Impact of Dietary Fructose and High Salt Diet: Are Preclinical Studies Relevant to Asian Societies?
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Susceptibility to Hypertension: Genetics
3.2. Salt-Sensitive Blood Pressure
3.3. Fructose Intake, Blood Pressure, and Cardiovascular Risk
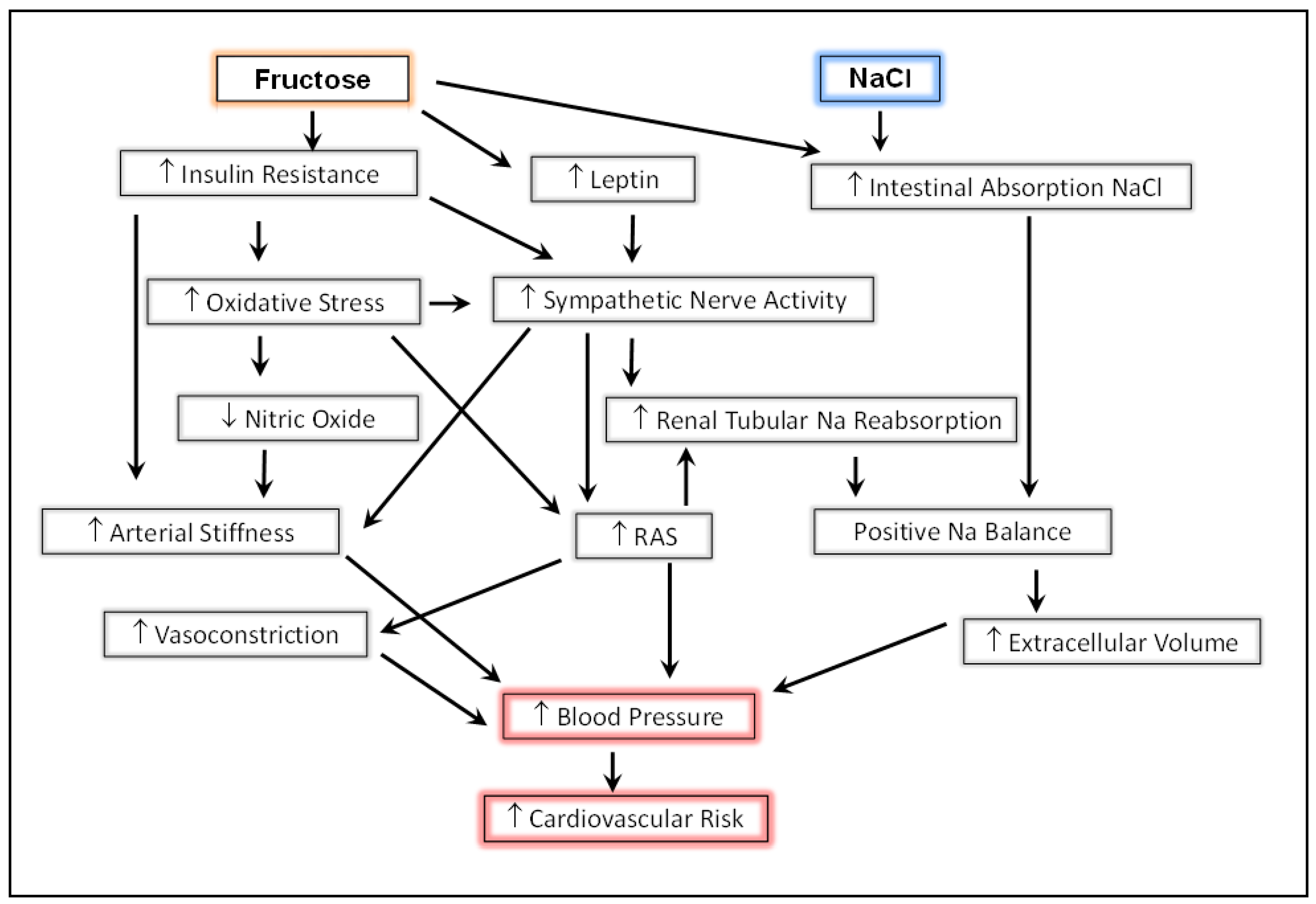
3.4. Preclinical Data on Dietary Fructose, High Salt, and Blood Pressure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Folkow, B.; Svanborg, A. Physiology of cardiovascular aging. Physiol. Rev. 1993, 73, 725–764. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Murphy, R.A.; Newman, A.B.; Bauer, D.C.; Harris, T.B.; Yang, Z.; Applegate, W.B.; Kritchevsky, S.B. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: The Health, Aging, and Body Composition (Health ABC) Study. JAMA Intern. Med. 2015, 175, 410–419. [Google Scholar] [CrossRef]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart, A.; Obesity Committee of the Council on Nutrition, P.A.; Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of Cardiovascular Events and All-Cause Mortality with Arterial Stiffness A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Eren, O.C.; Ortiz, A.; Afsar, B.; Covic, A.; Kuwabara, M.; Lanaspa, M.A.; Johnson, R.J.; Kanbay, M. Multilayered Interplay Between Fructose and Salt in Development of Hypertension. Hypertension 2019, 73, 265–272. [Google Scholar] [CrossRef] [PubMed]
- DeChristopher, L.R.; Auerbach, B.J.; Tucker, K.L. High fructose corn syrup, excess-free-fructose, and risk of coronary heart disease among African Americans- the Jackson Heart Study. BMC Nutr. 2020, 6, 70. [Google Scholar] [CrossRef]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef]
- Appel, L.J. The Effects of Dietary Factors on Blood Pressure. Cardiol. Clin. 2017, 35, 197–212. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Xiang, Y.B.; Yang, G.; Li, H.; Gao, Y.T.; Zheng, W.; Shu, X.O. Adherence to dietary guidelines and mortality: A report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am. J. Clin. Nutr. 2014, 100, 693–700. [Google Scholar] [CrossRef]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell. Biochem. 2009, 332, 145–159. [Google Scholar] [CrossRef]
- Park, J.H.; Ku, H.J.; Kim, J.K.; Park, J.W.; Lee, J.H. Amelioration of High Fructose-Induced Cardiac Hypertrophy by Naringin. Sci. Rep. 2018, 8, 9464. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Soares, E.; Fernandes, J.; Viana, S.; Carvalho, E.; Pereira, F.C.; Reis, F. Early cardiac changes in a rat model of prediabetes: Brain natriuretic peptide overexpression seems to be the best marker. Cardiovasc. Diabetol. 2013, 12, 44. [Google Scholar] [CrossRef]
- Komnenov, D.; Levanovich, P.E.; Rossi, N.F. Hypertension Associated with Fructose and High Salt: Renal and Sympathetic Mechanisms. Nutrients 2019, 11, 569. [Google Scholar] [CrossRef]
- Gordish, K.L.; Kassem, K.M.; Ortiz, P.A.; Beierwaltes, W.H. Moderate (20%) fructose-enriched diet stimulates salt-sensitive hypertension with increased salt retention and decreased renal nitric oxide. Physiol. Rep. 2017, 5, e13162. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Cabral, P.D.; Hong, N.J.; Asirwatham, J.; Saez, F.; Garvin, J.L. Fructose reabsorption by rat proximal tubules: Role of Na(+)-linked cotransporters and the effect of dietary fructose. Am. J. Physiol. Renal Physiol. 2019, 316, F473–F480. [Google Scholar] [CrossRef]
- Galipeau, D.; Verma, S.; McNeill, J.H. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2478–H2484. [Google Scholar] [CrossRef] [PubMed]
- Caldiz, C.I.; de Cingolani, G.E. Insulin resistance in adipocytes from spontaneously hypertensive rats: Effect of long-term treatment with enalapril and losartan. Metabolism 1999, 48, 1041–1046. [Google Scholar] [CrossRef]
- Ares, G.R.; Kassem, K.M.; Ortiz, P.A. Fructose acutely stimulates NKCC2 activity in rat thick ascending limbs by increasing surface NKCC2 expression. Am. J. Physiol. Renal Physiol. 2019, 316, F550–F557. [Google Scholar] [CrossRef] [PubMed]
- Coronati, M.; Baratta, F.; Pastori, D.; Ferro, D.; Angelico, F.; Del Ben, M. Added Fructose in Non-Alcoholic Fatty Liver Disease and in Metabolic Syndrome: A Narrative Review. Nutrients 2022, 14, 1127. [Google Scholar] [CrossRef]
- Stricker, S.; Rudloff, S.; Geier, A.; Steveling, A.; Roeb, E.; Zimmer, K.P. Fructose Consumption-Free Sugars and Their Health Effects. Dtsch. Arztebl. Int. 2021, 118, 71–78. [Google Scholar] [CrossRef]
- Student, J.; Sowers, J.; Lockette, W. THIRSTY FOR FRUCTOSE: Arginine Vasopressin, Fructose, and the Pathogenesis of Metabolic and Renal Disease. Front. Cardiovasc. Med. 2022, 9, 883365. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R.; Packard, C.J.; Boren, J. Dietary Fructose and the Metabolic Syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef]
