Changes in Sleep Patterns during Pregnancy and Predictive Factors: A Longitudinal Study in Saudi Women
Abstract
1. Introduction
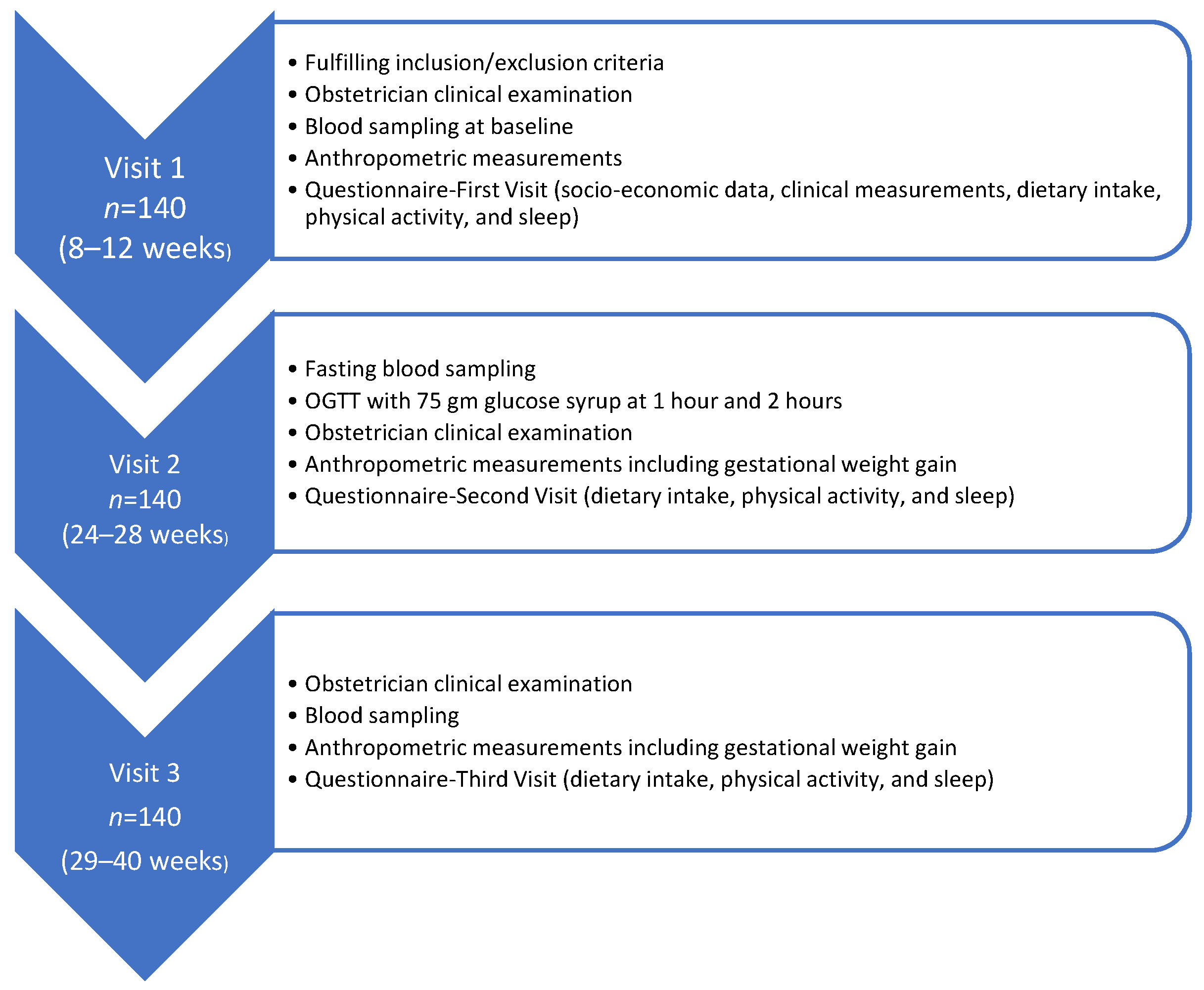
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Data Collection
2.3.1. Sleep Assessment
2.3.2. Biochemical Assessment
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of Participants
3.2. Changes in Biochemical and Physical Parameters during Pregnancy
3.3. Percentage and Characterization of Abnormal Sleep Patterns during Pregnancy
3.4. Factors Predictive of Poor Sleep
3.4.1. Predictors for Sleep in Each Trimester
3.4.2. Predictors for Sleep Changes across Time
- Vitamin D levels
- Income
- Energy Intake
- Sitting
4. Discussion
4.1. Alterations in Sleep Patterns
4.2. Factors Associated with Worsening Sleep Patterns
4.2.1. Demographic Factors and Parity
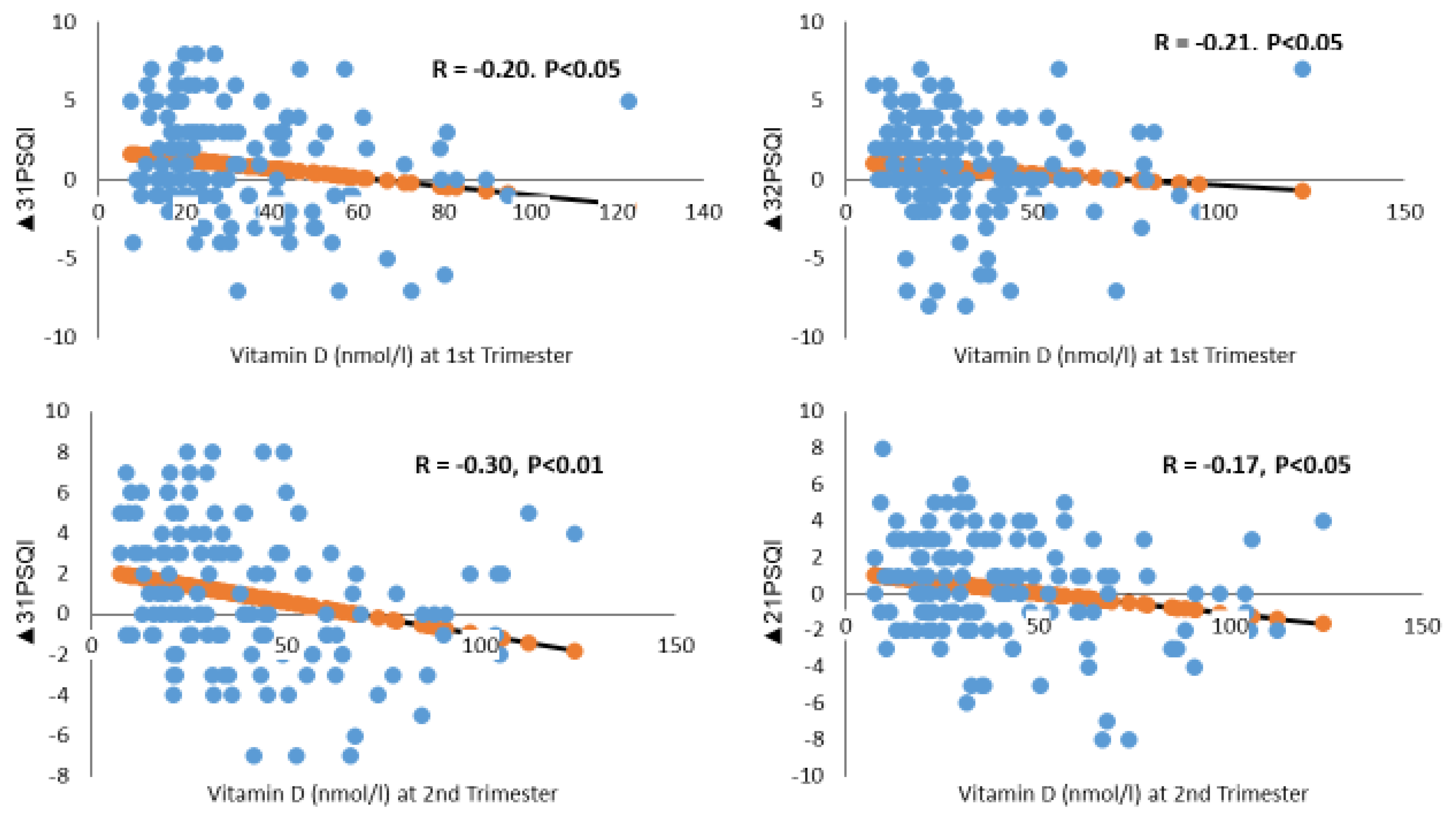
4.2.2. Low Vitamin D Levels
4.2.3. Energy Intake and Sitting
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.A. Alterations in Sleep during Pregnancy and Postpartum: A Review of 30 Years of Research. Sleep Med. Rev. 1998, 2, 231–242. [Google Scholar] [CrossRef]
- Fatima, Y.; Doi, S.A.; Najman, J.M.; Mamun, A.A. Exploring Gender Difference in Sleep Quality of Young Adults: Findings from a Large Population Study. Clin. Med. Res. 2016, 14, 138–144. [Google Scholar] [CrossRef]
- Nodine, P.M.; Matthews, E.E. Common Sleep Disorders: Management Strategies and Pregnancy Outcomes. J. Midwifery Womens Health 2013, 58, 368–377. [Google Scholar] [CrossRef]
