Vitamin D Treatment Prevents Uremia-Induced Reductions in Aortic microRNA-145 Attenuating Osteogenic Differentiation despite Hyperphosphatemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Design of the Study
2.2. In Vivo Study
2.2.1. Experimental Design
2.2.2. Blood Chemistries
2.2.3. Immunofluorescence
2.2.4. Alizarin Red Staining
2.3. Ex Vivo and In Vitro Studies
2.3.1. Experimental Design
2.3.2. Transfection with Antagomirs and Mimics
2.4. Common Analytical and Technical Procedures Used in All the Studies
2.4.1. Total Calcium Measurement
2.4.2. Quantitative PCR
2.4.3. Statistical Analysis
3. Results
3.1. In Vivo Study
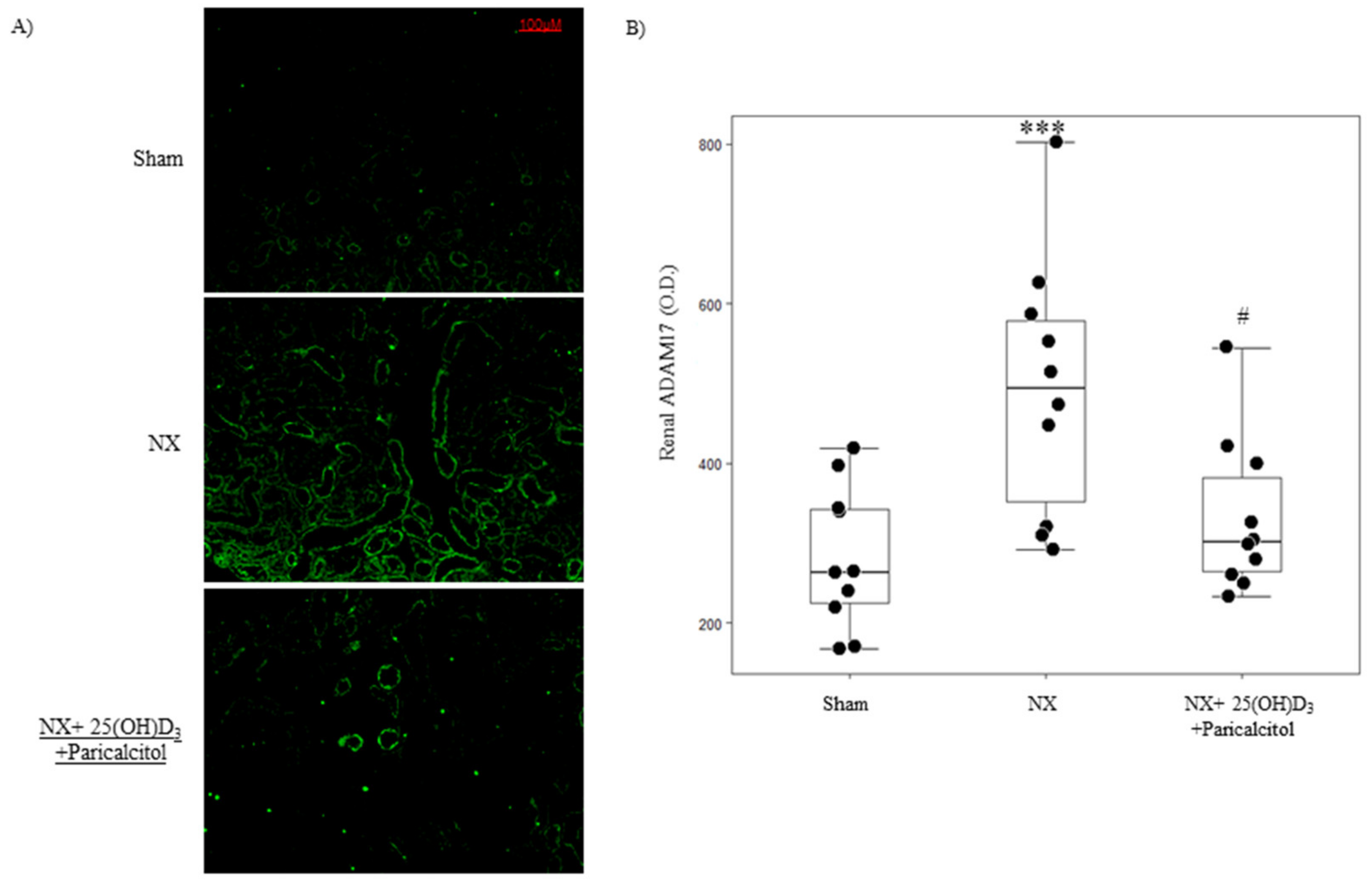
3.1.1. Biochemical Parameters and Kidney Function
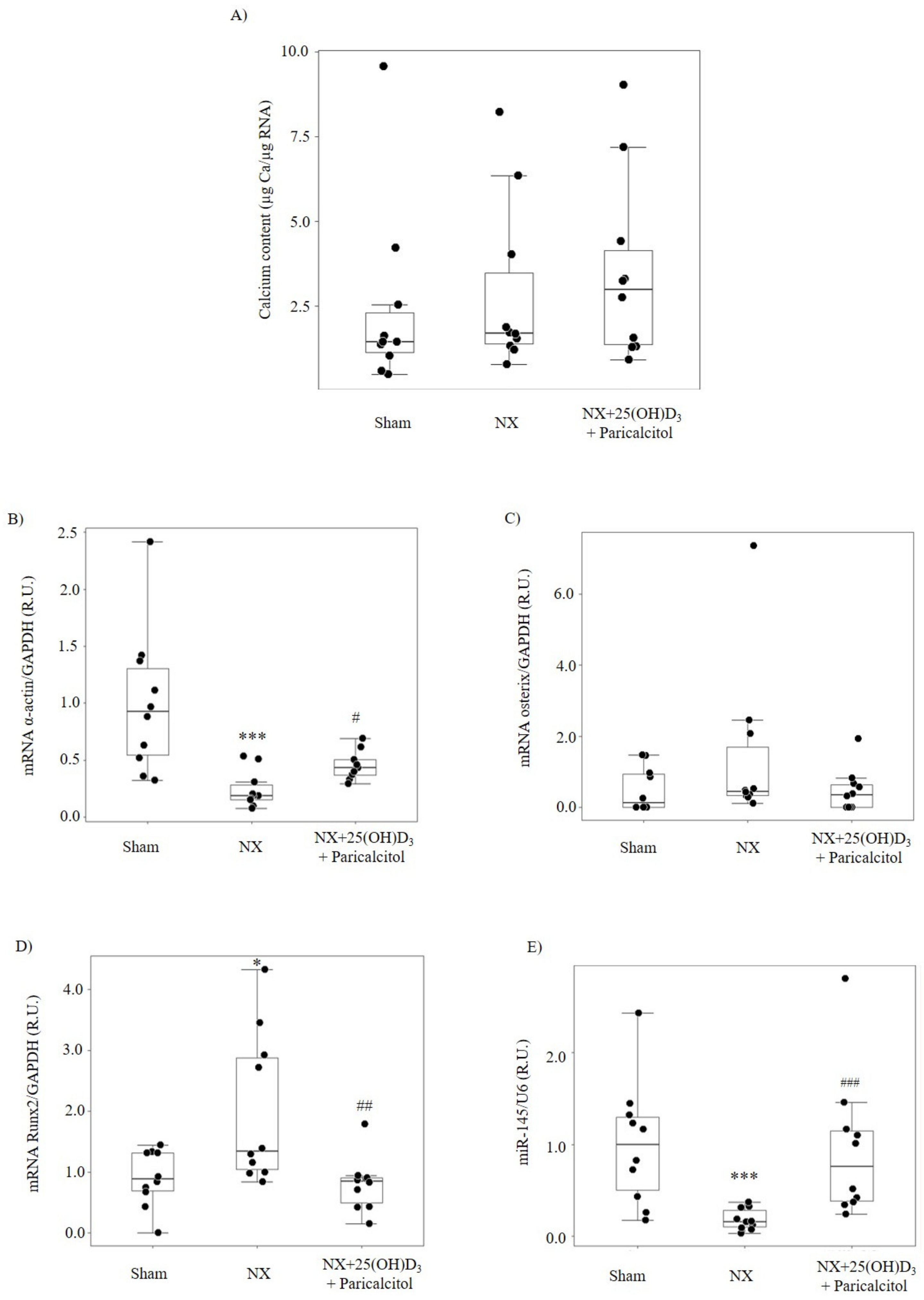
3.1.2. Aortic Osteogenic Differentiation and Calcification
3.2. Ex Vivo and In Vitro Studies
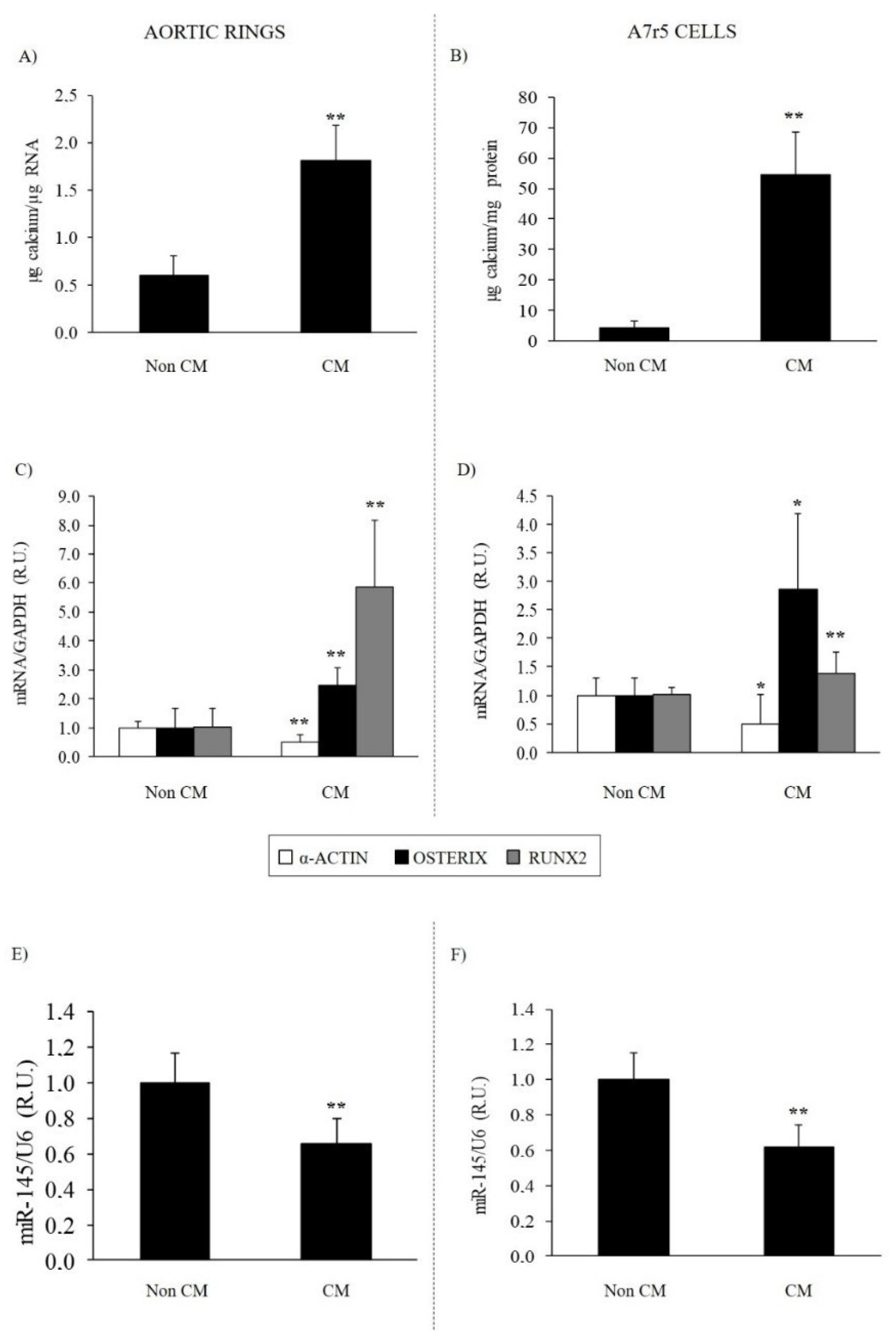
3.2.1. Effect of Calcifying Medium on Aortic Rings and VSMC Osteogenic Differentiation and Calcification
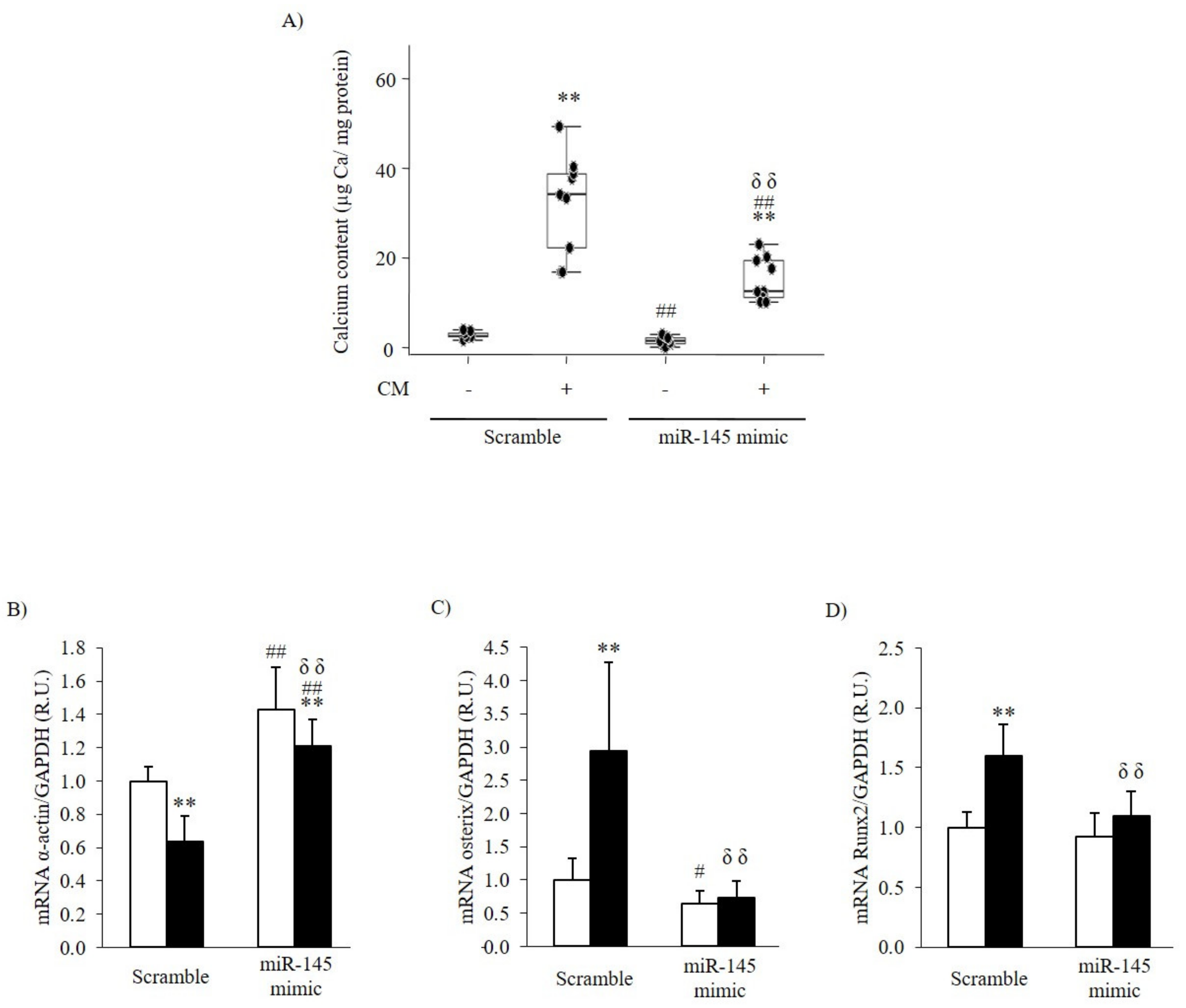
3.2.2. Effect of the Inhibition or Overexpression of miR-145 on the VSMC Phenotype
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; London, G.M. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001, 38, 938–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raggi, P.; Boulay, A.; Chasan-Taber, S.; Amin, N.; Dillon, M.; Burke, S.K.; Chertow, G.M. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J. Am. Coll. Cardiol. 2002, 39, 695–701. