp. Psychol. 2011, 64, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-P.; Lin, Y.Q.; Zhang, L.; Wilson, Y.; Oyston, L.; Cotterell, J.; Qi, Y.; Khuong, T.M.; Bakhshi, N.; Planchenault, Y.; et al. Sucralose Promotes Food Intake through NPY and a Neuronal Fasting Response. Cell Metab. 2016, 24, 75–90. [Google Scholar] [CrossRef] [PubMed]
- von Poser Toigo, E.; Huffell, A.; Mota, C.; Bertolini, D.; Pettenuzzo, L.; Dalmaz, C. Metabolic and feeding behavior alterations provoked by prenatal exposure to aspartame. Appetite 2014, 87, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-H.; Chen, M.-L.; Liu, S.-S.; Zhan, Y.-H.; Quan, Y.; Qin, Y.-M.; Deng, S.-P. Effects of mother’s dietary exposure to acesulfame-K in Pregnancy or lactation on the adult offspring’s sweet preference. Chem. Senses 2011, 36, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Collison, K.S.; Makhoul, N.J.; Zaidi, M.Z.; Al-Rabiah, R.; Inglis, A.; Andres, B.L.; Ubungen, R.; Shoukri, M.; A Al-Mohanna, F. Interactive effects of neonatal exposure to monosodium glutamate and aspartame on glucose homeostasis. Nutr. Metab. 2012, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Lertrit, A.; Srimachai, S.; Saetung, S.; Chanprasertyothin, S.; Chailurkit, L.-O.; Areevut, C.; Katekao, P.; Ongphiphadhanakul, B.; Sriphrapradang, C. Effects of sucralose on insulin and glucagon-like peptide-1 secretion in healthy subjects: A randomized, double-blind, placebo-controlled trial. Nutrition 2018, 55-56, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Abou-Donia, M.B.; El-Masry, E.M.; Abdel-Rahman, A.A.; McLendon, R.E.; Schiffman, S.S. Splenda Alters Gut Microflora and Increases Intestinal P-Glycoprotein and Cytochrome P-450 in Male Rats. J. Toxicol. Environ. Health Part A 2008, 71, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Sylvetsky, A.C.; Sem, S.; Merkel, P.; Dore, F.; Stern, D.B.; Henry, C.J.; Cai, H.; Walter, P.J.; Crandall, K.A.; Rother, K.I.; et al. Consumption of Diet Soda Sweetened with Sucralose and Acesulfame-Potassium Alters Inflammatory Transcriptome Pathways in Females with Overweight and Obesity. Mol. Nutr. Food Res. 2020, 64, e1901166. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Sharma, A.K.; de Souza, R.; Dolinsky, V.W.; Becker, A.B.; Mandhane, P.J.; Turvey, S.; Subbarao, P.; Lefebvre, D.L.; Sears, M.R.; et al. Association Between Artificially Sweetened Beverage Consumption During Pregnancy and Infant Body Mass Index. JAMA Pediatr. 2016, 170, 662–670. [Google Scholar] [CrossRef]
- Zhu, Y.; Olsen, S.; Mendola, P.; Halldorsson, T.; Rawal, S.; Hinkle, S.; Yeung, E.; Chavarro, J.; Grunnet, L.G.; Granström, C.; et al. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: A prospective cohort study. Int. J. Epidemiol. 2017, 46, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Plows, J.F.; Ventura, E.E. Effects of consuming sugars and alternative sweeteners during pregnancy on maternal and child health: Evidence for a secondhand sugar effect. Proc. Nutr. Soc. 2018, 78, 262–271. [Google Scholar] [CrossRef]
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.J.; Bergström, A.; Charles, M.-A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: An individual participant data meta-analysis of European, North American and Australian cohorts. Bjog 2019, 126, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, T.I.; Strøm, M.; Petersen, S.B.; Olsen, S.F. Intake of artificially sweetened soft drinks and risk of preterm delivery: A prospective cohort study in 59,334 Danish pregnant women. Am. J. Clin. Nutr. 2010, 92, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Sylvetsky, A.C.; Gardner, A.L.; Bauman, V.; Blau, J.E.; Garraffo, H.M.; Walter, P.J.; Rother, K.I. Nonnutritive Sweeteners in Breast Milk. J. Toxicol. Environ. Heal. Part A 2015, 78, 1029–1032. [Google Scholar] [CrossRef]
- Rother, K.I.; Sylvetsky, A.C.; Walter, P.J.; Garraffo, H.M.; Fields, D.A. Pharmacokinetics of Sucralose and Acesulfame-Potassium in Breast Milk Following Ingestion of Diet Soda. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 466–470. [Google Scholar] [CrossRef]
- Murtaugh, M.A.; Ferris, A.M.; Capacchione, C.M.; Reece, E. Energy Intake and Glycemia in Lactating Women with Type 1 Diabetes. J. Am. Diet. Assoc. 1998, 98, 642–648. [Google Scholar] [CrossRef]
- Bentley-Lewis, R.; Goldfine, A.B.; Green, D.E.; Seely, E.W. Lactation After Normal Pregnancy Is Not Associated With Blood Glucose Fluctuations. Diabetes Care 2007, 30, 2792–2793. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greibe, E.; Leth-Møller, M.; Stampe, S.; Ovesen, P.; Pedersen, M.; Hoffmann-Lücke, E. Development and validation of an LC-MS/MS method for quantification of artificial sweeteners in human matrixes. Biomed Chromatogr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Berger, A.; Kiefer, M. Comparison of Different Response Time Outlier Exclusion Methods: A Simulation Study. Front. Psychol. 2021, 12, 675558. [Google Scholar] [CrossRef]
- Fitch, S.E.; Payne, L.E.; van de Ligt, J.L.G.; Doepker, C.; Handu, D.; Cohen, S.M.; Anyangwe, N.; Wikoff, D. Use of acceptable daily intake (ADI) as a health-based benchmark in nutrition research studies that consider the safety of low-calorie sweeteners (LCS): A systematic map. BMC Public Heal. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Boron, W.F.; Boulpaep, E.L. Medical Physiology: A cellular and Molecular Approach, 3rd ed.; Saunders Elsevier: Philadelphia, PA, USA, 2009; p. 129. [Google Scholar]
- Reinhart, W.H. The optimum hematocrit. Clin. Hemorheol. Microcirc. 2016, 64, 575–585. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Smith, M.; Tokuda, M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clin. Nutr. ESPEN 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Carakostas, M.C.; Moore, N.H.; Poulos, S.; Renwick, A.G. Biological fate of low-calorie sweeteners. Nutr. Rev. 2016, 74, 670–689. [Google Scholar] [CrossRef]
- Franz, M. Amounts of Sweeteners in Popular Diet Sodas. 2010. Available online: https://static.diabetesselfmanagement.com/pdfs/DSM0310_012.pdf (accessed on 25 October 2021).