- Yannakoulia, M.; Anastasiou, C.A.; Karfopoulou, E.; Pehlivanidis, A.; Panagiotakos, D.B.; Vgontzas, A. Sleep Quality Is Associated with Weight Loss Maintenance Status: The MedWeight Study. Sleep Med. 2017, 34, 242–245. [Google Scholar] [CrossRef]
- Stamatakis, K.A.; Punjabi, N.M. Effects of Sleep Fragmentation on Glucose Metabolism in Normal Subjects. Chest 2010, 137, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Hertz, G.; Fast, A.; Feinsilver, S.H.; Albertario, C.L.; Schulman, H.; Fein, A.M. Sleep in Normal Late Pregnancy. Sleep 1992, 15, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.A.; Hauck, Y.L.; Carty, E.M.; Hutton, E.K.; Fenwick, J.; Stoll, K. Childbirth Fear, Anxiety, Fatigue, and Sleep Deprivation in Pregnant Women. J. Obstet. Gynecol. Neonatal. Nurs. 2009, 38, 567–576. [Google Scholar] [CrossRef]
- Tan, X.; Cook, J.D.; Cedernaes, J.; Benedict, C. Consumer Sleep Trackers: A New Tool to Fight the Hidden Epidemic of Obstructive Sleep Apnoea? Lancet Respir. Med. 2019, 7, 1012. [Google Scholar] [CrossRef]
- Mindell, J.A.; Cook, R.A.; Nikolovski, J. Sleep Patterns and Sleep Disturbances across Pregnancy. Sleep Med. 2015, 16, 483–488. [Google Scholar] [CrossRef] [PubMed]
- National Sleep Foundation. Pregnancy and Sleep. Available online: http://sleepfoundation.org/sleep-topics/pregnancy-and-sleep (accessed on 25 May 2022).