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Vervloet, M.; Cozzolino, M. Vascular calcification in chronic kidney disease: Different bricks in the wall? Kidney Int. 2017, 91, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Slatopolsky, E.; Brown, A.; Dusso, A. Role of phosphorus in the pathogenesis of secondary hyperparathyroidism. Am. J. Kidney Dis. 2001, 37, S54–S57. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Gao, L.; Yang, Y.; Tong, D.; Guo, B.; Liu, L.; Li, Z.; Song, T.; Huang, C. miR-145 mediates the antiproliferative and gene regulatory effects of vitamin D3 by directly targeting E2F3 in gastric cancer cells. Oncotarget 2015, 6, 7675–7685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taibi, F.; Meuth, V.M.-L.; M’Baya-Moutoula, E.; Djelouat, M.; Louvet, L.; Bugnicourt, J.M.; Poirot, S.; Bengrine, A.; Chillon, J.M.; Massy, Z.A.; et al. Possible involvement of microRNAs in vascular damage in experimental chronic kidney disease. Biochim. Biophys. Acta 2014, 1842, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Cordes, K.R.; Sheehy, N.T.; White, M.P.; Berry, E.C.; Morton, S.U.; Muth, A.N.; Lee, T.H.; Miano, J.M.; Ivey, K.N.; Srivastava, D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009, 460, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C. MicroRNA-145 in vascular smooth muscle cell biology: A new therapeutic target for vascular disease. Cell Cycle 2009, 8, 3469–3473. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Liu, X.; Yang, J.; Lin, Y.; Xu, D.Z.; Lu, Q.; Deitch, E.A.; Huo, Y.; Delphin, E.S.; Zhang, C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ. Res. 2009, 105, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Cheng, Y.; Yue, J.; Yang, J.; Liu, X.; Chen, H.; Dean, D.B.; Zhang, C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ. Res. 2007, 100, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Ellam, T.J.; Chico, T.J. Phosphate: The new cholesterol? The role of the phosphate axis in non-uremic vascular disease. Atherosclerosis 2012, 220, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Rangrez, A.Y.; M’Baya-Moutoula, E.; Metzinger-Le Meuth, V.; Henaut, L.; Djelouat, M.S.; Benchitrit, J.; Massy, Z.A.; Metzinger, L. Inorganic phosphate accelerates the migration of vascular smooth muscle cells: Evidence for the involvement of miR-223. PLoS ONE 2012, 7, e47807. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Tian, Q.; Ling, S.; Liu, Y.; Yang, S.; Shao, Z. miR-145 suppresses osteogenic differentiation by targeting Sp7. FEBS Lett. 2013, 587, 3027–3031. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Papagiannakopoulos, T.; Pan, G.; Thomson, J.A.; Kosik, K.S. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 2009, 137, 647–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Zhang, N.; Yan, R.; Yang, W.; Cong, G.; Yan, N.; Ma, W.; Hou, J.; Yang, L.; Jia, S. Hyperhomocysteinemia induces vascular calcification by activating the transcription factor RUNX2 via Krüppel-like factor 4 up-regulation in mice. J. Biol. Chem. 2019, 294, 19465–19474. [Google Scholar] [CrossRef]
- Yoshida, T.; Yamashita, M.; Hayashi, M. Kruppel-like factor 4 contributes to high phosphate-induced phenotypic switching of vascular smooth muscle cells into osteogenic cells. J. Biol. Chem. 2012, 287, 25706–25714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terzi, F.; Burtin, M.; Hekmati, M.; Federici, P.; Grimber, G.; Briand, P.; Friedlander, G. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J. Clin. Investig. 