- Ehret, G.B.; Ferreira, T.; Chasman, D.I.; Jackson, A.U.; Schmidt, E.M.; Johnson, T.; Thorleifsson, G.; Luan, J.; Donnelly, L.A.; Kanoni, S.; et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat. Genet. 2016, 48, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- International Consortium for Blood Pressure Genome-Wide Association, S; Ehret, G.B.; Munroe, P.B.; Rice, K.M.; Bochud, M.; Johnson, A.D.; Chasman, D.I.; Smith, A.V.; Tobin, M.D.; Verwoert, G.C.; et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011, 478, 103–109. [Google Scholar] [CrossRef]
- Surendran, P.; Drenos, F.; Young, R.; Warren, H.; Cook, J.P.; Manning, A.K.; Grarup, N.; Sim, X.; Barnes, D.R.; Witkowska, K.; et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat. Genet. 2016, 48, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kraja, A.T.; Smith, J.A.; Brody, J.A.; Franceschini, N.; Bis, J.C.; Rice, K.; Morrison, A.C.; Lu, Y.; Weiss, S.; et al. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat. Genet. 2016, 48, 1162–1170. [Google Scholar] [CrossRef]
- Warren, H.R.; Evangelou, E.; Cabrera, C.P.; Gao, H.; Ren, M.X.; Mifsud, B.; Ntalla, I.; Surendran, P.; Liu, C.Y.; Cook, J.P.; et al. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat. Genet. 2017, 49, 403–415. [Google Scholar] [CrossRef]
- Kato, N.; Loh, M.; Takeuchi, F.; Verweij, N.; Wang, X.; Zhang, W.; Kelly, T.N.; Saleheen, D.; Lehne, B.; Leach, I.M.; et al. Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat. Genet. 2015, 47, 1282–1293. [Google Scholar] [CrossRef]
- Kanai, M.; Akiyama, M.; Takahashi, A.; Matoba, N.; Momozawa, Y.; Ikeda, M.; Iwata, N.; Ikegawa, S.; Hirata, M.; Matsuda, K.; et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat. Genet. 2018, 50, 390–400. [Google Scholar] [CrossRef]
- Takeuchi, F.; Akiyama, M.; Matoba, N.; Katsuya, T.; Nakatochi, M.; Tabara, Y.; Narita, A.; Saw, W.Y.; Moon, S.; Spracklen, C.N.; et al. Interethnic analyses of blood pressure loci in populations of East Asian and European descent. Nat. Commun. 2018, 9, 5052. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, H. Explanation for the Japanese paradox: Prevention of increase in coronary heart disease and reduction in stroke. J. Atheroscler. Thromb. 2007, 14, 278–286. [Google Scholar] [CrossRef]
- Cutler, J.A.; Follmann, D.; Allender, P.S. Randomized trials of sodium reduction: An overview. Am. J. Clin. Nutr. 1997, 65, 643S–651S. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; D’Elia, L.; Kandala, N.B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef]
- Weinberger, M.H.; Fineberg, N.S.; Fineberg, S.E.; Weinberger, M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 2001, 37, 429–432. [Google Scholar] [CrossRef]
- Elijovich, F.; Weinberger, M.H.; Anderson, C.A.; Appel, L.J.; Bursztyn, M.; Cook, N.R.; Dart, R.A.; Newton-Cheh, C.H.; Sacks, F.M.; Laffer, C.L.; et al. Salt Sensitivity of Blood Pressure: A Scientific Statement from the American Heart Association. Hypertension 2016, 68, e7–e46. [Google Scholar] [CrossRef]
- Morimoto, A.; Uzu, T.; Fujii, T.; Nishimura, M.; Kuroda, S.; Nakamura, S.; Inenaga, T.; Kimura, G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 1997, 350, 1734–1737. [Google Scholar] [CrossRef]
- Stamler, J. The INTERSALT Study: Background, methods, findings, and implications. Am. J. Clin. Nutr. 1997, 65, 626S–642S. [Google Scholar] [CrossRef]
- Qi, H.; Liu, B.; Guo, C.; Liu, Z.; Cao, H.; Liu, K.; Sun, W.; Zhang, L. Effects of environmental and genetic risk factors for salt sensitivity on blood pressure in northern China: The systemic epidemiology of salt sensitivity (EpiSS) cohort study. BMJ 2018, 8, e023042. [Google Scholar] [CrossRef]
- Whelton, P.K.; Appel, L.J.; Sacco, R.L.; Anderson, C.A.; Antman, E.M.; Campbell, N.; Dunbar, S.B.; Frohlich, E.D.; Hall, J.E.; Jessup, M.; et al. Sodium, blood pressure, and cardiovascular disease: Further evidence supporting the American Heart Association sodium reduction recommendations. Circulation 2012, 126, 2880–2889. [Google Scholar] [CrossRef]
- Kamitani, A.; Rakugi, H.; Higaki, J.; Yi, Z.; Mikami, H.; Miki, T.; Ogihara, T. Association analysis of a polymorphism of the angiotensinogen gene with essential hypertension in Japanese. J. Hum. Hypertens 1994, 8, 521–524. [Google Scholar]
- Bianchi, G.; Tripodi, G.; Casari, G.; Salardi, S.; Barber, B.R.; Garcia, R.; Leoni, P.; Torielli, L.; Cusi, D.; Ferrandi, M.; et al. 2 Point Mutations within the Adducin Genes Are Involved in Blood-Pressure Variation. Proc. Natl. Acad. Sci. USA 1994, 91, 3999–4003. [Google Scholar] [CrossRef] [PubMed]