- Yang, Y.; Li, W.; Ma, T.J.; Zhang, L.; Hall, B.J.; Ungvari, G.S.; Xiang, Y.T. Prevalence of Poor Sleep Quality in Perinatal and Postnatal Women: A Comprehensive Meta-analysis of Observational Studies. Front. Psychiatry 2020, 11, 161. [Google Scholar] [CrossRef]
- Sedov, I.D.; Cameron, E.E.; Madigan, S.; Tomfohr-Madsen, L.M. Sleep Quality during Pregnancy: A Meta-analysis. Sleep Med. Rev. 2018, 38, 168–176. [Google Scholar] [CrossRef]
- August, E.M.; Salihu, H.M.; Biroscak, B.J.; Rahman, S.; Bruder, K.; Whiteman, V.E. Systematic Review on Sleep Disorders and Obstetric Outcomes: Scope of Current Knowledge. Am. J. Perinatol. 2013, 30, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Facco, F.L.; Grobman, W.A.; Kramer, J.; Ho, K.H.; Zee, P.C. Self-Reported Short Sleep Duration and Frequent Snoring in Pregnancy: Impact on Glucose Metabolism. Am. J. Obstet. Gynecol. 2010, 203, 142.e1–142.e5. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, X.; Wang, Y.; Li, J.; Xu, Y.; Song, X.; Su, S.; Zhu, X.; Vitiello, M.V.; Shi, J.; et al. Sleep Disturbances during Pregnancy and Adverse Maternal and Fetal Outcomes: A Systematic Review and Meta-analysis. Sleep Med. Rev. 2021, 58, 101436. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, H.; Li, C.; Chen, L.; Zhang, C.; Liu, Z.; Wu, Y.; Huang, H. Associations between Maternal Sleep Quality throughout Pregnancy and Newborn Birth Weight. Behav. Sleep Med. 2021, 19, 57–69. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Lee, P.L.; Lin, J.W.; Lee, C.N. Cross-Sectional and Longitudinal Associations between Sleep and Health-Related Quality of Life in Pregnant Women: A Prospective Observational Study. Int. J. Nurs. Stud. 2016, 56, 45–53. [Google Scholar] [CrossRef]
- Christian, L.M.; Carroll, J.E.; Porter, K.; Hall, M.H. Sleep Quality across Pregnancy and Postpartum: Effects of Parity and Race. Sleep Health 2019, 5, 327–334. [Google Scholar] [CrossRef]
- Balieiro, L.; Gontijo, C.A.; Fahmy, W.M.; Maia, Y.; Crispim, C.A. Does Sleep Influence Weight Gain during Pregnancy? A Prospective Study. Sleep Sci. 2019, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Hedman, C.; Pohjasvaara, T.; Tolonen, U.; Suhonen-Malm, A.S.; Myllylä, V.V. Effects of Pregnancy on Mothers’ Sleep. Sleep Med. 2002, 3, 37–42. [Google Scholar] [CrossRef]
- Román-Gálvez, R.M.; Amezcua-Prieto, C.; Salcedo-Bellido, I.; Martínez-Galiano, J.M.; Khan, K.S.; Bueno-Cavanillas, A. Factors Associated with Insomnia in Pregnancy: A Prospective Cohort Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 221, 70–75. [Google Scholar] [CrossRef]
- Zhao, P.; Bedrick, B.S.; Brown, K.E.; McCarthy, R.; Chubiz, J.E.; Ju, Y.S.; Raghuraman, N.; Fay, J.C.; Jungheim, E.S.; Herzog, E.D.; et al. Sleep Behavior and Chronotype before and throughout Pregnancy. Sleep Med. 2022, 94, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Mindell, J.A.; Jacobson, B.J. Sleep Disturbances during Pregnancy. J. Obstet. Gynecol. Neonatal. Nurs. 2000, 29, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Silva-Perez, L.J.; Gonzalez-Cardenas, N.; Surani, S.; Etindele Sosso, F.A.; Surani, S.R. Socioeconomic Status in Pregnant Women and Sleep Quality during Pregnancy. Cureus 2019, 11, e6183. [Google Scholar] [CrossRef] [PubMed]