2000, 106, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcidiacono, M.V.; Yang, J.; Fernandez, E.; Dusso, A. The induction of C/EBPbeta contributes to vitamin D inhibition of ADAM17 expression and parathyroid hyperplasia in kidney disease. Nephrol. Dial. Transpl. 2015, 30, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lautrette, A.; Li, S.; Alili, R.; Sunnarborg, S.W.; Burtin, M.; Lee, D.C.; Friedlander, G.; Terzi, F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: A new therapeutic approach. Nat. Med. 2005, 11, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Melenhorst, W.B.; Visser, L.; Timmer, A.; van den Heuvel, M.C.; Stegeman, C.A.; van Goor, H. ADAM17 upregulation in human renal disease: A role in modulating TGF-alpha availability? Am. J. Physiol. Renal Physiol. 2009, 297, F781–F790. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Torremade, N.; Bozic, M.; Panizo, S.; Barrio-Vazquez, S.; Fernandez-Martin, J.L.; Encinas, M.; Goltzman, D.; Arcidiacono, M.V.; Fernandez, E.; Valdivielso, J.M. Vascular Calcification Induced by Chronic Kidney Disease Is Mediated by an Increase of 1alpha-Hydroxylase Expression in Vascular Smooth Muscle Cells. J. Bone Miner. Res. 2016, 31, 1865–1876. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- London, G.M.; Guérin, A.P.; Verbeke, F.H.; Pannier, B.; Boutouyrie, P.; Marchais, S.J.; Mëtivier, F. Mineral metabolism and arterial functions in end-stage renal disease: Potential role of 25-hydroxyvitamin D deficiency. J. Am. Soc. Nephrol. 2007, 18, 613–620. [Google Scholar] [CrossRef]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef]