- Brand, E.; Chatelain, N.; Mulatero, P.; Fery, I.; Curnow, K.; Jeunemaitre, X.; Corvol, P.; Pascoe, L.; Soubrier, F. Structural analysis and evaluation of the aldosterone synthase gene in hypertension. Hypertension 1998, 32, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Siffert, W.; Rosskopf, D.; Siffert, G.; Busch, S.; Moritz, A.; Erbel, R.; Sharma, A.M.; Ritz, E.; Wichmann, H.E.; Jakobs, K.H.; et al. Association of a human G-protein beta3 subunit variant with hypertension. Nat. Genet. 1998, 18, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, T.; Ishikawa, K.; Sugimoto, K.; Rakugi, H.; Ogihara, T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens. Res. 2003, 26, 521–525. [Google Scholar] [CrossRef]
- Gong, X.; Han, X.; Lu, X.; Chen, J.; Huang, J.; Kelly, T.N.; Chen, C.S.; He, J.; Gu, D.; Chen, S. Association of Kir genes with blood pressure responses to dietary sodium intervention: The GenSalt study. Hypertens. Res. 2018, 41, 1045–1053. [Google Scholar] [CrossRef]
- Han, C.; Hu, Z.; Liu, F.; Yang, X.; Kelly, T.N.; Chen, J.; Huang, J.; Chen, C.S.; He, J.; Chen, S.; et al. Genetic variants of cGMP-dependent protein kinase genes and salt sensitivity of blood pressure: The GenSalt study. J. Hum. Hypertens. 2019, 33, 62–68. [Google Scholar] [CrossRef]
- Gu, X.; Gu, D.; He, J.; Rao, D.C.; Hixson, J.E.; Chen, J.; Li, J.; Huang, J.; Wu, X.; Rice, T.K.; et al. Resequencing Epithelial Sodium Channel Genes Identifies Rare Variants Associated with Blood Pressure Salt-Sensitivity: The GenSalt Study. Am. J. Hypertens. 2018, 31, 205–211. [Google Scholar] [CrossRef]
- Ko, J.; Lee, M.; Patel, D.I.; Nguyen, V.; Wang, J. Examining the Potential Effect of a Salt Sensitivity Biomarker in Korean American Immigrants: A Pilot Study. J. Immigr. Minor. Health 2021. [Google Scholar] [CrossRef]
- Lee, H.S.; Duffey, K.J.; Popkin, B.M. Sodium and potassium intake patterns and trends in South Korea. J. Hum. Hypertens. 2013, 27, 298–303. [Google Scholar] [CrossRef]
- Song, E.K.; Moser, D.K.; Kang, S.M.; Lennie, T.A. Self-reported Adherence to a Low-Sodium Diet and Health Outcomes in Patients with Heart Failure. J. Cardiovasc. Nurs. 2016, 31, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Chang, J.H.; Kim, J.H.; Kang, J.W. Is high sodium intake associated with hearing impairment? The association between spot urine sodium concentration and hearing threshold in Korean adolescents. Asia Pac. J. Clin. Nutr. 2018, 27, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, S.H.; Shim, Y.S. Association of sodium intake with insulin resistance in Korean children and adolescents: The Korea National Health and Nutrition Examination Survey 2010. J. Pediatr. Endocrinol. Metab. 2018, 31, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, K.; Lee, B.K.; Ahn, J. Association of the Healthy Eating Index with Estimated Cardiovascular Age in Adults from the KNHANES 2013–2017. Nutrients 2020, 12, 2912. [Google Scholar] [CrossRef]
- Uechi, K.; Sugimoto, M.; Kobayashi, S.; Sasaki, S. Urine 24-Hour Sodium Excretion Decreased between 1953 and 2014 in Japan, but Estimated Intake Still Exceeds the WHO Recommendation. J. Nutr. 2017, 147, 390–397. [Google Scholar] [CrossRef]
- Okuda, M.; Sasaki, S. Assessment of Foods Associated with Sodium and Potassium Intake in Japanese Youths Using the Brief-Type Self-Administered Diet History Questionnaire. Nutrients 2021, 13, 2345. [Google Scholar] [CrossRef]
- Wen, X.; Zhou, L.; Stamler, J.; Chan, Q.; Van Horn, L.; Daviglus, M.L.; Dyer, A.R.; Elliott, P.; Ueshima, H.; Miura, K.; et al. Agreement between 24-h dietary recalls and 24-h urine collections for estimating sodium intake in China, Japan, UK, USA: The International Study of Macro- and Micro-nutrients and Blood Pressure. J. Hypertens. 2019, 37, 814–819. [Google Scholar] [CrossRef]
- Tan, M.; He, F.J.; Wang, C.; MacGregor, G.A. Twenty-Four-Hour Urinary Sodium and Potassium Excretion in China: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e012923. [Google Scholar] [CrossRef]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D.; et al. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef]
- Zhu, Z.; Cui, X.; Wei, X.; Zang, J.; Feng, J.; Wang, Z.; Shi, Z. Dietary Sodium Intake Is Positively Associated with Sugar-Sweetened Beverage Consumption in Chinese Children and Adolescents. Nutrients 2021, 13, 3949. [Google Scholar] [CrossRef]
- Xi, B.; Shen, Y.; Reilly, K.H.; Wang, X.; Mi, J. Recapitulation of four hypertension susceptibility genes (CSK, CYP17A1, MTHFR, and FGF5) in East Asians. Metabolism 2013, 62, 196–203. [Google Scholar] [CrossRef]