- Rutters, F.; Besson, H.; Walker, M.; Mari, A.; Konrad, T.; Nilsson, P.M.; Balkau, B.; Dekker, J.M. The Association between Sleep Duration, Insulin Sensitivity, and Β-cell Function: The Egir-Risc Study. J. Clin. Endocrinol. Metab. 2016, 101, 3272–3280. [Google Scholar] [CrossRef]
- Gay, C.L.; Richoux, S.E.; Beebe, K.R.; Lee, K.A. Sleep Disruption and Duration in Late Pregnancy Is Associated with Excess Gestational Weight Gain among Overweight and Obese Women. Birth 2017, 44, 173–180. [Google Scholar] [CrossRef]
- Chua, E.C.; Shui, G.; Cazenave-Gassiot, A.; Wenk, M.R.; Gooley, J.J. Changes in Plasma Lipids during Exposure to Total Sleep Deprivation. Sleep 2015, 38, 1683–1691. [Google Scholar] [CrossRef]
- Kaneita, Y.; Uchiyama, M.; Yoshiike, N.; Ohida, T. Associations of Usual Sleep Duration with Serum Lipid and Lipoprotein Levels. Sleep 2008, 31, 645–652. [Google Scholar] [CrossRef]
- Xu, Y.H.; Shi, L.; Bao, Y.P.; Chen, S.J.; Shi, J.; Zhang, R.L.; Lu, L. Association between Sleep Duration during Pregnancy and Gestational Diabetes Mellitus: A Meta-analysis. Sleep Med. 2018, 52, 67–74. [Google Scholar] [CrossRef]
- Flanagan, E.W.; Most, J.; Broskey, N.T.; Altazan, A.D.; Beyl, R.A.; Keadle, S.K.; Drews, K.L.; Singh, P.; Redman, L.M. Identification of Changes in Sleep across Pregnancy and the Impact on Cardiometabolic Health and Energy Intake in Women with Obesity. Sleep Med. 2021, 77, 120–127. [Google Scholar] [CrossRef]
- Bennett, C.J.; Cain, S.W.; Blumfield, M.L. Monounsaturated Fat Intake Is Associated with Improved Sleep Quality in Pregnancy. Midwifery 2019, 78, 64–70. [Google Scholar] [CrossRef]
- Lee, K.A.; Zaffke, M.E.; Baratte-Beebe, K. Restless Legs Syndrome and Sleep Disturbance during Pregnancy: The Role of Folate and Iron. J. Womens Health Gend. Med. 2001, 10, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.S.; Loy, S.L.; Cheung, Y.B.; Cai, S.; Colega, M.T.; Godfrey, K.M.; Chong, Y.S.; Tan, K.H.; Shek, L.P.; Lee, Y.S.; et al. Plasma Vitamin D Deficiency Is Associated with Poor Sleep Quality and Night-Time Eating at Mid-pregnancy in Singapore. Nutrients 2017, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Tous, M.; Villalobos, M.; Iglesias, L.; Fernández-Barrés, S.; Arija, V. Vitamin D Status during Pregnancy and Offspring Outcomes: A Systematic Review and Meta-analysis of Observational Studies. Eur. J. Clin. Nutr. 2020, 74, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Al-Musharaf, S.; Fouda, M.A.; Turkestani, I.Z.; Al-Ajlan, A.; Sabico, S.; Alnaami, A.M.; Wani, K.; Hussain, S.D.; Alraqebah, B.; Al-Serehi, A.; et al. Vitamin D Deficiency Prevalence and Predictors in Early Pregnancy among Arab Women. Nutrients 2018, 10, 489. [Google Scholar] [CrossRef] [PubMed]
- Barrett, H.; McElduff, A. Vitamin D and Pregnancy: An Old Problem Revisited. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 527–539. [Google Scholar] [CrossRef]
- Gao, Q.; Kou, T.; Zhuang, B.; Ren, Y.; Dong, X.; Wang, Q. The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-analysis. Nutrients 2018, 10, 1395. [Google Scholar] [CrossRef]
- Sağlam, G.; Pektaş, G.; Karakullukçu, S.; Pektaş, A.; Aykut, S.D. Vitamin D Deficiency Is Associated with Depression, Anxiety and Sleep Disturbance in Pregnant Women. J. Acad. Res. Med. 2021, 11, 51. [Google Scholar] [CrossRef]