- Zhao, M.M.; Xu, M.J.; Cai, Y.; Zhao, G.; Guan, Y.; Kong, W.; Tang, C.; Wang, X. Mitochondrial reactive oxygen species promote p65 nuclear translocation mediating high-phosphate-induced vascular calcification in vitro and in vivo. Kidney Int. 2011, 79, 1071–1079. [Google Scholar] [CrossRef] [Green Version]
- Byon, C.H.; Javed, A.; Dai, Q.; Kappes, J.C.; Clemens, T.L.; Darley-Usmar, V.M.; McDonald, J.M.; Chen, Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 2008, 283, 15319–15327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paloian, N.J.; Giachelli, C.M. A current understanding of vascular calcification in CKD. Am. J. Physiol. Renal Physiol. 2014, 307, F891–F900. [Google Scholar] [CrossRef] [Green Version]
- Goettsch, C.; Rauner, M.; Pacyna, N.; Hempel, U.; Bornstein, S.R.; Hofbauer, L.C. miR-125b regulates calcification of vascular smooth muscle cells. Am. J. Pathol. 2011, 179, 1594–1600. [Google Scholar] [CrossRef]
- Arcidiacono, M.V.; Yang, J.; Fernandez, E.; Dusso, A. Parathyroid-specific epidermal growth factor-receptor inactivation prevents uremia-induced parathyroid hyperplasia in mice. Nephrol. Dial. Transpl. 2015, 30, 434–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Graciolli, F.G.; Neves, K.R.; dos Reis, L.M.; Graciolli, R.G.; Noronha, I.L.; Moyses, R.M.; Jorgetti, V. Phosphorus overload and PTH induce aortic expression of Runx2 in experimental uraemia. Nephrol. Dial. Transpl. 2009, 24, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lin, H.; Zhang, L.; Sun, Q.; Yuan, G.; Zhang, L.; Chen, S.; Chen, Z. miR-145 and miR-143 regulate odontoblast differentiation through targeting Klf4 and Osx genes in a feedback loop. J. Biol. Chem. 2013, 288, 