- Tsuchihashi, T. Dietary salt intake in Japan—Past, present, and future. Hypertens. Res. 2022, 45, 748–757. [Google Scholar] [CrossRef]
- Wang, S.S.; Lay, S.; Yu, H.N.; Shen, S.R. Dietary Guidelines for Chinese Residents (2016): Comments and comparisons. J. Zhejiang Univ. Sci. B 2016, 17, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Shin, J.; Kim, G.H.; Park, S.; Ihm, S.H.; Kim, H.C.; Kim, K.I.; Kim, J.H.; Lee, J.H.; Park, J.M.; et al. 2018 Korean Society of Hypertension Guidelines for the management of hypertension: Part II-diagnosis and treatment of hypertension. Clin. Hypertens. 2019, 25, 20. [Google Scholar] [CrossRef]
- Bakris, G.; Ali, W.; Parati, G. ACC/AHA Versus ESC/ESH on Hypertension Guidelines: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2019, 73, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Ferder, L.; Ferder, M.D.; Inserra, F. The role of high-fructose corn syrup in metabolic syndrome and hypertension. Curr. Hypertens. Rep. 2010, 12, 105–112. [Google Scholar] [CrossRef]
- Madero, M.; Perez-Pozo, S.E.; Jalal, D.; Johnson, R.J.; Sanchez-Lozada, L.G. Dietary fructose and hypertension. Curr. Hypertens. Rep. 2011, 13, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.J.; Stamler, J.; Van Horn, L.; Robertson, C.E.; Chan, Q.N.; Dyer, A.R.; Huang, C.C.; Rodriguez, B.L.; Zhao, L.C.; Daviglus, M.L.; et al. Sugar-Sweetened Beverage, Sugar Intake of Individuals, and Their Blood Pressure International Study of Macro/Micronutrients and Blood Pressure. Hypertension 2011, 57, 695–701. [Google Scholar] [CrossRef]
- Chan, Q.; Stamler, J.; Griep, L.M.; Daviglus, M.L.; Horn, L.V.; Elliott, P. An Update on Nutrients and Blood Pressure. J. Atheroscler. Thromb. 2016, 23, 276–289. [Google Scholar] [CrossRef]
- Soleimani, M. Dietary fructose, salt absorption and hypertension in metabolic syndrome: Towards a new paradigm. Acta Physiol. 2011, 201, 55–62. [Google Scholar] [CrossRef]
- Segal, M.S.; Gollub, E.; Johnson, R.J. Is the fructose index more relevant with regards to cardiovascular disease than the glycemic index? Eur. J. Nutr. 2007, 46, 406–417. [Google Scholar] [CrossRef]
- Jayalath, V.H.; de Souza, R.J.; Ha, V.; Mirrahimi, A.; Blanco-Mejia, S.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; Wolever, T.M.; Beyene, J.; et al. Sugar-sweetened beverage consumption and incident hypertension: A systematic review and meta-analysis of prospective cohorts. Am. J. Clin. Nutr. 2015, 102, 914–921. [Google Scholar] [CrossRef]
- Johnson, M.D.; Zhang, H.Y.; Kotchen, T.A. Sucrose does not raise blood pressure in rats maintained on a low salt intake. Hypertension 1993, 21, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Knapka, J.J.; MacArthy, P.; Yousufi, A.K.; Sabnis, S.G.; Antonovych, T.T. High sucrose diets increase blood pressure of both salt-sensitive and salt-resistant rats. Am. J. Hypertens. 1992, 5, 585–591. [Google Scholar] [CrossRef]
- Khan, T.A.; Tayyiba, M.; Agarwal, A.; Mejia, S.B.; de Souza, R.J.; Wolever, T.M.S.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Sievenpiper, J.L. Relation of Total Sugars, Sucrose, Fructose, and Added Sugars with the Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Mayo Clin. Proc. 2019, 94, 2399–2414. [Google Scholar] [CrossRef] [PubMed]
- Marriott, B.P.; Cole, N.; Lee, E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 2009, 139, 1228S–1235S. [Google Scholar] [CrossRef]
- Takeichi, H.; Taniguchi, H.; Fukinbara, M.; Tanaka, N.; Shikanai, S.; Sarukura, N.; Hsu, T.F.; Wong, Y.; Yamamoto, S. Sugar intakes from snacks and beverages in Japanese children. J. Nutr. Sci. Vitaminol. 2012, 58, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Uchida, K.; Ohnaka, K.; Morita, M.; Toyomura, K.; Kono, S.; Ueki, T.; Tanaka, M.; Kakeji, Y.; Maehara, Y.; et al. Sugars, sucrose and colorectal cancer risk: The Fukuoka colorectal cancer study. Scand. J. Gastroenterol. 2014, 49, 581–588. [Google Scholar] [CrossRef]
- Fujiwara, A.; Murakami, K.; Asakura, K.; Uechi, K.; Sugimoto, M.; Wang, H.C.; Masayasu, S.; Sasaki, S. Estimation of Starch and Sugar Intake in a Japanese Population Based on a Newly Developed Food Composition Database. Nutrients 2018, 10, 1474. [Google Scholar] [CrossRef]
- Yamakawa, M.; Wada, K.; Koda, S.; Mizuta, F.; Uji, T.; Oba, S.; Nagata, C. High Intake of Free Sugars, Fructose, and Sucrose Is Associated with Weight Gain in Japanese Men. J. Nutr. 2020, 150, 322–330. [Google Scholar] [CrossRef]
- Cho, H.; Budhathoki, S.; Kanehara, R.; Goto, A.; Yamaji, T.; Kakugawa, Y.; Saito, Y.; Matsuda, T.; Iwasaki, M.; Tsugane, S. Association between dietary sugar intake and colorectal adenoma among cancer screening examinees in Japan. Cancer Sci. 2020, 111, 3862–3872. [Google Scholar] [CrossRef] [PubMed]