- Gunduz, S.; Kosger, H.; Aldemir, S.; Akcal, B.; Tevrizci, H.; Hizli, D.; Celik, H.T. Sleep Deprivation in the Last Trimester of Pregnancy and Inadequate Vitamin D: Is There a Relationship? J. Chin. Med. Assoc. 2015, 79, 34–38. [Google Scholar] [CrossRef]
- Woo, J.; Penckofer, S.; Giurgescu, C.; Yeatts, P.E. Vitamin D Deficiency and Sleep Quality in Minority Pregnant Women. MCN Am. J. Matern. Child Nurs. 2020, 45, 155–160. [Google Scholar] [CrossRef]
- Suleiman, K.H.; Yates, B.C.; Berger, A.M.; Pozehl, B.; Meza, J. Translating the Pittsburgh Sleep Quality Index into Arabic. West. J. Nurs. Res. 2010, 32, 250–268. [Google Scholar] [CrossRef]
- Jomeen, J.; Martin, C.R. Assessment and Relationship of Sleep Quality to Depression in Early Pregnancy. J. Reprod. Infant. Psychol. 2007, 25, 87–99. [Google Scholar] [CrossRef]
- Wani, K.; Sabico, S.; Alnaami, A.M.; Al-Musharaf, S.; Fouda, M.A.; Turkestani, I.Z.; Al-Ajlan, A.; Alshingetti, N.M.; Alokail, M.S.; Al-Daghri, N.M. Early-Pregnancy Metabolic Syndrome and Subsequent Incidence in Gestational Diabetes Mellitus in Arab Women. Front. Endocrinol. 2020, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Saleh, Y.; Aljohani, N.; Sulimani, R.; Al-Othman, A.M.; Alfawaz, H.; Fouda, M.; Al-Amri, F.; Shahrani, A.; Alharbi, M.; et al. Vitamin D Status Correction in Saudi Arabia: An Experts’ Consensus under the Auspices of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases (ESCEO). Arch. Osteoporos. 2017, 12, 1. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Palacios, C.; Ansary, A.; Kulier, R.; Peña-Rosas, J.P. Vitamin D Supplementation for Women during Pregnancy. Cochrane Database Syst. Rev. 2012, 2, CD008873. [Google Scholar]
- Skouteris, H.; Germano, C.; Wertheim, E.H.; Paxton, S.J.; Milgrom, J. Sleep Quality and Depression during Pregnancy: A Prospective Study. J. Sleep Res. 2008, 17, 217–220. [Google Scholar] [CrossRef]
- Shariat, M.; Abedinia, N.; Noorbala, A.A.; Raznahan, M. The Relationship between Sleep Quality, Depression, and Anxiety in Pregnant Women: A Cohort Study. J. Sleep Sci. 2017, 2, 20–27. [Google Scholar]
- Ahmed, A.E.; Al-Jahdali, F.; AlALwan, A.; Abuabat, F.; Bin Salih, S.A.; Al-Harbi, A.; Baharoon, S.; Khan, M.; Ali, Y.Z.; Al-Jahdali, H. Prevalence of Sleep Duration among Saudi Adults. Saudi Med. J. 2017, 38, 276. [Google Scholar] [CrossRef]
- Okun, M.L.; Schetter, C.D.; Glynn, L.M. Poor Sleep Quality Is Associated with Preterm Birth. Sleep 2011, 34, 1493–1498. [Google Scholar] [CrossRef]
- Naghi, I.; Keypour, F.; Ahari, S.B.; Tavalai, S.A.; Khak, M. Sleep Disturbance in Late Pregnancy and Type and Duration of Labour. J. Obstet. Gynaecol. 2011, 31, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.H.; Matthews, K.A.; Kravitz, H.M.; Gold, E.B.; Buysse, D.J.; Bromberger, J.T.; Owens, J.F.; Sowers, M. Race and Financial Strain Are Independent Correlates of Sleep in Midlife Women: The Swan Sleep Study. Sleep 2009, 32, 73–82. [Google Scholar] [PubMed]