- Butchko, H.H.; Stargel, W. Aspartame: Scientific Evaluation in the Postmarketing Period. Regul. Toxicol. Pharmacol. 2001, 34, 221–233. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Pretorius, E. Revisiting the safety of aspartame. Nutr. Rev. 2017, 75, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, A.; Tarannum, N. Water-compatible ‘aspartame’-imprinted polymer grafted on silica surface for selective recognition in aqueous solution. Anal. Bioanal. Chem. 2013, 405, 4245–4252. [Google Scholar] [CrossRef] [PubMed]
- Lange, F.T.; Scheurer, M.; Brauch, H.-J. Artificial sweeteners—a recently recognized class of emerging environmental contaminants: A review. Anal. Bioanal. Chem. 2012, 403, 2503–2518. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.O. How Sweet It Is: Sweeteners in Breast Milk. Breastfeed. Med. 2019, 14, 14–16. [Google Scholar] [CrossRef]
- Sundhedsstyrelsen. Amning. Available online: https://www.sst.dk/da/viden/ernaering/ernaering-til-spaedboern/amning (accessed on 11 May 2022).
- WHO. Breastfeeding. 2022. Available online: https://www.who.int/health-topics/breastfeeding#tab=tab_1 (accessed on 11 May 2022).
| All | BMI < 25 | BMI > 27 | T1DM | |
|---|---|---|---|---|
| Women, n | 49 | 20 | 21 | 8 |
| BMI, kg/m2 | 25.9 (7.6) | 21.3 (2.9) | 28.8 (6.4) | 25.3 (7.7) |
| Age, years | 29 (3) | 28 (2) | 31 (4) | 28.5 (6) |
| Parity, number | 1 (3) | 1 (0) | 2 (1) | 1 (1.8) |
| Offspring birth weight, g | 3780 (508) | 3662 (504) | 3855 (545) | 3981 (417) |
| Offspring birth length, cm | 53 (3) | 52.5 (3) | 53 (3) | 52 (2.8) |
| Offspring GA, days | 218 (17) | 282 (14) | 288 (17.5) | 268 (16.2) |
| Offspring age, days | 121 (154.5) | 179.5 (123.5) | 94 (141.5) | 77.5 (84.5) |
| n | Plasma | Breast Milk | ||||
|---|---|---|---|---|---|---|
| Peak Concentration | Time to Peak | Peak Concentration | Time to Peak | AUC Ratio | ||
| Ace-k | 46 | 1548 ng/mL | 120 min | 936 ng/mL | 240 min | 88.93% |
| Saccharin | 46 | 350.7 ng/mL | 30 min | 81.5 ng/mL | 240 min | 38.91% |
| Cyclamate | 44 | 160.6 ng/mL | 60 min | 2.56 ng/mL | 300 min | 1.86% |
| Sucralose | 46 | 134.6 ng/mL | 120 min | <LOQ | - | - |
| Ace-K | Saccharin | Cyclamate | Sucralose | |
|---|---|---|---|---|
| ADI (mg/kg/day) [26] | 9 | 5 | 7 | 15 |
| Dose given (mg) | 85 | 20 | 60 | 75 |
| Woman max doses (n) | 8 | 19 | 9 | 15 |
| Offspring max doses (n) | 14 | 81 | 5444 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stampe, S.; Leth-Møller, M.; Greibe, E.; Hoffmann-Lücke, E.; Pedersen, M.; Ovesen, P. Artificial Sweeteners in Breast Milk: A Clinical Investigation with a Kinetic Perspective. Nutrients 2022, 14, 2635. https://doi.org/10.3390/nu14132635
Stampe S, Leth-Møller M, Greibe E, Hoffmann-Lücke E, Pedersen M, Ovesen P. Artificial Sweeteners in Breast Milk: A Clinical Investigation with a Kinetic Perspective. Nutrients. 2022; 14(13):2635. https://doi.org/10.3390/nu14132635
Chicago/Turabian StyleStampe, Sofie, Magnus Leth-Møller, Eva Greibe, Elke Hoffmann-Lücke, Michael Pedersen, and Per Ovesen. 2022. "Artificial Sweeteners in Breast Milk: A Clinical Investigation with a Kinetic Perspective" Nutrients 14, no. 13: 2635. https://doi.org/10.3390/nu14132635
APA StyleStampe, S., Leth-Møller, M., Greibe, E., Hoffmann-Lücke, E., Pedersen, M., & Ovesen, P. (2022). Artificial Sweeteners in Breast Milk: A Clinical Investigation with a Kinetic Perspective. Nutrients, 14(13), 2635. https://doi.org/10.3390/nu14132635