- Edo, A.; Pertiwi, Y.D.; Hirooka, K.; Masuda, S.; Kamaruddin, M.I.; Yanagi, M.; Nagao, A.; Ohno, H.; Yoneda, M.; Kiuchi, Y. Association of Dietary Nutrient Intake with Early Age-Related Macular Degeneration in Japanese-Americans. Metabolites 2021, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Shikanai, S.; Koung Ry, L.; Takeichi, H.; Emiko, S.; San, P.; Sarukura, N.; Kamoshita, S.; Yamamoto, S. Sugar intake and body weight in Cambodian and Japanese children. J. Med. Investig. 2014, 61, 72–78. [Google Scholar] [CrossRef]
- Pang, S.; Song, P.; Sun, X.; Qi, W.; Yang, C.; Song, G.; Wang, Y.; Zhang, J. Dietary fructose and risk of metabolic syndrome in Chinese residents aged 45 and above: Results from the China National Nutrition and Health Survey. Nutr. J. 2021, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.; Elliott, P.; Dennis, B.; Dyer, A.R.; Kesteloot, H.; Liu, K.; Ueshima, H.; Zhou, B.F.; Group, I.R. INTERMAP: Background, aims, design, methods, and descriptive statistics (nondietary). J. Hum. Hypertens. 2003, 17, 591–608. [Google Scholar] [CrossRef]
- Shay, C.M.; Stamler, J.; Dyer, A.R.; Brown, I.J.; Chan, Q.; Elliott, P.; Zhao, L.; Okuda, N.; Miura, K.; Daviglus, M.L.; et al. Nutrient and food intakes of middle-aged adults at low risk of cardiovascular disease: The international study of macro-/micronutrients and blood pressure (INTERMAP). Eur. J. Nutr. 2012, 51, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kwon, S.O.; Lee, Y. Weight Status and Dietary Factors Associated with Sugar-Sweetened Beverage Intake among Korean Children and Adolescents—Korea National Health and Nutrition Examination Survey, 2008–2011. Clin. Nutr. Res. 2013, 2, 135–142. [Google Scholar] [CrossRef][Green Version]
- Bae, J.; Chun, B.Y.; Park, P.S.; Choi, B.Y.; Kim, M.K.; Shin, M.H.; Lee, Y.H.; Shin, D.H.; Kim, S.K. Higher consumption of sugar-sweetened soft drinks increases the risk of hyperuricemia in Korean population: The Korean Multi-Rural Communities Cohort Study. Semin. Arthritis Rheum. 2014, 43, 654–661. [Google Scholar] [CrossRef]
- Ha, K.; Chung, S.; Lee, H.S.; Kim, C.I.; Joung, H.; Paik, H.Y.; Song, Y. Association of Dietary Sugars and Sugar-Sweetened Beverage Intake with Obesity in Korean Children and Adolescents. Nutrients 2016, 8, 31. [Google Scholar] [CrossRef]
- Song, H.J.; Paek, Y.J.; Choi, M.K.; Yoo, K.B.; Kang, J.H.; Lee, H.J. Gender Differences in the relationship between carbonated sugar-sweetened beverage intake and the likelihood of hypertension according to obesity. Int. J. Public Health 2017, 62, 573–581. [Google Scholar] [CrossRef]
- Lim, H.; Lee, H.J.; Choue, R.; Wang, Y. Trends in Fast-Food and Sugar-Sweetened Beverage Consumption and Their Association with Social Environmental Status in South Korea. J. Acad. Nutr. Diet. 2018, 118, 1228–1236.e1221. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Jo, G.; Chung, H.K.; Shin, M.J. Association between sugar-sweetened beverage consumption and incident hypertension in Korean adults: A prospective study. Eur. J. Nutr. 2019, 58, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Nakamura, K.; Miura, K.; Takamura, T.; Yoshita, K.; Nagasawa, S.Y.; Morikawa, Y.; Ishizaki, M.; Kido, T.; Naruse, Y.; et al. Sugar-sweetened beverage and diet soda consumption and the 7-year risk for type 2 diabetes mellitus in middle-aged Japanese men. Eur. J. Nutr. 2014, 53, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.T.; Chan, T.F.; Huang, H.L.; Lee, C.Y.; Tsai, S.; Wu, P.W.; Yang, Y.C.; Wang, T.N.; Lee, C.H. Fructose-Rich Beverage Intake and Central Adiposity, Uric Acid, and Pediatric Insulin Resistance. J. Pediatr. 2016, 171, 90–96.e91. [Google Scholar] [CrossRef]
- Li, D.; Yu, D.; Zhao, L. Trend of sugar-sweetened beverage consumption and intake of added sugar in China nine provinces among adults. Wei Sheng Yan Jiu 2014, 43, 70–72. [Google Scholar]
- Gui, Z.H.; Zhu, Y.N.; Cai, L.; Sun, F.H.; Ma, Y.H.; Jing, J.; Chen, Y.J. Sugar-Sweetened Beverage Consumption and Risks of Obesity and Hypertension in Chinese Children and Adolescents: A National Cross-Sectional Analysis. Nutrients 2017, 9, 1302. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, M.; Yang, C.; Zheng, H.; Zhu, Y. Association of sugar-sweetened beverage intake with risk of metabolic syndrome among children and adolescents in urban China. Public Health Nutr. 2020, 23, 2770–2780. [Google Scholar] [CrossRef]
- Gan, Q.; Xu, P.; Yang, T.; Cao, W.; Xu, J.; Li, L.; Pan, H.; Zhao, W.; Zhang, Q. Sugar-Sweetened Beverage Consumption Status and Its Association with Childhood Obesity among Chinese Children Aged 6–17 Years. Nutrients 2021, 13, 2211. [Google Scholar] [CrossRef]