- Conlon, R.; Wang, B.; Germeroth, L.J.; Cheng, Y.; Buysse, D.J.; Levine, M.D. Demographic, Pregnancy-Related, and Health-Related Factors in Association with Changes in Sleep among Pregnant Women with Overweight or Obesity. Int. J. Behav. Med. 2020, 28, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Aukia, L.; Paavonen, E.J.; Jänkälä, T.; Tolvanen, M.; Korja, R.; Karlsson, L.; Karlsson, H.; Polo-Kantola, P. Insomnia Symptoms Increase during Pregnancy, but No Increase in Sleepiness-Associations with Symptoms of Depression and Anxiety. Sleep Med. 2020, 72, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ates, S.; Batmaz, G.; Sevket, O.; Molla, T.; Dane, C.; Dane, B. Pregnancy Outcome of Multiparous Women Aged over 40 Years. Int. J. Reprod. Med. 2013, 2013, 287519. [Google Scholar] [CrossRef]
- Bärebring, L.; Schoenmakers, I.; Glantz, A.; Hulthén, L.; Jagner, Å.; Ellis, J.; Bärebring, M.; Bullarbo, M.; Augustin, H. Vitamin D Status during Pregnancy in a Multi-Ethnic Population-Representative Swedish Cohort. Nutrients 2016, 8, 655. [Google Scholar] [CrossRef]
- Lundqvist, A.; Sandström, H.; Stenlund, H.; Johansson, I.; Hultdin, J. Vitamin D Status during Pregnancy: A Longitudinal Study in Swedish Women from Early Pregnancy to Seven Months Postpartum. PLoS ONE 2016, 11, e0150385. [Google Scholar]
- McCarty, D.E.; Reddy, A.; Keigley, Q.; Kim, P.Y.; Marino, A.A. Vitamin D, Race, and Excessive Daytime Sleepiness. J. Clin. Sleep Med. 2012, 8, 693–697. [Google Scholar] [CrossRef]
- Shiue, I. Low Vitamin D Levels in Adults with Longer Time to Fall Asleep: US NHANES, 2005–2006. Age 2013, 168, 5074–5075. [Google Scholar] [CrossRef]
- Massa, J.; Stone, K.L.; Wei, E.K.; Harrison, S.L.; Barrett-Connor, E.; Lane, N.E.; Paudel, M.; Redline, S.; Ancoli-Israel, S.; Orwoll, E.; et al. Vitamin D and Actigraphic Sleep Outcomes in Older Community-Dwelling Men: The MrOS Sleep Study. Sleep 2015, 38, 251–257. [Google Scholar] [CrossRef]
- Musiol, I.M.; Stumpf, W.E.; Bidmon, H.J.; Heiss, C.; Mayerhofer, A.; Bartke, A. Vitamin D Nuclear Binding to Neurons of the Septal, Substriatal and Amygdaloid Area in the Siberian Hamster (Phodopus Sungorus) Brain. Neuroscience 1992, 48, 841–848. [Google Scholar] [CrossRef]
- Stumpf, W.E.; Bidmon, H.J.; Li, L.; Pilgrim, C.; Bartke, A.; Mayerhofer, A.; Heiss, C. Nuclear Receptor Sites for Vitamin D-Soltriol in Midbrain and Hindbrain of Siberian Hamster (Phodopus sungorus) Assessed by Autoradiography. Histochemistry 1992, 98, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, A.; Eguchi, N.; Kimura, K.; Kiyohara, Y.; Qu, W.M.; Huang, Z.L.; Mochizuki, T.; Lazarus, M.; Kobayashi, T.; Kaneko, T.; et al. Dominant Localization of Prostaglandin D Receptors on Arachnoid Trabecular Cells in Mouse Basal Forebrain and Their Involvement in the Regulation of Non-rapid Eye Movement Sleep. Proc. Natl. Acad. Sci. USA 2001, 98, 11674–11679. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.; Shirvani, J.S.; Firouzjahi, A.; Heidari, P.; Hajian-Tilaki, K.O. Association between Nonspecific Skeletal Pain and Vitamin D Deficiency. Int. J. Rheum. Dis. 2010, 13, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Okura, K.; Lavigne, G.J.; Huynh, N.; Manzini, C.; Fillipini, D.; Montplaisir, J.Y. Comparison of Sleep Variables between Chronic Widespread Musculoskeletal Pain, Insomnia, Periodic Leg Movements Syndrome and Control Subjects in a Clinical Sleep Medicine Practice. Sleep Med. 2008, 9, 352–361. [Google Scholar] [CrossRef]