9261–9271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapustin, A.N.; Chatrou, M.L.; Drozdov, I.; Zheng, Y.; Davidson, S.M.; Soong, D.; Furmanik, M.; Sanchis, P.; De Rosales, R.T.; Alvarez-Hernandez, D.; et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ. Res. 2015, 116, 1312–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Wei, D.; Yan, F. MicroRNA-145 functions as a tumor suppressor by targeting matrix metalloproteinase 11 and Rab GTPase family 27a in triple-negative breast cancer. Cancer Gene Ther. 2016, 23, 258–265. [Google Scholar] [CrossRef]
- Teng, M.; Wolf, M.; Lowrie, E.; Ofsthun, N.; Lazarus, J.M.; Thadhani, R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N. Engl. J. Med. 2003, 349, 446–456. [Google Scholar] [CrossRef] [PubMed]
| Sham (n = 10) | NX (n = 10) | NX + 25(OH)D3 + Paricalcitol (n = 10) | |
|---|---|---|---|
| BUN (mg/dL) | 26.20 ± 6.78 | 51.00 ± 10.27 *** | 52.00 ± 7.16 |
| Calcium (mg/dL) | 13.08 ± 0.89 | 13.09 ± 0.40 | 14.81 ± 1.10 ### |
| Phosphate (mg/dL) | 9.94 ± 1.16 | 9.52 ± 2.67 | 10.28 ± 1.47 # |
| PTH (pg/mL) | 190.00 ± 89.79 | 399.70 ± 292.21 * | 50.80 ± 23.98 ## |
| FGF23 (ng/mL) | 0.66 (0.62−0.71) | 0.81 (0.67−0.83) | 7.16 (4.21−11.43) ### |
| Scramble | miR-145 Antagomir | |||
|---|---|---|---|---|
| Non-CM | CM | Non-CM | CM | |
| Ca content (µg Ca/mg protein) | 2.31 [2.20–3.02] | 33.96 [22.05–38.72] ** | 3.65 [3.43–5.56] ## | 50.90 [48.29–57.47] **,##,δδ |
| mRNA α-actin/GAPDH (R.U.) | 1.00 ± 0.09 | 0.64 ± 0.15 ** | 0.73 ± 0.10 ## | 0.59 ± 0.21 |
| mRNA Osterix/GAPDH (R.U.) | 1.00 ± 0.33 | 2.94 ± 1.34 ** | 1.73 ± 0.74 # | 2.61 ± 1.07 |