- Gui, Z.; Huang, S.; Chen, Y.; Zhao, Y.; Jiang, N.; Zhang, S.; Chen, Y. Association between Sugar-Sweetened Beverage Consumption and Executive Function in Children. Nutrients 2021, 13, 4563. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, C.; Qu, S.; Wei, X.; Feng, J.; Zhang, S.; Wang, Y.; Su, J. Effects of School-Based Interventions on Reducing Sugar-Sweetened Beverage Consumption among Chinese Children and Adolescents. Nutrients 2021, 13, 1862. [Google Scholar] [CrossRef]
- Cai, W.; Li, J.; Shi, J.; Yang, B.; Tang, J.; Truby, H.; Li, D. Acute metabolic and endocrine responses induced by glucose and fructose in healthy young subjects: A double-blinded, randomized, crossover trial. Clin. Nutr. 2018, 37, 459–470. [Google Scholar] [CrossRef]
- Singh, A.K.; Amlal, H.; Haas, P.J.; Dringenberg, U.; Fussell, S.; Barone, S.L.; Engelhardt, R.; Zuo, J.; Seidler, U.; Soleimani, M. Fructose-induced hypertension: Essential role of chloride and fructose absorbing transporters PAT1 and Glut5. Kidney Int. 2008, 74, 438–447. [Google Scholar] [CrossRef]
- Soleimani, M.; Alborzi, P. The role of salt in the pathogenesis of fructose-induced hypertension. Int. J. Nephrol. 2011, 2011, 392708. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Hong, N.J.; Yang, N.; Cabral, P.D.; Berthiaume, J.M.; Dominici, F.P.; Garvin, J.L. Dietary Fructose Increases the Sensitivity of Proximal Tubules to Angiotensin II in Rats Fed High-Salt Diets. Nutrients 2018, 10, 1244. [Google Scholar] [CrossRef]
- Petrovic, S.; Wang, Z.; Ma, L.; Seidler, U.; Forte, J.G.; Shull, G.E.; Soleimani, M. Colocalization of the apical Cl-/HCO3- exchanger PAT1 and gastric H-K-ATPase in stomach parietal cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G1207–G1216. [Google Scholar] [CrossRef]
- Petrovic, S.; Wang, Z.; Ma, L.; Soleimani, M. Regulation of the apical Cl-/HCO-3 exchanger pendrin in rat cortical collecting duct in metabolic acidosis. Am. J. Physiol. Renal Physiol. 2003, 284, F103–F112. [Google Scholar] [CrossRef]
- Dudeja, P.K.; Rao, D.D.; Syed, I.; Joshi, V.; Dahdal, R.Y.; Gardner, C.; Risk, M.C.; Schmidt, L.; Bavishi, D.; Kim, K.E.; et al. Intestinal distribution of human Na+/H+ exchanger isoforms NHE-1, NHE-2, and NHE-3 mRNA. Am. J. Physiol. 1996, 271, G483–G493. [Google Scholar] [CrossRef]
- Levanovich, P.E.; Chung, C.S.; Komnenov, D.; Rossi, N.F. Fructose plus High-Salt Diet in Early Life Results in Salt-Sensitive Cardiovascular Changes in Mature Male Sprague Dawley Rats. Nutrients 2021, 13, 3129. [Google Scholar] [CrossRef]
- Queiroz-Leite, G.D.; Crajoinas, R.O.; Neri, E.A.; Bezerra, C.N.; Girardi, A.C.; Reboucas, N.A.; Malnic, G. Fructose acutely stimulates NHE3 activity in kidney proximal tubule. Kidney Blood Press. Res. 2012, 36, 320–334. [Google Scholar] [CrossRef]
- Cabral, P.D.; Hong, N.J.; Hye Khan, M.A.; Ortiz, P.A.; Beierwaltes, W.H.; Imig, J.D.; Garvin, J.L. Fructose stimulates Na/H exchange activity and sensitizes the proximal tubule to angiotensin II. Hypertension 2014, 63, e68–e73. [Google Scholar] [CrossRef]
- Soncrant, T.; Komnenov, D.; Beierwaltes, W.H.; Chen, H.; Wu, M.; Rossi, N.F. Bilateral renal cryodenervation decreases arterial pressure and improves insulin sensitivity in fructose-fed Sprague-Dawley rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R529–R538. [Google Scholar] [CrossRef]
- Johns, E.J.; Kopp, U.C.; DiBona, G.F. Neural control of renal function. Compr. Physiol. 2011, 1, 731–767. [Google Scholar] [CrossRef]
- Chen, H.H.; Chu, C.H.; Wen, S.W.; Lai, C.C.; Cheng, P.W.; Tseng, C.J. Excessive Fructose Intake Impairs Baroreflex Sensitivity and Led to Elevated Blood Pressure in Rats. Nutrients 2019, 11, 2581. [Google Scholar] [CrossRef]
- Komnenov, D.; Levanovich, P.E.; Perecki, N.; Chung, C.S.; Rossi, N.F. Aortic Stiffness and Diastolic Dysfunction in Sprague Dawley Rats Consuming Short-Term Fructose Plus High Salt Diet. Integr. Blood Press. Control 2020, 13, 111–124. [Google Scholar] [CrossRef]
- Xu, C.; Yu, J. Pathophysiological mechanisms of hypertension development induced by fructose consumption. Food Funct. 2022, 13, 1702–1717. [Google Scholar] [CrossRef]
- Kamata, K.; Yamashita, K. Insulin resistance and impaired endothelium-dependent renal vasodilatation in fructose-fed hypertensive rats. Res. Commun. Mol. Pathol. Pharmacol. 1999, 103, 195–210. [Google Scholar]
- Verma, S.; Bhanot, S.; Yao, L.; McNeill, J.H. Vascular insulin resistance in fructose-hypertensive rats. Eur. J. Pharmacol. 1997, 322, R1–R2. [Google Scholar] [CrossRef]
- Zenner, Z.P.; Gordish, K.L.; Beierwaltes, W.H. Free radical scavenging reverses fructose-induced salt-sensitive hypertension. Integr. Blood Press. Control 2018, 11, 1–9. [Google Scholar] [CrossRef]