- Khoo, A.L.; Chai, L.Y.; Koenen, H.J.; Sweep, F.C.; Joosten, I.; Netea, M.G.; van der Ven, A.J. Regulation of Cytokine Responses by Seasonality of Vitamin D Status in Healthy Individuals. J. Clin. Exp. Immunol. 2011, 164, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Shahi, M.; Hosseini, S.; Helli, B.; Haghighyzade, M.; Abolfathi, M. The Effect of Vitamin D Supplement on Quality of Sleep in Adult People with Sleep Disorders. Tehran Univ. Med. J. 2017, 75, 443–448. [Google Scholar]
- Patterson, R.E.; Emond, J.A.; Natarajan, L.; Wesseling-Perry, K.; Kolonel, L.N.; Jardack, P.; Ancoli-Israel, S.; Arab, L. Short Sleep Duration Is Associated with Higher Energy Intake and Expenditure among African-American and Non-Hispanic White Adults. J. Nutr. 2014, 144, 461–466. [Google Scholar] [CrossRef]
- Pauley, A.M.; Moore, G.A.; Mama, S.K.; Molenaar, P.; Downs, D.S. Systematic Review of the Associations between Prenatal Sleep Behaviours and Components of Energy Balance for Regulating Weight Gain. J. Sleep Res. 2022, e13619. [Google Scholar] [CrossRef]
- Sivertsen, B.; Hysing, M.; Dørheim, S.K.; Eberhard-Gran, M. Trajectories of Maternal Sleep Problems before and after Childbirth: A Longitudinal Population-Based Study. BMC Pregnancy Childbirth 2015, 15, 129. [Google Scholar] [CrossRef]


| Characteristics | Values |
|---|---|
| Age, years | 28.0 ± 5.2 |
| University graduate or higher | 86 (63.7) |
| Employment | 40 (28.0) |
| Income | |
| >1300 USD | 43 (30.0) |
| Parity | |
| Multipara | 91 (65.0) |
| Pre-pregnancy BMI | 26.8 ± 6.2 |
| Overweight | 37 (26.0) |
| Obesity | 29 (21.0) |
| First Trimester | Second Trimester | Third Trimester | p-Value | |
|---|---|---|---|---|
| n | 141 | 141 | 141 | |
| Gestational age (weeks) | 12.3 ± 3.1 | 26.0 ± 4.8 A | 34.4 ± 3.0 AB | <0.001 |
| Anthropometric parameters | ||||
| BMI (Kg/m2) | 27.6 ± 6.0 | 29.8 ± 6.0 A | 30.5 ± 5.3 AB | <0.001 |
| Waist–hip ratio | 0.84 ± 0.08 | 0.93 ± 0.09 A | 0.97 ± 0.08 AB | <0.001 |
| Body fat % | 35.3 ± 5.2 | 37.8 ± 2.8 A | 38.2 ± 2.5 AB | <0.001 |
| Gestational weight gain (kg) | 0.35 ± 0.21 | 0.35 ± 0.16 | 0.867 | |
| Systolic blood pressure (mmHg) | 113.2 ± 12.3 | 110.6 ± 11.2 | 110.7 ± 10.9 | 0.154 |
| Diastolic blood pressure (mmHg) | 67.0 ± 8.8 | 66.7 ± 10.5 | 68.3 ± 9.1 | 0.544 |
| Dietary parameters | ||||
| Energy intake (%) | 60.1 ± 12.3 | 58.5 ± 14.8 | 51.3 ± 12.1 A | 0.010 |
| Carbohydrates intake (%) | 90.6 ± 20.7 | 94.9 ± 30.0 | 87.5 ± 34.4 | 0.353 |
| Protein intake (%) | 95.7 ± 32.1 | 89.9 ± 30.9 | 76.0 ± 31.6 | 0.093 |
| Fat intake (%) | 93.9 ± 28.5 | 90.3 ± 30.7 | 77.9 ± 21.9 | 0.130 |
| Vitamin D intake (IU/day) | 149.5 ± 146.4 | 174.1 ± 167.8 | 152.3 ± 201.7 | 0.740 |
| Calcium intake (mg/day) | 301.9 ± 380.8 | 353.4 ± 416.0 | 262.4 ± 396.2 | 0.710 |
| Water (mL/day) | 1407.8 ± 743.5 | 1422.9 ± 691.8 | 1562.5 ± 786.9 | 0.735 |
| Tea (mL/day) | 533.3 ± 696.4 | 187.3 ± 110.1 A | 177.0 ± 90.4 A | 0.005 |
| Coffee (mL/day) | 78.9 ± 65.7 | 78.9 ± 75.5 | 121.3 ± 80.0 | 0.161 |
| Physical activity | ||||
| Sitting (min/wk) | 1137.0 ± 728.0 | 1296.0 ± 721.0 | 1311.0 ± 601.0 | 0.440 |
| Low physical activity (min/wk) | 351.0 ± 474.0 | 532.0 ± 660.0 | 109.0 ± 62.0 | 0.140 |
| Biochemical parameters | ||||
| Calcium (mmol/L) | 2.1 ± 0.2 | 2.1 ± 0.2 | 2.1 ± 0.3 | 0.174 |