| mRNA Runx2/GAPDH (R.U.) | 1.00 ± 0.13 | 1.60 ± 0.26 ** | 1.15 ± 0.10 # | 1.76 ± 0.26 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-López, N.; Panizo, S.; Arcidiacono, M.V.; de la Fuente, S.; Martínez-Arias, L.; Ottaviano, E.; Ulloa, C.; Ruiz-Torres, M.P.; Rodríguez, I.; Cannata-Andía, J.B.; et al. Vitamin D Treatment Prevents Uremia-Induced Reductions in Aortic microRNA-145 Attenuating Osteogenic Differentiation despite Hyperphosphatemia. Nutrients 2022, 14, 2589. https://doi.org/10.3390/nu14132589
Carrillo-López N, Panizo S, Arcidiacono MV, de la Fuente S, Martínez-Arias L, Ottaviano E, Ulloa C, Ruiz-Torres MP, Rodríguez I, Cannata-Andía JB, et al. Vitamin D Treatment Prevents Uremia-Induced Reductions in Aortic microRNA-145 Attenuating Osteogenic Differentiation despite Hyperphosphatemia. Nutrients. 2022; 14(13):2589. https://doi.org/10.3390/nu14132589
Chicago/Turabian StyleCarrillo-López, Natalia, Sara Panizo, Maria Vittoria Arcidiacono, Sandra de la Fuente, Laura Martínez-Arias, Emerenziana Ottaviano, Catalina Ulloa, María Piedad Ruiz-Torres, Isabel Rodríguez, Jorge B. Cannata-Andía, and et al. 2022. "Vitamin D Treatment Prevents Uremia-Induced Reductions in Aortic microRNA-145 Attenuating Osteogenic Differentiation despite Hyperphosphatemia" Nutrients 14, no. 13: 2589. https://doi.org/10.3390/nu14132589
APA StyleCarrillo-López, N., Panizo, S., Arcidiacono, M. V., de la Fuente, S., Martínez-Arias, L., Ottaviano, E., Ulloa, C., Ruiz-Torres, M. P., Rodríguez, I., Cannata-Andía, J. B., Naves-Díaz, M., & Dusso, A. S. (2022). Vitamin D Treatment Prevents Uremia-Induced Reductions in Aortic microRNA-145 Attenuating Osteogenic Differentiation despite Hyperphosphatemia. Nutrients, 14(13), 2589. https://doi.org/10.3390/nu14132589