| No. | Author, Year | Country | n | Population | Method Dietary Assessment | Sodium Intakes or Sodium Excretion | Dietary Energy Intake |
|---|---|---|---|---|---|---|---|
| 1 | Lee, 2013 [50] | South Korea | 9264 | Subjects > 1 year old | 24-h diet recall | Mean: 4.7 g | Not reported |
| 2 | Song, 2016 [51] | South Korea | 9869 | Adults (19–64 years) | Food Frequency Questionnaire + a 24-h diet recall | Mean sodium: 4943 mg/d Males: 6045 mg/d Females: 4240 mg/d | Energy: 1910 kcal/d Males: 2322 kcal/d Females:1648 kcal/d |
| 3 | Choi, 2018 [52] | South Korea | 10,672 | Adults > 18 years | 24-h urine sodium | Mean (SE): 3.3 (0.1) g/d | Not reported |
| 4 | Kim, 2018 [53] | South Korea | 718 | Children & adolescents (10–18 years) | 24-h urine sodium | Mean: 4190.55 mg | Mean 2270.70 kcal/d |
| 5 | Park, 2020 [54] | Korea | N/R | KNHANES 2014 (Subjects ≥ 1 year) | 24-h diet recall | Total sodium: 3889.9 mg Male: 4557 mg Female: 3222.6 mg By age 1–2 years: 1111.7 mg 3–5 years: 1775.1 mg 6–11 years: 2916.5 mg 12–18 years: 3766.5 mg 19–29 years: 4226.4 mg 30–49 years: 4448.4 mg 50–64 years: 4161.4 mg 65+ years: 2987.1 mg | Not reported |
| 6 | Uechi, 2017 [55] | Japan | N/R | Systematic Review | 24-h urine sodium | Mean (±SE) Overall: 4900 ± 190 mg Males: 5280 ± 310 mg Females: 4620 ± 290 mg | Not reported |
| 7 | Okuda, 2021 [56] | Japan | 2377 | Children (10–11 and 13–14 years) | Brief diet history questionnaire | Mean sodium: 4179 ± 1162 mg/d | Energy: 2005 ± 570 kcal/d |
| 8 | Wen, 2019 [57] | Japan China | 1,145,839 | Adults (40–59 years) | 24-h diet recall | Japan: 202.2 ± 55.6 mmol/d China: 173.5 ± 84.5 mmol | Not reported |
| 9 | Tan, 2019 [58] | China | N/R | Systematic Review | Systematic Review | Mean urinary sodium excretion (range) 3–6 years: 86.99 (69.88, 104.1) mmol/24 h 6–16 years: 151.09 (131.55, 170.63) mmol/24 h ≥16 years: 189.07 (182.14, 195.99) mmol/24 h | Not reported |
| 10 | Powles, 2013 [59] | China Japan Korea | N/R | Systematic Review | Systematic Review | All values in g/d China: Total: 4.83 (4.62–5.05) Male: 5.05 (4.71–5.39) Female: 4.60 (4.31–4.88) Japan Total: 4.89 (4.71–5.08) Male: 5.12 (4.85–5.41) Female: 4.68 (4.43–4.93) Korea Total: 3.79 (3.16–4.46) Male: 4.01 (3.07–5.21) Female: 3.59 (2.79–4.55) | Not reported |
| 11 | Zhu, 2021 [60] | China (Shanghai) | 3958 | Adolescents (6–17 years) | Three 24-h dietary recalls | Dietary sodium intake 4297.6 ± 2285.5 mg/d Dietary source of sodium: salt (57.4%); soy sauce (13.2%), fungi and algae (6.5%) Mono Na glutamate (4.6%) | Not reported |
| No. | Author, Year | Country | n | Population | Dietary Assessment Method | Dietary Fructose/Fructose-Rich Beverage Intakes |
|---|---|---|---|---|---|---|
| 1 | Takeichi, 2012 [77] | Japan | 283 | Children (7, 10 and 13 years) | Three 24-h dietary recalls | Fructose intake 7 year males: 3.5 ± 3.3 g/d 7 year females: 3.8 ± 3.6 g/d 10 year males: 3.4 ± 3.7 g/d 10 year females: 3.6 ± 2.8 g/d 13 year males: 4.4 ± 4.6 g/d 13 year females: 2.1 ± 2.7 g/d Overall: 3.5 ± 3.5 g/d |
| 2 | Wang, 2014 [78] | Japan | 1631 | Healthy control and individuals with colorectal cancer | Personal computer assisted dietary interview | Fructose intake Males (colorectal cancer): 6.17 (2.70–11.65) g/d Males (control): 6.11 (2.82–10.77) g/d Females (colorectal cancer): 8.98 (5.67–14.58) g/d Females (control): 9.63 (5.98–14.87) g/d |
| 3 | Fujiwara, 2018 [79] | Japan | 2335 | Toddlers (18–35 month) preschool children (3–6 years) schoolchildren (8–14 years) adults (20–69 years) | Weighted Dietary Record | Fructose intake Toddlers (Boys): 7.8 ± 4.3 g/d Toddlers (Girls): 7.6 ± 5.4 g/d Preschool children (Boys): 10.0 ± 3.7 g/d Preschool children (Girls): 9.3 ± 2.7 g/d Schoolchildren (Boys): 11.1 ± 3.8 g/d Schoolchildren (Girls): 10.3 ± 4.0 g/d Adults (Males): 10.1 ± 4.3 g/d Adults (Females): 9.9 ± 4.0 g/d |
| 4 | Yamakawa, 2020 [80] | Japan | 22394 | Adults (≥35 years) | Food Frequency Questionnaire | Fructose intake Males: 15.0 ± 10.0 g/d Females: 13.7 ± 8.1 g/d |
| 5 | Cho, 2020 [81] | Japan | 1435 | Healthy control and individuals with colorectal adenoma | Food Frequency Questionnaire | Fructose intake Colorectal adenoma (n = 738): 10.4 (7.0–14.8) g/d Control (n = 697): 11.1 (7.6–15.1) g/d |
| 6 | Edo, 2021 [82] | Japanese-American | 555 | Healthy control and individuals with early age-related macular degeneration (AMD) | Food Frequency Method | Fructose intake AMD (n = 111): 2.8 ± 3.6 g/d Control (n = 444): 3.7 ± 4.2 g/d |
| 7 | Shikanai, 2016 [83] | Taiwan | 410 | Children (7, 10 and 12 years) | Three 24-h dietary recalls | Fructose intake 7 year males: 7.3 ± 7.1 g/d 7 year females: 8.7 ± 6.9 g/d 10 year males: 9.5 ± 8.7 g/d 10 year females: 7.2 ± 6.6 g/d 12 year males: 8.5 ± 10.7 g/d 12 year females: 7.5 ± 7.4 g/d Overall: 8.8 ± 8.1 g/d |