| Total cholesterol (mmol/L) | 5.2 ± 1.1 | 6.6 ± 1.5 A | 6.6 ± 1.3 A | < 0.001 |
| HDL-cholesterol (mmol/L) | 1.3 ± 0.4 | 1.6 ± 0.5 A | 1.4 ± 0.5 A | < 0.001 |
| LDL-cholesterol (mmol/L) | 3.2 ± 0.8 | 4.1 ± 1.2 A | 4.1 ± 1.1 A | < 0.001 |
| Glucose (mmol/L) | 4.9 ± 1.1 | 4.8 ± 1.0 | 5.1 ± 1.5 B | 0.020 |
| Triglycerides (mmol/L) | 1.4 ± 0.5 | 2.1 ± 0.8 A | 2.4 ± 1.0 AB | < 0.001 |
| Vitamin D (nmol/L) | 32.9 ± 20.2 | 40.2 ± 25.6 A | 38.3 ± 22.9 A | < 0.001 |
| HbA1c | 5.1 ± 0.5 | 4.8 ± 0.5 A | 5.1 ± 0.6 B | < 0.001 |
| First Trimester | Second Trimester | Third Trimester | p-Value | |
|---|---|---|---|---|
| n | 141 | 141 | 141 | |
| Week of gestation | 12.3 ± 3.1 | 26.0 ± 4.8 A | 34.4 ± 3.0 AB | <0.001 |
| Sleep components | ||||
| Habitual sleep efficiency | 1.3 ± 1.4 | 1.2 ± 1.3 | 1.5 ± 1.3 | 0.104 |
| Sleep duration | 0.8 ± 1.0 | 0.9 ± 1.1 | 1.3 ± 1.2 A | 0.001 |
| Sleep latency | 1.0 ± 1.1 | 1.1 ± 1.1 | 1.1 ± 1.1 | 0.514 |
| Sleep disturbance | 0.8 ± 0.7 | 0.9 ± 0.7 | 0.7 ± 0.7 | 0.064 |
| Sleep quality | 0.7 ± 0.6 | 0.9 ± 0.7 | 1.2 ± 0.9 AB | <0.001 |
| Sleep medication | 0.0 ± 0.2 | 0.0 ± 0.0 | 0.1 ± 0.2 | 0.532 |
| Day dysfunction | 0.4 ± 0.7 | 0.3 ± 0.6 | 0.3 ± 0.6 | 0.465 |
| Fall asleep (in minutes) | 51.9 ± 41.6 | 30.7 ± 27.0 A | 38.0 ± 31.0 A | <0.001 |
| Total sleep hours (hours/day) | 7.9 ± 2.8 | 7.1 ± 2.5 A | 8.8 ± 4.9 B | <0.001 |
| Total PSQI score | 5.1 ± 2.6 | 5.3 ± 2.6 B | 6.1 ± 2.4 A | 0.001 |
| Change in PSQI | Vitamin D Status in 1st Trimester | Vitamin D Status in 2nd Trimester | ||||
|---|---|---|---|---|---|---|
| Sufficient | Deficient | p-Value | Sufficient | Deficient | p-Value | |
| ∆31PSQI | −0.3 ± 3.8 | 1.2 ± 3.3 | 0.060 | −0.9 ± 3.0 | 1.6 ± 3.3 | <0.001 |
| ∆21PSQI | −0.9 ± 3.5 | 0.5 ± 2.7 | 0.034 | −0.8 ± 3.2 | 0.7 ± 2.6 | 0.008 |
| ∆32PSQI | 0.6 ± 3.2 | 0.7 ± 3.1 | 0.926 | −0.1 ± 3.3 | 0.9 ± 3.0 | 0.113 |
| ∆31PSQI | ∆21PSQI | ∆32PSQI | ||||
|---|---|---|---|---|---|---|
| B ± SE | p-Value | B ± SE | p-Value | B ± SE | p-Value | |
| High income | −0.60 ± 0.26 | 0.025 | −0.11 ± 0.22 | 0.611 | −0.49 ± 0.25 | 0.054 |
| First trimester | ||||||
| NS | ||||||
| Second trimester | ||||||
| Vitamin D (nmol/L) # | −0.20 ± 0.01 | 0.039 | −0.01 ± 0.01 | 0.621 | −0.03 ± 0.02 | 0.107 |
| Sitting (in min/wk) # | 0.00 ± 0.00 | 0.216 | 0.00 ± 0.00 | 0.373 | 0.01 ± 0.00 | 0.044 |
| Third trimester | ||||||
| Energy intake (%) Kcal/day # | 0.01 ± 0.04 | 0.771 | −0.03 ± 0.05 | 0.589 | 0.15 ± 0.07 | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Musharaf, S. Changes in Sleep Patterns during Pregnancy and Predictive Factors: A Longitudinal Study in Saudi Women. Nutrients 2022, 14, 2633. https://doi.org/10.3390/nu14132633
Al-Musharaf S. Changes in Sleep Patterns during Pregnancy and Predictive Factors: A Longitudinal Study in Saudi Women. Nutrients. 2022; 14(13):2633. https://doi.org/10.3390/nu14132633
Chicago/Turabian StyleAl-Musharaf, Sara. 2022. "Changes in Sleep Patterns during Pregnancy and Predictive Factors: A Longitudinal Study in Saudi Women" Nutrients 14, no. 13: 2633. https://doi.org/10.3390/nu14132633
APA StyleAl-Musharaf, S. (2022). Changes in Sleep Patterns during Pregnancy and Predictive Factors: A Longitudinal Study in Saudi Women. Nutrients, 14(13), 2633. https://doi.org/10.3390/nu14132633