| 8 | Pang, 2021 [84] | China | 25528 | Adults (≥45 years) | Three 24-h dietary records | Total fructose intake average (median) City: 11.6 (8.3) g/d Rural: 7.6 (5.3) g/d Free-fructose City: 6.7 (4.2) g/d Rural: 4.6 (2.7) g/d Bound-fructose intake City: 4.9 (3.7) g/d Rural: 3.1 0 (2.4) g/d |
| No. | Author, Year | Country | n | Population | Dietary Assessment Method | Dietary Fructose/Fructose-Rich Beverage Intakes | Dietary Energy Intake |
|---|---|---|---|---|---|---|---|
| 1 | Lee, 2013 [87] | South Korea | 5421 | Children & adolescent (7–18 years) | 24-h dietary recall | SSB intake Overall: 7–18 year: 98.7 mL/d Males 7–18 year: 114.1 mL/d Females 7–18 year: 82.1 mL/d Overall: 7–12 year: 64.7 mL/d Overall: 13–18 year: 120.2 mL/d | Mean energy intake for all: 1991 kcal/day |
| 2 | Bae, 2014 [88] | Korea | 9400 | Apparently healthy adult (Korean Multi-Rural Communities Cohort Study) | Food Frequency Questionnaire | Soft drink intake Males (n = 3564): 16.2 ± 56.7 mL/d Females (n = 5836): 8.7 ± 45.0 mL/d | Energy intake Males (n = 3564): 1698.5 ± 494.1 mL/d Females (n = 5836): 1481.0 ± 437.0 mL/d |
| 3 | Ha, 2016 [89] | South Korea | 2599 | Children (9–14 years) | Dietary records 3–7 days | Mean percent energy from SSB Males: 5.8% Females: 6.0% | Mean energy intake: Males: 1846 kcal/day Females: 1617 kcal/day |
| 4 | Song, 2016 [90] | South Korea | 9869 | Adults (19–64 years) | Food Frequency Questionnaire + 24-h diet recall | Mean carbonated SSB Overall: 21.8 g/d Males: 28.9 g/d Females: 17.2 g/d | Energy intake: 1910 kcal/d Males: 2322 kcal/d Females: 1648 kcal/d |
| 5 | Lim, 2017 [91] | South Korea | 49,826 | Subject aged > 1 year | 24-h dietary recall | SSB consumption per consumer as energy intake Year 1998: 123 kcal Year 2001: 141 kcal Year 2005: 126 kcal Year 2007–09: 166 kcal | Not reported |
| 6 | Kwak, 2018 [92] | South Korea | 5775 | Adult (≥40 years old) | Food Frequency Questionnaire | Mean SSB intake: 1.5 serving/week | Energy intake: 1938 kcal/d |
| 7 | Sakurai, 2013 [93] | Japan | 2037 | Adult men (35–44 years) | Diet History Questionnaire | Median (range) SSB intake: 0.2 (0.0–9.6) servings †/d | Mean energy intake: 2194 kcal/d |
| 8 | Lin, 2016 [94] | Taiwan | 1454 | Adolescents (12–16 years) | Food Frequency Questionnaire | SSB intake Nonuser = 11% 1–350 mL/d = 38% 351–750 mL/d = 44% >750 mL/d = 7% | Energy intake (kcal/d) Nonuser: 1930 ± 52.7 1–350 mL/d: 1901 ± 27.9 351–750 mL/d: 2194 ± 42 >750 mL/d: 2425 ± 151 |
| 9 | Li, 2014 [95] | China | 29,215 | Adult (≥18 years old) | Not reported | SSB consumption rate 2004: 15.1% 2006: 14.9% 2009: 29.3% | Not reported |
| 10 | Gui, 2017 [96] | China | 53,151 | Adolescents (6–17 years) | Self-reported dietary questionnaire | SSB per capita: 0.41 serving */d SSB per consumer: 0.61 serving */d | Not reported |
| 11 | Li, 2020 [97] | China | 5,258 | Adolescents (7–18 years) | Simplified self-reported dietary questionnaire | SSB intake Non-SSB drinker = 33.4% 0–0.3 serving */d = 36.8% >0.3 serving */d = 29.8% | Not reported |
| 12 | Gan, 2021 [98] | China | 25,553 | Adolescents (6–17 years) | Food Frequency Questionnaire | SSB intake: 181 g/d 6–10 year old: 129.5 g/d 11–14 year old: 208 g/d 15–17 year old: 285 g/d | Median energy intake: 1892.3 (1419.7–2572.6) kcal/d |
| 13 | Gui, 2021 [99] | China | 6,387 | Children (6–12 years) | Self-reported dietary questionnaire | Mean SSB intake per week per SSB consumer: 2.45 servings * | Not reported |
| 14 | Zhu, 2021 [100] | China (Shanghai) | 3958 | Adolescents (6–17 years) | Three 24-h dietary recalls | SSB consumption 59.4 ± 126.3 g/d | Not reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khor, B.H.; Komnenov, D.; Rossi, N.F. Impact of Dietary Fructose and High Salt Diet: Are Preclinical Studies Relevant to Asian Societies? Nutrients 2022, 14, 2515. https://doi.org/10.3390/nu14122515
Khor BH, Komnenov D, Rossi NF. Impact of Dietary Fructose and High Salt Diet: Are Preclinical Studies Relevant to Asian Societies? Nutrients. 2022; 14(12):2515. https://doi.org/10.3390/nu14122515
Chicago/Turabian StyleKhor, Ban Hock, Dragana Komnenov, and Noreen F. Rossi. 2022. "Impact of Dietary Fructose and High Salt Diet: Are Preclinical Studies Relevant to Asian Societies?" Nutrients 14, no. 12: 2515. https://doi.org/10.3390/nu14122515
APA StyleKhor, B. H., Komnenov, D., & Rossi, N. F. (2022). Impact of Dietary Fructose and High Salt Diet: Are Preclinical Studies Relevant to Asian Societies? Nutrients, 14(12), 2515. https://doi.org/10.3390/nu14122515







